Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Chronic Non-Healing Ulcers Associated with Atopic Inflammation: A Case Report
Authors Yang X, Wang H, Song Z, Chen Q
Received 17 May 2022
Accepted for publication 20 July 2022
Published 26 July 2022 Volume 2022:15 Pages 1429—1434
DOI https://doi.org/10.2147/CCID.S374964
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Xianjie Yang, Huan Wang, Zhiqiang Song, Qiquan Chen
Department of Dermatology, Southwest Hospital, Army Medical University, Chongqing, People’s Republic of China
Correspondence: Qiquan Chen, Department of Dermatology, Southwest Hospital, Army Medical University, No. 28 Gaotanyan Steet, Shapingba District, Chongqing, People’s Republic of China, Tel +86-15683418212, Email [email protected]
Abstract: Chronic non-healing ulcers are the undesirable outcome of delayed wound healing influenced by many factors. It can be seen in patients with diabetes, autoimmune conditions and multiple primary skin conditions. But chronic non-healing ulcers secondary to atopic inflammation are rarely reported in the literature. In this study, we reported a case with wounds caused by the wrong tattoo and surgery, activation of atopic inflammation caused delayed wound healing and the formation of chronic non-healing ulcers. The patient’s atopic inflammation was relieved and stabilized with oral cyclosporine and topical application of halometasone cream and subsequently 0.1% tacrolimus cream, and then the chronic non-healing ulcers healed well, without recurrence at a follow-up visit 3 months later.
Keywords: chronic non-healing ulcers, wound healing, type 2 inflammation, atopic inflammation, eosinophils, cyclosporine
Introduction
Skin wounds, one of the most common clinical issues, can be classified as acute or chronic, depending on whether the wound has been healing for more than three months, chronic wounds account for nearly 35% of all skin wounds, which not only make great impacts on the quality of patients’ life, but also place huge economic burden on the health-care systems.1 Wound healing is a complex process including hemostasis, inflammation, proliferation, and remodeling. In this process, angiogenesis plays a key role in improving the rate and quality of the healing process.2 Many underlying diseases can affect the angiogenesis and disrupt the wound healing process, resulting in chronic wounds and ultimately presenting as chronic non-healing ulcers, for example, diabetes, cardiovascular diseases, obesity, sensory neuropathies, autoimmune conditions such as scleroderma, rheumatoid arthritis, cutaneous lupus erythematosus inflammatory bowel disease, and primary skin conditions like necrobiosis lipoidica, sarcoidosis, ulcerative pyoderma gangrenosum, panniculitis (including erythema induratum), bullous diseases (including bullous pemphigoid, pemphigus, bullous lichen planus, and porphyria cutanea tarda), Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).3 In all chronic inflammatory settings, chronic non-healing ulcers secondary to atopic inflammation has never been reported. Atopic inflammation is typical of type 2 inflammation, which is mainly mediated by Th2 cells, eosinophils, mast cells and basophils, and the disease models include atopic dermatitis (AD), allergic rhinitis, and asthma.4 In this study, we present a case with chronic non-healing ulcers associated with atopic inflammation.
Case Report
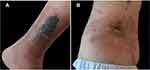
A 31-year-old young man presented to the department of dermatology (Southwest Hospital, Army Medical University, Chongqing, China) because of unhealed ulcers on his right waist and right leg for more than six months. The patient had tattoos on his right waist and lower leg five years ago. The area with red dye appeared induration after 1 year, accompanied by local redness and itching, without obvious heat pain and systemic symptoms. He visited a local hospital for a diagnosis considering hypertrophic scars or foreign body granuloma, but no biopsy or histopathological examination was performed. One year ago, he received several local superficial radiation treatments at a local hospital, and the induration did not go away. Six months ago, the patient underwent surgery to remove the induration at a local hospital. The excised tissue was not examined for pathology and etiology. After surgery, the patient’s wound healing was poor, and obvious swelling exudation and erosion gradually appeared in the wound area, and finally formed deep ulcers. He was treated with oral minocycline, rifampicin, clarithromycin, and topical fusidic acid cream and polymyxin B ointment, and regularly received dressing change at the wound care clinic in the local hospital. But the ulcer still did not heal or even aggravate, and large patchy erythema with pruritus surrounding the ulcer. The patient was otherwise healthy and had nothing unusual in his past history. Physical examination revealed a 0.8×1.8 cm ulcer on the inner lower third of the right leg, with small amount of yellow exudate on the ulcer base, large patchy erythema with edema around the ulcer, and blue tattoos near the ulcer (Figure 1A). Another 1.5×2.0 cm ulcer, with a deep surface and regular margin, was seen on the right side of the loin, and large flaky dry desquamation erythema surrounding the ulcer, blue Chinese character tattoos were nearby (Figure 1B).
Histopathological examination of the ulcer of the right leg showed epidermal hyperkeratosis with incomplete keratosis, the stratum spinosum was hypertrophic (Figure 2A), the dermis was permeated with dense lymphocytes and numerous eosinophils infiltrated, some histiocytes and neutrophils were also seen (Figure 2B), no obvious inflammatory cell infiltration was observed in subcutaneous tissue. Fungal culture and nucleic acid tests of tuberculosis and atypical mycobacterium were negative with the tissue. Only staphylococcus epidermidis was positive in wound secretion culture. Routine blood tests, blood glucose, liver and kidney function, and autoantibody spectrum were all negative. Chest radiographs and abdominal ultrasonography showed no abnormal findings. However, serum total IgE was 621.00IU/ mL, significantly higher than the upper limit of normal value (165 IU/mL). The specific IgE detection showed that dust mite allergen specific IgE level was grade 5 (50–100 IU/mL), and Artemisia allergen specific IgE level was grade 3 (17.5–50 IU/mL), ragweed allergen specific IgE and cockroach allergen IgE were both grade 1 (0.35–0.75 IU/mL), a detected content under 0.35 U/mL was considered negative reactivity. He denied any previous history of allergic disease, but his father and sister had a history of allergic rhinitis, and his sister’s daughter had a history of infantile eczema. His atopy background was defined. After ruling out other possibilities, we speculated that his chronic non-healing ulcers might be related to the atopic inflammation.
A history of hypertension and abnormal renal function were excluded, and the patient was treated with cyclosporine 200mg/day, combined with halometasone cream and moisturizing care for the erythema surrounding the ulcer. One month later, ulcers were completely healed, and the erythema around the ulcer subsided, leaving only pigmentation (Figure 3A and B). The patient’s itching symptoms were also significantly relieved, and the self-report was satisfied with the treatment effect. After maintaining the original dose for 2 weeks, the cyclosporine dosage was reduced to 100mg/day, and the dose was maintained for 2 weeks, then it was eventually stopped. Intermittent topical application of 0.1% tacrolimus cream maintains topical anti-inflammatory effects for two months. Ulcer and its surrounding eczema lesions did not recur at a follow-up visit 3 months later.
Discussion
Atopy is a predisposition to respond immunologically to diverse antigens/allergens, leading to overproduction of immunoglobulin E (IgE).5 The genetic factors play a crucial role in the propensity for atopy, regulating the total IgE synthesis, and in the production of IgE antibodies to specific epitopes. IgE plays a central role in allergen-induced inflammatory processes in various atopic diseases. For example, IgE presents at increased levels both in the serum and skin of AD patients, and there is a significant association between higher levels of IgE and the severity of the disease.6 High levels of serum total IgE and allergen-specific IgE are markers of atopic background, and they are important diagnostic evidences in the diagnosis of atopic dermatitis and other allergic diseases.5 Our patient had high levels of total IgE and very high levels of dust mite specific IgE; in addition, the skin biopsy of his skin lesion showed obvious eosinophil infiltration, which also suggested allergic inflammation. What is more, he had a definite family history of allergies. Given these evidences, AD was diagnosed with the eczema lesions surrounding the ulcer initially. Clinically, there are some patients with adult-onset atopic dermatitis who develop the disease in adulthood due to specific triggers, some of these sporadic cases do not have a clear personal allergic history. It seems that the patient had an underlying atopic background, but without prior symptoms, the implantation of exogenous allergens in tattoos might induce the onset of his atopic march. Therefore, we will continue to closely follow the patient to see if he will have recurrent eczema lesions or secondary allergic diseases.
The predominance of type 2 inflammation is a key driver of allergic diseases. Atopic inflammation, which is a typical type 2 inflammation, is mainly mediated by Th2 cells and type 2 cytokines including interleukin-4 (IL-4), IL-5 and IL-13. IL-4 and IL-13 promote IgE switching, and IL-5 stimulates eosinophil growth and activation. Besides, epithelial-derived cytokines such as IL‑25, IL‑33 and thymic stromal lymphopoietin (TSLP) also serve to amplify existing atopic inflammation.7 This inflammatory environment may have an adverse effect on delayed wound healing. Zhao et al had showed that an inflamed skin condition induced by IL-4 has a pronounced negative influence on the healing process. In the context of Th2 environment with a high level of IL-4, wound closure was significantly delayed and accompanied by vigorous inflammation, epidermal hyper-proliferation, increased angiogenesis, and decreased collagen deposition and tensile strength.8 However, some of these cytokines may play a different role in wound healing. He et al found that IL-33 improves wound healing through enhanced M2 macrophage polarization in diabetic mice.9 A recent study defines a positive regulatory role of IL-25-mediated-IL-17RB signaling in diabetic wound healing, and the group also found that IL-25 promoted M2 macrophage polarization and fibroblast activation in diabetic mice and high-glucose environment through PI3K/AKT/mTOR and TGF-β/SMAD signaling.10 Since these studies were all based on a diabetic mice model, the specific role of IL-33 and IL-25 in post-traumatic wound healing in normal humans needs to be further studied. Atopic inflammation is a complex inflammatory response involving a large number of cytokines. The influence of this inflammatory background on wound healing has not been studied so far, and our case may have certain clinical implications.
In our case, there was extensive infiltration of eosinophils in the ulcerated lesions. Eosinophils are involved in protection from parasites, fungi, viruses, and bacteria. A detailed examination of our patient did not reveal any evidence other than specific microbial infection. Staphylococcus epidermis, which is one of the resident floras of human skin, was only cultured in wound secretions, and its infection-induced inflammation is pathologically dominated by neutrophils rather than eosinophils. Eosinophils are implicated in the pathogenesis of atopic inflammation diseases like atopic dermatitis, allergic rhinitis, and asthma. Eosinophil infiltration in the lesions is one of the features of these diseases.11 Normal wound healing goes through four stages: hemostasis, inflammation, proliferation, and remodeling. The inflammatory stage, mainly mediated by neutrophils and macrophages, is the key to determining whether the wound healing is delayed.12 The infiltration of eosinophils may be detrimental to wound healing. Leitch et al found that wound healing is delayed in IL-5-overexpressing mice and this corresponds to significantly increased levels of eosinophils within the wound site that may contribute to and exacerbate the inflammatory response, resulting in detrimental wound repair.13 This may explain our patient’s delayed wound healing and the formation of his chronic non-healing ulcers.
The diagnosis of our case needs to be differentiated from other diseases. Auto-sensitization dermatitis (ASD) should be firstly considered. ASD is a cutaneous phenomenon in which acute secondary dermatitis develops at a location distant from a primary inflammatory focus. ASD occurs in patients with venous stasis and cases of infection, and the typical example of the latter is tinea pedis causing an eczematous eruption on the hands and/or legs. ASD usually presents as eczematous eruptions, but urticarial, lichenoid, morbilliform, psoriatic, rosacea-like reaction, or scarlatiniform in morphology could also be seen.14 In our case, there was neither infection nor venous stasis at the ulcer site, and the formation of ulcer is caused by the local abnormal inflammatory environment affecting the delay of wound healing. In addition, eczema lesions only surround the ulcer, not locations distant away from the primary ulcer. Instead of treating the ulcer locally, systemic anti-inflammatory therapy for atopic inflammation resulted in ulcer healing and eczema lesions resolving in our case. All of these suggest that this case is different from ASD. Since our case has marked eosinophil infiltration in the lesions, other skin disorders associated with eosinophils need to be identified, such as parasitic infections, Wells syndrome, eosinophilic fasciitis, and eosinophilic granulomatosis with polyangiitis. However, a careful examination found no evidence of parasitic infection, no vasculitis or granuloma. All of the above differential diagnoses can be ruled out in our case by pathological results and clinical features.
For the treatment of atopic inflammation, systemic or topical anti-inflammatory agents may be considered depending on the severity and distribution of the lesions. Cyclosporine, a calcineurin inhibitor, is a useful immunosuppressive agent for achieving disease control in patients with moderate to severe atopic dermatitis. The mechanism of cyclosporine played in treating chronic non-healing ulcers with atopic inflammation including inhibit the expression of T activated cells, reduce the number of eosinophils, inhibit the expression of IL-4, IL-13, TSLP and other inflammatory mediators.15 As adjunctive therapies to enhance the local anti-inflammatory effects, we choice halometasone cream, a topical corticosteroids with high potency, to treat the acute stage of eczema lesions around the ulcers, and topical maintenance therapy was administered with 0.1% tacrolimus cream, which is a topical calcineurin inhibitor and steroid-sparing with a good safety profile, it has been widely used in the maintenance treatment of various inflammatory dermatosis.16
Conclusion
Our case suggests that chronic non-healing ulcers can be associated with atopic inflammation. It is of great clinical significance to identify and diagnose the possibility of atopic inflammation inducing chronic non-healing ulcers as soon as possible, and to treat atopic inflammation in a timely manner after excluding other contraindications. For the treatment of chronic non-healing ulcers induced by this atopic inflammation, oral cyclosporine combined with topical anti-inflammatory drugs such as corticosteroids and topical calcineurin inhibitors can be considered. Since this is only a case report, as the inherent limitations, clinical studies with a larger sample size are needed to confirm this clinical phenomenon in the future.
Consent Statement
Informed consent for publication of the case details and associated images was obtained from the patient and all procedures were performed in accordance with the Helsinki Declaration. Institutional approval was not required to publish the case details.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82003359).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nosrati H, Khodaei M, Alizadeh Z, et al. Cationic, anionic and neutral polysaccharides for skin tissue engineering and wound healing applications. Int J Biol Macromol. 2021;192:298–322. doi:10.1016/j.ijbiomac.2021.10.013
2. Nosrati H, Aramideh Khouy R, Nosrati A, et al. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J Nanobiotechnology. 2021;19(1):1. doi:10.1186/s12951-020-00755-7
3. Suthar M, Gupta S, Bukhari S, et al. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci. 2017;24(1):16. doi:10.1186/s12929-017-0324-1
4. Folci M, Ramponi G, Arcari I, et al. Eosinophils as major player in type 2 inflammation: autoimmunity and beyond. Adv Exp Med Biol. 2021;1347:197–219. doi:10.1007/5584_2021_640
5. Diaz-Cabrera NM, Sánchez-Borges MA, Ledford DK. Atopy: a collection of comorbid conditions. J Allergy Clin Immunol Pract. 2021;9(11):3862–3866. doi:10.1016/j.jaip.2021.09.002
6. Wollenberg A, Thomsen SF, Lacour JP, et al. Targeting immunoglobulin E in atopic dermatitis: a review of the existing evidence. World Allergy Organ J. 2022;14(3):100519. doi:10.1016/j.waojou.2021.100519
7. Gandhi NA, Bennett BL, Graham NM, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35–50. doi:10.1038/nrd4624
8. Zhao Y, Bao L, Chan LS, et al. Aberrant wound healing in an epidermal interleukin-4 transgenic mouse model of atopic dermatitis. PLoS One. 2016;11(1):e0146451. doi:10.1371/journal.pone.0146451
9. He R, Yin H, Yuan B, et al. IL-33 improves wound healing through enhanced M2 macrophage polarization in diabetic mice. Mol Immunol. 2017;90:42–49. doi:10.1016/j.molimm.2017.06.249
10. Li S, Ding X, Zhang H, et al. IL-25 improves diabetic wound healing through stimulating M2 macrophage polarization and fibroblast activation. Int Immunopharmacol. 2022;106:108605. doi:10.1016/j.intimp.2022.108605
11. O’Sullivan JA, Bochner BS. Eosinophils and eosinophil-associated diseases: an update. J Allergy Clin Immunol. 2018;141(2):505–517. doi:10.1016/j.jaci.2017.09.022
12. Adib Y, Bensussan A, Michel L. Cutaneous wound healing: a review about innate immune response and current therapeutic applications. Mediators Inflamm. 2022;2022:5344085. doi:10.1155/2022/5344085
13. Leitch VD, Strudwick XL, Matthaei KI, et al. IL-5-overexpressing mice exhibit eosinophilia and altered wound healing through mechanisms involving prolonged inflammation. Immunol Cell Biol. 2009;87(2):131–140. doi:10.1038/icb.2008.72
14. Ferree SD, Yang C, Kourosh AS. Autosensitization dermatitis: a case of rosacea-like id reaction. JAAD Case Rep. 2019;5(5):410–412. doi:10.1016/j.jdcr.2019.02.029
15. Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63(6):925–946. doi:10.1016/j.jaad.2010.02.063
16. Gutfreund K, Bienias W, Szewczyk A, et al. Topical calcineurin inhibitors in dermatology. Part I: properties, method and effectiveness of drug use. Postepy Dermatol Alergol. 2013;30(3):165–169. doi:10.5114/pdia.2013.35619
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.