Back to Journals » Cancer Management and Research » Volume 13
Chronic Progression of Lung Cancer Recurrence After Surgery: Warning Role of Postoperative Pneumonia
Authors Lin D, Zhu J, Xu X, Xiao K, Wen X, Zheng Q, Zhou Y, Cai X
Received 2 July 2021
Accepted for publication 11 September 2021
Published 24 September 2021 Volume 2021:13 Pages 7387—7398
DOI https://doi.org/10.2147/CMAR.S327646
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Dong-qi Lin,1 Jin-guo Zhu,1 Xiao-hua Xu,1 Ke Xiao,1 Xu-qing Wen,1 Qi-fa Zheng,1 Yu-hua Zhou,2 Xin-ying Cai3
1Department of Cardiothoracic Surgery, The Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, People’s Republic of China; 2Nursing Department, Shantou Central Hospital, Shantou, Guangdong, People’s Republic of China; 3Clinical Research Center, Shantou Central Hospital, Shantou, Guangdong, People’s Republic of China
Correspondence: Xin-ying Cai
Clinical Research Center, Shantou Central Hospital, Wai-ma Road 114, Shantou, Guangdong, People’s Republic of China
Tel +86 754-88903584
Fax +86 754-88548117
Email [email protected]
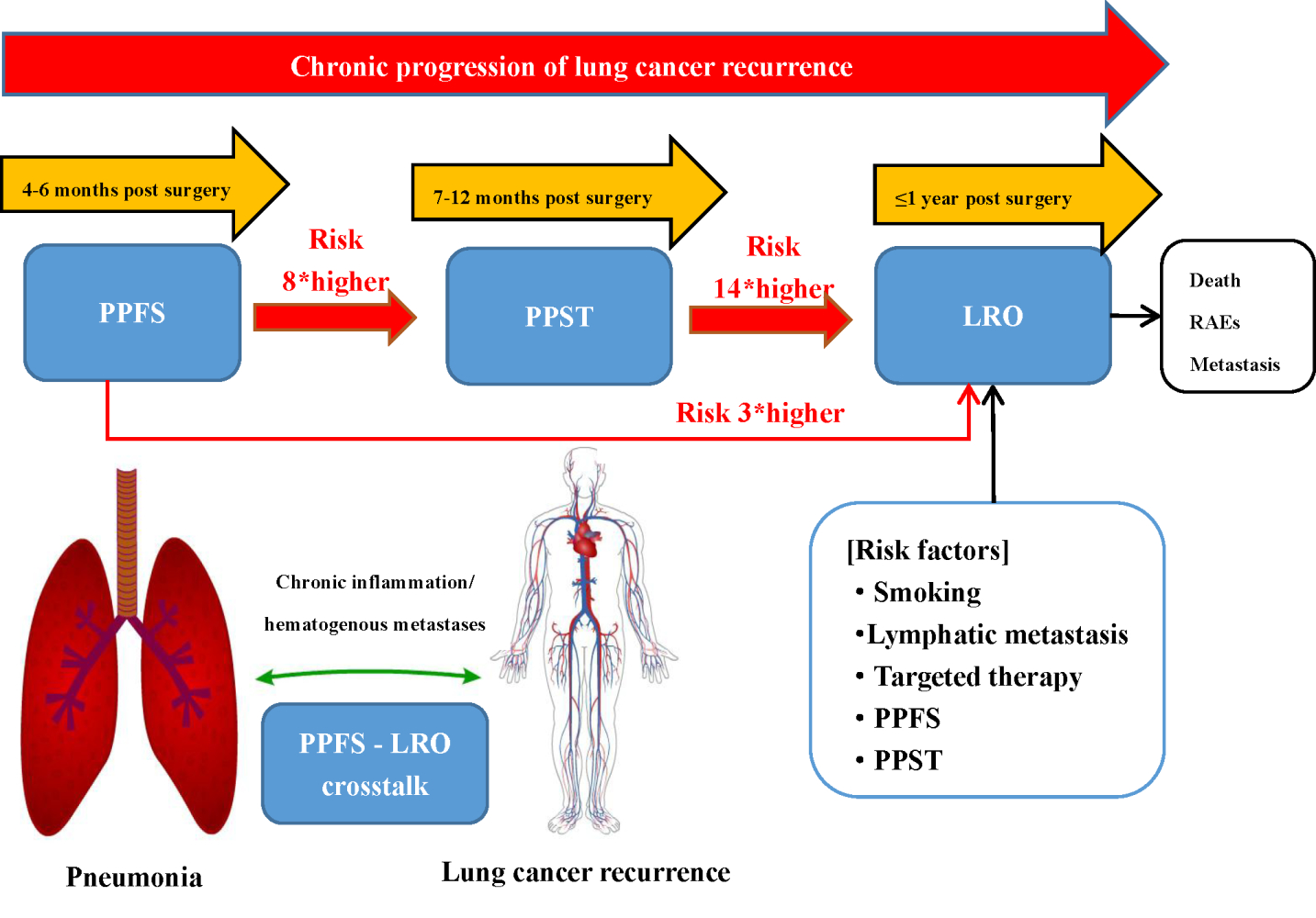
Purpose: The association between the process of postoperative pneumonia and lung cancer recurrence remains elusive in lung cancer surgery. Herein, the association between postoperative pneumonia and lung cancer recurrence was investigated, emphasizing the warning role of postoperative specific pneumonia in primary lung cancer resection patients.
Methods: The occurrence of postoperative pneumonia was assessed in 4– 6 months (PPFS), 7– 12 months (PPST), and lung cancer recurrence within 1 year (LRO) in 332 patients. The primary outcome was the development of PPST and LRO according to PPFS occurrence. The relevant risk factors of PPFS, PPST, and LRO were identified through multivariable regression analysis.
Results: During follow-up, 151 (45.48%) participants experienced PPFS. Irrespective of the existing postoperative pneumonia in 1– 3 months (PPOT), PPFS significantly increased the risk of PPST (P < 0.01) and LRO (P < 0.01), and persistent PPST further increased the risk of LRO (P < 0.001). The generalized estimating equation identified chemotherapy as an independent risk factor for PPFS and PPST.
Conclusion: PPFS was associated with the increased risk of PPST and LRO. Postoperative pulmonary inflammation assessed 4 months post-surgery also significantly influenced LRO development, indicating a need for close follow-up of lung inflammatory conditions to improve patient outcomes.
Keywords: chemotherapy, targeted therapy, inflammatory environment, risk factors
Graphical Abstract:
Introduction
Lung cancer is the leading cause of cancer deaths worldwide, as it is a malignant tumor with a high recurrence rate.1 Hitherto, surgery is the leading curative therapy for early-stage lung cancer.2 The risk factors of postoperative pneumonia are related to several causes, such as age, length of hospital stay, and previous antibiotic treatment.3–5 The occurrence of postoperative pneumonia affects the outcome of tumor treatment and has a significant correlation with the overall survival rate of patients.6,7
Smoking is the major risk factor for lung cancer.8 Some studies reported that the estimated prevalence rate of smoking is five times greater for men than women. Recently, lung cancer in women who are nonsmokers is on the rise, especially in Asia.9,10 Wu et al11 also reported that women with pulmonary adenocarcinoma were likely to be nonsmokers. Thus, female gender and a family history of lung cancer are two major predicted groups of lung cancer recurrence. This association between pulmonary adenocarcinoma and nonsmokers has also been shown by Hsu et al in 2018.12
Reportedly, during the first 4 years after surgery, the risk of lung cancer recurrence ranged from 6% to 10% per person-year.13 According to previous studies, the overall mortality was 6% in the first year after surgery. The risk of death due to lung cancer was 36%.14 The mortality analysis of lung cancer patients revealed that the number of cancer-related deaths is associated with postoperative pneumonia.15–17 Pneumonia is a common complication after lung cancer surgery. Also, an association between postoperative pneumonia and LRO was observed in lung cancer surgery patients. Postoperative pneumonia following lung cancer resection is an immediate and common complication referred to as postoperative pneumonia referred in 30 days (PPT). Previous studies about postoperative pneumonia on risk assessment mainly focused on the short term after lung cancer resection. The long-term incidence of developing pneumonia after discharge and subsequent complications has rarely attracted attention.
No comprehensive study has yet shown any correlation between the occurrence of postoperative discharged pneumonia and lung cancer recurrence. To address the drawbacks of these studies, we conducted a longitudinal retrospective analysis to elucidate the correlation between the chronic progression of postoperative pneumonia in patients with lung cancer surgery and the recurrence of lung cancer at 1 year after surgery. This study would provide a valuable framework for selecting patients who would benefit from close post-surgical follow-up.
Materials and Methods
Patients
This study included lung cancer patients who underwent complete surgical resection in two comprehensive hospitals from January 2015 to April 2020. The inclusion criteria were as follows: histologically confirmed primary lung cancer without metastatic tumors according to the 7th American Joint Committee on Cancer (AJCC) staging manual. Initial diagnosis revealed that all patients ≥18-years-old underwent extensive examinations, including chest computed tomography (CT), abdominal ultrasonography, whole-body bone scan, and brain magnetic resonance imaging (MRI). The exclusion criteria presented clinical evidence of congenital lung disease. Patients diagnosed with fourth-stage lung cancer or severe lung dysfunction were not included. In addition, patients lost to follow-up were also excluded from the study. The present study was approved by the Ethics Committee of The Second Affiliated Hospital of Shantou University Medical College and Shantou Central Hospital (2021–38). This study complied with the Declaration of Helsinki. Informed consent was obtained from all the patients.
Data Collection
Data on patients, including age, sex, smoking history, time of quitting smoking, preoperative comorbidities, and medications, from the electronic database of the medical record system, were reviewed retrospectively. Intraoperative data included the type of lung cancer surgery, TNM-stage, histological data, lymphatic metastasis, operation time, and intraoperative blood loss. Postoperative data included hospital stay, intensive care unit (ICU) stay, 30-day mortality, in-hospital mortality, wound infection, failed intubation, reoperation due to blood loss, and postoperative treatments. Respiratory adverse events (RAEs) were defined as upper respiratory tract infections (URTIs) and lower respiratory tract infections (LRTIs), including nasopharyngitis and laryngitis, bronchitis, influenza, respiratory-related opportunistic infections (OIs), interstitial lung disease (ILD), and pulmonary embolism (PE), including deep vein thrombosis (DVT) or venous thromboembolism (VTE).18 Laboratory data included white blood cell count, neutrophil ratio, and albumin level. In addition, we reviewed the reports of preoperative pneumonia at discharge and 3, and 6, and 12 months post-surgery.
The most recent preoperatively pulmonary imaging report within 30 days was used for the initial evaluation of lung inflammation, and chest imaging reports within the postoperative 30 days as well as during 1–3-, 4–6-, and 7–12-month follow-ups were used for diagnosis of PPT, PPOT, PPFS, and PPST, respectively. The primary outcome was a recrudescent lung outcome compared to the occurrence of postoperative pneumonia.19 The inclusion criteria were postoperative pneumonia diagnosed by either pulmonologist or thoracic surgeon according to the Infectious Diseases Society of America/American Thoracic Society guidelines for diagnosing community-acquired pneumonia (CAP) in adults. These guidelines provided criteria for the diagnosis of pneumonia: the presence of clinical features of lung infiltration through chest X-rays and CT scans.20,21
The primary outcome was the development of PPST and LRO according to PPFS occurrence in patients. For this outcome, the risk of PPFS, PPST, and LRO was assessed according to the occurrence and period of preoperative and postoperative pneumonia. The secondary outcome was the influence of PPFS on recurrence rate, the incidence of RAEs, and mortality in the first year after lung cancer surgery. In addition, the risk factors contributing to the development of postoperative PPST and LRO were identified.
Surgical Procedures and Postoperative Follow-Up Protocol
The data of a total of 451 patients who underwent lobectomy and lymph node dissection for lung cancer were enrolled. The surgical procedures include routinely performed lymph node dissection with lobectomy and pneumonectomy. The postoperative follow-up, adjuvant chemotherapy, targeted therapy, and radiotherapy were performed in the two institutions. The detection of pneumonia and lung cancer recurrence protocol adhered to the following schedule in this study. Chest X-rays and CT scans were conducted in discharge at 1–3-, 4–6-, and 7–12-months post-surgery. Patients who do not return on time for each treated course were considered as lost to follow-up. Brain imaging and bone scintigraphy were scheduled to detect metastasis. Recurrence included both locoregional and systemic diseases according to chest X-rays and CT scans.
Statistical Analysis
Statistical analyses were performed using SPSSAU 2016–2021 (Qing-Si Technology Ltd, Beijing, China). First, we compared the participants’ baseline characteristics and PPFS. Categorical variables were compared using chi-square or Fisher’s exact test and expressed as absolute number (percent). The multivariate analyses using a generalized estimating equation (GEE) with the logit function and multivariable logistic regression analysis to evaluate the association of PPFS with the development of PPST and LRO to account for the correlation of events. Variables, including PPOT, PPFS, and PPST potentially affecting LRO were entered into a multivariable logistic model to assess their impact on the chronic development of LRO. Additionally, variables with a P-value <0.05 in the univariate analysis were included within the scope of satisfying analysis to increase the predictive power of the model. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated using logistic regression models. P-values <0.05 indicated statistical significance.
Results
Baseline Characteristics
Of the 406 cases, 74 lost to follow-up were excluded. The remaining 332 patients were enrolled in this study (Figure 1). The cohort consisted of 203 (61.14%) males. Subsequently, 151 (45.48%) patients developed PPFS during the follow-up period. A total of 26 patients underwent molecular screening and received targeted therapy (Figure 2). Participants who were treated with chemotherapy after surgery or suffered intraoperative blood loss tended to have PPFS. Also, the in-hospital mortality rate was 0%, and failed intubation and reoperation due to blood loss did not occur (Table 1, Supplemental Table 1).
 |
Table 1 Clinical Characteristics of Patients with or without PPFS |
 |
Figure 1 Flow of the retrospective study. |
PPFS and Development of PPST and LRO
The incidence of preoperative pneumonia and PPOT was significantly higher in PPFS patients than in non-PPFS patients (59.60% vs 46.96%, P < 0.05; 80.13% s. 23.76%, P < 0.001). Compared to PPFS patients, non-PPFS patients had a significantly higher incidence of early recovery (69.61% vs 36.42%, P < 0.001), defined as an absence of postoperative pneumonia diagnostic criteria 30 days after surgery. The incidence of PPST (81.46% vs 0.24.31%, P < 0.001) and LRO (49.67% vs 12.15%, P < 0.001) was significantly higher in PPFS than in non-PPFS patients (Table 2).
 |
Table 2 Preoperative and Postoperative Pneumonia Occurrence, Recovery, and Outcome of Patients with or without PPFS |
On the other hand, PPOT was at an increased risk for PPST with a relative risk of 4.604. Also, PPFS occurrence increased the risk of PPST (OR: 2.886, 95% CI: 1.193–6.978, P < 0.05) and LRO (OR: 2.793, 95% CI: 1.406–5.552, P < 0.001) according to the GEE method for analyzing the primary outcomes of the study (Table 3). Furthermore, PPST occurrence had a significantly higher incidence with LRO (53.29% vs 4.85%, P < 0.001) compared to non-PPST patients. LRO itself was associated with increased risk of PPST (OR: 16.271, 95% CI: 6.757–39.182, P < 0.001).
 |
Table 3 Risks for Development of PPST According to Postoperative Pneumonia Occurrence and Stage |
Risk Factors for PPFS, PPST, and LRO
According to multivariable logistic regression analysis, chemotherapy was the risk factor for PPFS and PPST. Significantly, PPOT was identified as the risk factor for PPFS and PPST development, which was not revealed as an independent risk factor for LRO. PPFS was an independent risk factor associated with the development of both PPST (OR: 8.382, 95% CI: 4.304–16.323, P < 0.001) and LRO (OR: 2.911, 95% CI: 1.290–6.569, P < 0.01) after adjusting for other influencing factors (Table 4). Smoking and lymphatic metastasis were the risk factors for LRO (OR: 2.380, 95% CI: 1.107–5.118, P < 0.05; OR: 4.518, 95% CI: 1.566–13.039, P < 0.01), while the targeted therapy was identified as the protective factor (OR: 0.100, 95% CI: 0.027–0.376, P < 0.01). When introducing PPST instead of PPFS to the multivariable model for LRO, PPST was identified as the robust risk factor for LRO (OR: 21.770, 95% CI: 8.273–57.283, P < 0.001).
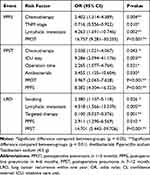 |
Table 4 Multivariable Logistic Regression Analysis of Risk Factors for PPFS, PPST, and LRO Patients |
Development of a LRO-Predicting Nomogram
Based on the independent predictors in the multivariate analysis identified in this study, nomogram was formulated to predict LRO (Figure 3). The model’s explanatory covariables consisted of smoking, lymphatic metastasis, targeted therapy, PPFS and PPST. Each level of the above variable was assigned a score on the scale. By adding the score for each of the selected variables, a total score was obtained for each patient. The LRO probability of each patient could be easily calculated by adding the scores for each variable. Patients with lower scores in the nomogram corresponded to inferior recurrence.
 |
Figure 3 Nomogram was used for predicting the risk of LRO. Abbreviations: PPFS, postoperative pneumonia in 4–6 months; PPST, postoperative pneumonia in 7–12. |
Discussion
Lung cancer is a common malignant tumor with poor therapeutic effects. Currently, comprehensive treatment is recommended as an effective strategy for lung cancer patients.16 However, the high recurrence rate at 1 year after lung cancer surgery is challenging in clinical treatment.22 The majority of the studies have focused on the risk factors for overall survival after lung cancer resection. Also, there is a lack of real-world data to predict and evaluate the clinical risk of lung cancer recurrence in the first year after surgery. Currently, the FDA attaches importance to real-world evidence, including electronic health records that can add and evaluate information on how factors, such as clinical setting and health system characteristics, affect the therapeutic effect and guide the outcomes.17 To the best of our knowledge, this is the first study to provide some real-world evidence to assess the recurrence of cancer within 1 year after lung cancer resection. A previous study suggested that the first local recurrence rate after lung cancer resection is 27–36%.19 In this retrospective review, we found that LRO incidence reached 29.22%, which was consistent with previous reports. The present study showed a close link between the occurrence of PPST and LRO development with a distinct warning role for PPFS. The PPFS-associated risk for LRO development was independent of preoperative pulmonary conditions and PPOT. PPFS is a clinical time window for critical interventions to modify disease deterioration and progression.
In the current analyses, PPFS significantly increased the risk of development of LRO, regardless of the existing preoperative pneumonia and PPT. Although preoperative pneumonia and PPT groups have a higher incidence of PPFS than the preoperative non-pneumonia and non-PPT groups, the risk of preoperative pneumonia and PPT on the development of LRO did not differ in patients without preoperative pneumonia and PPT. A poor early recovery of postoperative pneumonia was not associated with the incidence of PPST and PPFS. Although one study established a close link between PPT and the prognosis of lung cancer resection, it focused on the overall survival within 5 years after surgery.4 It did not assess the actual progression of LRO, which restricted further direct comparisons to our study. Herein, we found that PPOT patients had four times higher risk of progression to PPST than non-PPOT patients, whereas the risk was developing LRO was unchanged. However, the risk of PPST and LRO was increased up to eight and three times in PPFS patients compared with non-PPFS, respectively.
The association of the history of chemotherapy and the occurrence of PPFS and PPST was well-demonstrated. The PPFS and PPST groups had a higher incidence in the history of postoperative chemotherapy than the non-history groups. The history of chemotherapy was an independent risk factor for the progress of PPFS and PPST. A previous study showed that adjuvant chemotherapy after surgery is a high-risk factor for postoperative pneumonia.23 Chemotherapy played a major role after lung cancer surgery. Previous studies suggested that chemotherapy was related to the mechanism of alveolar injury and immune function. The level of inflammatory cells and interleukins increases in the bronchoalveolar lavage systemic inflammatory response in chemotherapy patients compared to those not undergoing neoadjuvant treatment24. Higenbottam et al reported that chemotherapy agents affect the lungs. The drug acts directly on alveolar cells in the metabolic process and induces an immune response to produce several biochemical substances. Lung damage is directly related to the dose of the drug and is affected by drug–drug, drug–diet, and drug–environment interactions.25 In addition, relevant clinical studies also showed that the commonly used chemotherapeutics for lung cancer, such as docetaxel, paclitaxel, gemcitabine, and vinorelbine, cause alveolar epithelial cell damage. Therefore, we hypothesized that chemotherapy weakens the immune system function over a period. The current study found that chemotherapy history is directly related to the incidence of PPFS but is not a risk factor for the occurrence of LRO. About 47.59% of patients in the current study did not experience chemotherapy. Therefore, PPFS patients without a history of chemotherapy should be treated with caution to prevent LRO occurrence.
Recent studies showed that small-molecule agents that target EGFR are potential causes of pneumonitis in patients with lung cancer.26 Furthermore, a post-marketing study found that crizotinib was associated with pneumonitis in 5.77% of ALK-positive NSCLC patients, of which 3.45% had pneumonitis of at least grade 3.27 However, the current study has shown that targeted therapy did not increase the incidence of PPFS and PPST, which was not consistent with the previous reports. With the progress of driving mutations, lung cancer has shifted from cytotoxic chemotherapy to molecular-tailored therapy. The results demonstrated that the targeted therapy was defined as the protective factor of LRO. The identification and characterization of driver mutations include epidermal growth factor receptor gene (EGFR) mutations, echinoderm microtubule-associated protein-like 4 gene (EML4), anaplastic lymphoma receptor tyrosine kinase gene (ALK) fusion, and reactive oxygen species (ROS) proto-oncogene 1 fusion in non–small cell lung cancer (NSCLC). Several large clinical trials provided evidence that appropriate targeted therapies for patients with NSCLC with defined molecular aberrations have a transformed treatment response and better outcomes than with chemotherapies. Sharma et al28 reported that EGFR overexpression was observed in tumors in >60% of patients with metastatic NSCLC and is correlated with poor prognosis. Zhong et al29 reported that EGFR tyrosine kinase inhibitors (TKIs) constitute the standard treatment for patients with EGFR mutations. The treatment with TKIs resulted in prolonged progression-free survival compared to conventional chemotherapy. The current study indicated that targeted therapy is crucial for advanced NSCLC and for the treatment of lung cancer within 1 year after surgery, which was consistent with previous reports. Therefore, molecular targeted therapy has critical therapeutic significance for LRO. However, the sample size was extremely small in this study for statistically significant results. The identification of driver mutations in the current study included the EGFR mutation, ALK fusion, or ROS1 fusion. The association between other driver mutation-related pneumonia and LRO was not analyzed in this study, thereby necessitating to expand the sample size for verification.
Previous studies have shown that smoking is associated with the recurrence of lung cancer. Next, we found that the LRO group had a higher incidence of smoking than the non-LRO group. The findings were consistent with previous reports. Accordingly, it was suggested that smoking is the risk factor for the development of LRO. Some studies reported that the carcinogenic compounds of cigarette smoke induce a direct cytotoxicity and mutagenic action on lung epithelial cells by generating somatic mutations,30 epigenetic events, epithelial-mesenchymal cell transformations,31,32 and chronic cell damage. Cigarette smoke-induced chronic lung inflammatory microenvironment, oxidative stress, and cell structure alterations, such as an increase in cell proliferation, angiogenesis, and apoptosis arrest, are irreversible processes with a significant influence in lung tumor growth.33 Okudela et al34 reported that a subset of adenocarcinoma patients showed a remarkable response to the targeted drugs. This phenomenon was frequent in the Japanese population in women and never-smokers.35 The occurrence and recurrence of lung adenocarcinoma may be induced by KRAS mutation in nonsmokers, while EGFR mutation could lead to lung adenocarcinoma relapse in smokers. Therefore, specific molecular targeted therapy and histological diagnosis could be combined in the treatment of smoking and non-smoking patients with lung cancer after surgery. Early diagnosis and follow-up of postoperative patients according to gene mutation and smoking status could prevent tumor metastasis.
The present study has shown that the perioperative rate was 26.5%, which was slightly lower than the LRO rate (29.2%). Lymph node metastasis was analyzed as the independent risk factor for LRO and related to the incidence of PPFS. Kotlyarov and Rukosuyev36 reported that the five-year local recurrence rate after radical resection of lung cancer was up to 33.8%, and the blood metastasis rate was 55.2%. Approximately 88.7% of blood metastases occurred within 1 year after surgery. Previous studies37,38 have established a correlation between hematogenous metastasis and lymph node metastasis. Leung et al39 reported that lymph nodes are often the first site of metastasis, deeming that lymphatics might serve as a part of the pathway contributing to the subsequent dissemination to distant organs. Experimental evidence has demonstrated that inhibiting lymphatic dissemination could be a promising method for preventing distant metastases. This study also provided evidence that lymph node metastasis has critical prognostic value after surgical resection of lung cancer, which might help to discover and guide the occurrence of LRO. Herein, we speculated that focusing on lymph node metastasis would predict the prognostic outcomes for lung cancer patients that would help the oncologists and physicians to make accurate and precise decisions.
There are two possible explanations about the association of PPFS with LRO. The first explanation is that systemic inflammation accelerates the adhesion of circulating tumor cells to the vascular endothelium of distant organs, which is the first step of forming distant metastases.40,41 Numerous studies have demonstrated that these two statuses of postoperative inflammatory and lung cancer metastasis are inter-related. The inflammatory status of PPOT might be related to the postoperative wound repair or the prognosis of lung tumors. Hence, PPOT is not the risk factor for predicting LRO. In the absence of PPOT, the recurrence of PPFS combined with the continuous attack state of PPST would form an inflammatory environment, which might accelerate the spread of tumor cells and further induce the development of LRO. The second explanation is that when the cause of inflammation, ie, PPOT persists, acute inflammation can be converted into chronic inflammation. The inflammatory cells could release chemicals, such as ROS, that promote carcinogenic evolution, and many factors released by inflammatory cells might inhibit the immune response.42,43 We observed that as a critical time window after lung cancer resection, PPFS played a warning role during the chronic inflammation of the development of LRO. Thus, PPFS, which was associated with the inflammatory environment, maybe a predictive factor for LRO.
In addition to avoiding further LRO, identifying the risk factors for lung cancer recurrence in the early stage could guide a timely follow-up and application of therapeutic measures in patients at risk for PPST progression after lung cancer surgery-associated PPFS. In this regard, our study has several strengths. This is the first study to encompass comprehensive datasets of real-world evidence of serially assessed lung inflammation and recurrence during the postoperative 12 months in a cohort of lung cancer surgery patients. Herein, we described a detailed overview of the factors associated with the occurrence of PPFS. In addition, this study provides primary evidence that regardless of PPOT, PPFS is associated with LRO development, thereby emphasizing the warning role for PPFS and the intermediary role for PPST, connecting these two diseases. Lastly, we reaffirmed the well-known risk factors for LRO, including smoking, lymphatic metastasis, blood loss, and radiotherapy. Chemotherapy or targeted therapy is a risk factor for PPFS and PPST. These findings might be helpful in identifying patients who had not received chemotherapy or targeted therapy and may benefit from close post-surgery follow-up because 40.99% and 19.88% of these patients in our study developed PPST and LRO, respectively. Future studies are warranted to confirm whether identifying and treating PPFS may improve LRO and the overall prognosis outcomes.
Conclusion
In conclusion, we observed a close correlation between PPST occurrence and LRO development with a warning role for PPFS in lung cancer surgery patients. Thus, all patients with lung cancer surgery should be followed and assessed for the pulmonary inflammation that reappeared and expanded after 3 months, especially the patients without a history of chemotherapy or targeted therapy. Multidisciplinary and close follow-ups to assess the recurrence and metastasis of lung cancer also should be considered in the case of patients without a history of chemotherapy or targeted therapy but with proposed risk factors or appearance of PPFS. In addition, animal studies are required to evaluate the molecular correlation between PPFs and LRO, and prospective studies need to be conducted to develop the management strategies to detect the postoperative intervention during lung cancer recurrence.
Acknowledgments
This work was supported by Shantou Science and Technology Plan Medical and Health Category Project Declaration Form, China.
Disclosure
The authors report no conflicts of interest in this study.
References
1. Lou F, Huang J, Sima C, Dycoco J, Rusch V, Bach P. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. The Journal of thoracic and cardiovascular surgery. 2013;145(1):75–81.
2. Deslauriers J, Ginsberg RJ, Piantadosi S, Fournier B. Prospective assessment of 30-day operative morbidity for surgical resections in lung cancer. Chest. 1994;106(6):329S–330S. doi:10.1378/chest.106.6_Supplement.329S
3. Mitás L, Horváth T, Sobotka M, Garajová B, Vomela J. [Complications in patients undergoing pulmonary oncological surgery]. Rozhledy v Chirurgii. 2010;89(2):113–117. Czech.
4. Simonsen DF, Søgaard M, Bozi I, Horsburgh CR, Thomsen RW. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med. 2015;109(10):1340–1346. doi:10.1016/j.rmed.2015.07.008
5. Höffken G, Niederman MS. Nosocomial pneumonia: the importance of a de-escalating strategy for antibiotic treatment of pneumonia in the ICU. Chest. 2002;122(6):2183–2196. doi:10.1378/chest.122.6.2183
6. Rosolem MM, Rabello L, Lisboa T, et al. Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J Crit Care. 2012;27(3):301. doi:10.1016/j.jcrc.2011.06.014
7. Farjah F, Backhus L, Cheng A. Failure to rescue and pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2015;149(5):1365–1373.e1363. doi:10.1016/j.jtcvs.2015.01.063
8. Samet JM, Avila-Tang E, Boffetta P, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15(18):5626–5645. doi:10.1158/1078-0432.CCR-09-0376
9. Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25(5):472–478. doi:10.1200/JCO.2006.07.2983
10. Lo YL, Tsai YH, Chang GC, et al. Risk factors for primary lung cancer among never smokers by gender in a matched case-control study. Cancer Causes Control. 2013;24(3):567–576. doi:10.1007/s10552-012-9994-x
11. Wu FZ, Huang YL, Wu C, et al. Assessment of selection criteria for low-dose lung screening CT among Asian Ethnic Groups in Taiwan: from mass screening to specific risk-based screening for non-smoker lung cancer. Clin Lung Cancer. 2016;17(5):e45–e56. doi:10.1016/j.cllc.2016.03.004
12. Hsu HT, Tang EK, Wu MT, et al. Modified lung-RADS improves performance of screening LDCT in a population with high prevalence of non–smoking-related lung cancer. Acad Radiol. 2018;25(10):1240–1251. doi:10.1016/j.acra.2018.01.012
13. Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013;145(1):75–82. doi:10.1016/j.jtcvs.2012.09.030
14. Bugge AS, Lund MB, Valberg M, Brustugun OT, Solberg S, Kongerud J. Cause-specific death after surgical resection for early-stage non-small-cell lung cancer. Eur J Cardio Thorac Surg. 2018;53(1):221–227.
15. Radu DM, Jauréguy F, Seguin A, et al. Postoperative pneumonia after major pulmonary resections: an unsolved problem in thoracic surgery. Ann Thorac Surg. 2007;84(5):1669–1673. doi:10.1016/j.athoracsur.2007.05.059
16. Chaft JE, Rimner A, Weder W, Azzoli CG, Cascone T. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol. 2021;18:547–557.
17. Sherman RE, Anderson SA, Pan GD, et al. Real-world evidence - what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–2297. doi:10.1056/NEJMsb1609216
18. Khoo JK, Barnes H, Key S, Glaspole IN, Östör J. Pulmonary adverse events of small molecule JAK inhibitors in autoimmune disease: systematic review and meta-analysis. Rheumatology. 2020;59(9):2217–2225. doi:10.1093/rheumatology/keaa117
19. Fedor D, Johnson WR, Singhal S. Local recurrence following lung cancer surgery: incidence, risk factors, and outcomes. Surg Oncol. 2013;22(3):156–161. doi:10.1016/j.suronc.2013.04.002
20. Watkins RR, Lemonovich TL. Diagnosis and management of community-acquired pneumonia in adults. Am Fam Physician. 2011;83(11):1299–1306.
21. Marrie TJ. Community-acquired pneumonia. Clin Infect Dis. 1994;18(4):501–513. doi:10.1093/clinids/18.4.501
22. Kobayashi S, Karube Y, Matsumura Y, Nishihira M, Chida M. Inflammatory risk factors for early recurrence of non-small cell lung cancer within one year following curative resection. World J Surg. 2020;44(10):3510–3521. doi:10.1007/s00268-020-05612-0
23. Sarıçam M. Efficacy of preoperative white blood cell count and lymphocyte/monocyte ratio in predicting post-lobectomy pneumonia. Turk J Thorac Cardiovasc Surg. 2021;29(1):84–91. doi:10.5606/tgkdc.dergisi.2021.19950
24. Leo F, Pelosi G, Sonzogni A, Chilosi M, Bonomo G, Spaggiari L. Structural lung damage after chemotherapy: fact or fiction? Lung Cancer. 2010;67(3):306–310. doi:10.1016/j.lungcan.2009.04.013
25. Higenbottam T, Kuwano K, Nemery B, Fujita Y. Understanding the mechanisms of drug-associated interstitial lung disease. Br J Cancer. 2004;91(S2):S31–37. doi:10.1038/sj.bjc.6602065
26. Mahmutovic Persson I, von Wachenfeldt K, Waterton J, Olsson L; On Behalf Of The Tristan Consortium. Imaging biomarkers in animal models of drug-induced lung injury: a Systematic Review. J Clin Med. 2020;10(1):107. doi:10.3390/jcm10010107
27. Gemma A, Kusumoto M, Kurihara Y, et al. Interstitial lung disease onset and its risk factors in Japanese patients with ALK-Positive NSCLC after treatment with crizotinib. J Thorac Oncol. 2019;14(4):672–682. doi:10.1016/j.jtho.2018.11.022
28. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181.
29. Zhong W, Wang Q, Mao W, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713–722. doi:10.1200/JCO.20.01820
30. Anderson GP, Bozinovski S. Acquired somatic mutations in the molecular pathogenesis of COPD. Trends Pharmacol Sci. 2003;24(2):71–76. doi:10.1016/S0165-6147(02)00052-4
31. Liu Y, Gao W, Zhang D. Effects of cigarette smoke extract on A549 cells and human lung fibroblasts treated with transforming growth factor-β1 in a coculture system. Clin Exp Med. 2010;10(3):159–167. doi:10.1007/s10238-009-0081-x
32. Veljkovic E, Jiricny J, Menigatti M, Rehrauer H, Han W. Chronic exposure to cigarette smoke condensate in vitro induces epithelial to mesenchymal transition-like changes in human bronchial epithelial cells, BEAS-2B - ScienceDirect. Toxicol Vitro. 2011;25(2):446–453. doi:10.1016/j.tiv.2010.11.011
33. Milara J, Cortijo J. Tobacco, inflammation, and respiratory tract cancer. Curr Pharm Des. 2012;18(26):3901–3938. doi:10.2174/138161212802083743
34. kudela K, Matsumura M, Arai H, Woo T. The nonsmokers' and smokers' pathways in lung adenocarcinoma: Histological progression and molecular bases. Cancer science. 2021.
35. Yatabe Y. EGFR mutations and the terminal respiratory unit. Cancer Metastasis Rev. 2010;29(1):23. doi:10.1007/s10555-010-9205-8
36. Kotlyarov EV, Rukosuyev AA. Long-term results and patterns of disease recurrence after radical operations for lung cancer. J Thorac Cardiovasc Surg. 1991;102(1):24–28. doi:10.1016/S0022-5223(19)36580-8
37. Hashimoto T. Prognostic value of genetically diagnosed lymph node micrometastasis in non-small cell lung carcinoma cases. Cancer Res. 2000;60:6472–6478.
38. Passlick B, Izbicki JR, Kubuschok B, Thetter O, Pantel K. Detection of disseminated lung cancer cells in lymph nodes: impact on staging and prognosis. Ann Thorac Surg. 1996;61(1):177. doi:10.1016/0003-4975(95)01012-2
39. Leung E, Xue A, Wang Y, Rougerie P, Sharma VP. Blood vessel endothelium-directed tumor cell streaming in breast tumors requires the HGF/C-Met signaling pathway. Oncogene. 2017;36(19):2680–2692. doi:10.1038/onc.2016.421
40. Mcdonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2010;125(6):1298–1305.
41. Kate MT, Hofland LJ, Grevenstein W, Koetsveld P, Jeekel J, Eijck C. Influence of proinflammatory cytokines on the adhesion of human colon carcinoma cells to lung microvascular endothelium. Int J Cancer. 2004;112(6):943–950. doi:10.1002/ijc.20506
42. Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15(2):73–86. doi:10.1038/nri3789
43. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

