Back to Journals » Patient Preference and Adherence » Volume 12
Chronic myeloid leukemia patients and treatment-free remission attitudes: a multicenter survey
Authors Lou J, Huang J , Wang Z , Wen B, Tu C, Huang W, Zhai Z, Du X
Received 22 January 2018
Accepted for publication 7 April 2018
Published 15 June 2018 Volume 2018:12 Pages 1025—1032
DOI https://doi.org/10.2147/PPA.S163393
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Naifeng Liu
Jin Lou,1,2 Junjie Huang,1 Zitong Wang,3 Bingbing Wen,1 Chuanqing Tu,4 Wangxiang Huang,5 Zhimin Zhai,2 Xin Du1
1Department of Hematology, Shenzhen Second People’s Hospital, The First Affiliated Hospital of Shenzhen University, Shenzhen 518000, China; 2Department of Hematology, The Second Affiliated Hospital of Anhui Medical University, Hefei 230601, China; 3School of Medicine, University of Sydney, Sydney, NSW, Australia; 4Department of Hematology, People’s Hospital of Baoan District, Shenzhen 518101, China; 5Department of Hematology, Longgang District Central Hospital of Shenzhen, Shenzhen 518116, China
Background: Treatment-free remission (TFR) is becoming an essential goal for chronic myeloid leukemia (CML) patients in clinical practice. Few studies have emphasized patient attitudes or preferences about discontinuing tyrosine kinase inhibitors treatment. This study aimed to evaluate the characteristics of Chinese CML patients and their views and perspectives on TFR.
Methods: A total of 329 CML patients participated in this multicenter, questionnaire-based, standardized, semi-structured, interview-guided, open-ended, cross-sectional study. Information about demographics, diagnosis information, treatment history, quality of life (QoL), and TFR preference was collected.
Results: The adherence rate was 50% (N=163) and sex dependent (males, OR=2.24, 95% CI=1.40–3.58). Physical activity, symptom burden, mood impact, and daily impact were found to be better among adherent patients. Thirty-four percent of the patients were willing to attempt TFR positively. The reasons for preferring TFR were due to side effects (56%) followed by high cost (52%), inconvenience (42%), and pregnancy need (41%). Multivariate analysis indicated that patients who were younger (OR=0.96, 95% CI=0.94–0.99) with shorter disease duration (OR=0.90, 95% CI=0.82–0.98) and higher disease symptom burden (OR=1.08, 95% CI=0.98–1.21) were more likely positive about TFR.
Conclusion: Patients who were younger with shorter disease duration and higher disease symptom burden were more likely to try TFR. They expressed several perceived noncost factors of TFR. Our data may help promote the management of CML and designing of clinical trials for TFR in some developed regions of China.
Keywords: CML, TKI, TFR, preference, perception
Background
Tyrosine kinase inhibitors (TKIs) were the well-accepted strategy for targeted therapy for patients with Philadelphia-positive (Ph+) chronic myeloid leukemia (CML).1 Receiving permission for clinical use by its remarkable survival benefit in CML patients, imatinib was the first-generation TKI.2 Subsequent generation TKIs, dasatinib, nilotinib, bosutinib, and ponatinib, were developed for obtaining a better therapeutic response.3 Patients with CML can have a life span identical to that of the overall population by having access to effective TKI therapy.4,5
Current recommendation for CML patients is to keep receiving TKI treatment regardless of the results of a deep molecular response.6–8 Nevertheless, long-term medication can lead to TKI treatment-related complications and financial difficulties, resulting in poor medical compliance.9 For these reasons, the prescription of life-long TKIs has been questioned. Treatment-free remission (TFR), the ability to obtain molecular response without receiving any TKI treatment,10 has become a promising management strategy for CML patients.11–13 However, patients with CML should have obtained deep molecular remission for a relatively long period before the TKI treatment is discontinued.14
Clinical trials have showed the feasibility of successful TFR.15,16 Thirty-eight percent to 47% of the participants remained deep molecular remission for 2 years after the discontinuation of imatinib.15,16 Subsequent trials have confirmed the feasibility of TFR in imatinib.17–19 TFR in the newer TKIs, such as dasatinib or nilotinib, was also studied. Compared to imatinib, novel generations of TKIs might promote better access to TFR success.20–24
TFR is becoming a target in clinical settings and is estimated to alter the guidelines of managing CML patients in the near future. Few studies have emphasized the attitudes of CML patients on discontinuing TKI treatment, which is crucial for the potential application of TFR study results. A recent study investigated the therapy goals and TFR preferences in CML patients in some cities in China, where medical insurances cover partial costs of TKIs.25 However, the outcome could be different in other areas, where TKI treatment expenses were covered by medical insurances and patient assistant program. This study aimed to evaluate the characteristics of CML patients in one of those areas, Shenzhen, and their attitudes and perspectives on TFR.
Methods
A total of 329 CML patients participated in this study. This study was approved by the Ethics Committee of Shenzhen Second People’s Hospital. Informed written consents were obtained from all participants from April 2016 to September 2017 at the Shenzhen Second People’s Hospital (N=246; 75%), People’s Hospital of Baoan District (N=46; 14%), and Longgang District Central Hospital of Shenzhen (N=37; 11%). Questionnaires were distributed in this standardized, semi-structured, interview-guided, open-ended, cross-sectional study.
Information about five major areas was collected: demographics, diagnosis information, treatment history, quality of life (QoL), and TFR preference. Demographic information consisted of domains of age, sex, marriage, medical insurance, and educational level. First-diagnosis information included records of dates, symptoms, physical examinations, and laboratory tests. The European Treatment Outcome Study (EUTOS) long-term survival (ELTS) score, a novel and the first long-term survival score developed in CML patients treated with TKIs, was adopted in prognosis scoring and compared with traditional Sokal scores.26 We also collected the history on treatments, including names of drugs, dates, related adverse events, adherence, real-time quantitative polymerase chain reaction (PCR) laboratory tests, and clinical expenses. The adherence was measured by two dimensions, consisting of unintentional incompliance (forgetfulness) and intentional incompliance (adverse reactions, better symptoms, and inconvenience). QoL was evaluated by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ C30) and EORTC QLQ CML 24.27 Satisfaction with treatment, medical insurance, and patient assistant program were also evaluated. Desired information on TKIs, treatment goals, attitudes, and reasons for TFR for CML patients were also investigated. We have asked whether the respondents desire for more information about TKIs on effectiveness, medical insurance, new development, expense, side effects, TFR, or other domains. Treatment goals for each individual, including wishing to be cured, aiming for a normal life expectancy with good QoL, wanting a normal life expectancy, or slowing down the progression of disease, were collected. Participants with a positive attitude towards TFR were defined as those who were willing to attempt TFR rather than those who were unsure or unwilling. As one of the reasons for TFR, participants with privacy need were defined as those who did not want others to know that they were CML patients.
Results were described by using proportions for categorical variables and medians/averages for continuous variables. Pearson’s chi-square test and Fisher’s exact test were adopted to assess the relationship between the categories of TKIs and TKI-related adverse reactions. Univariate analyses were conducted to determine factors associated with adherence and TFR attitudes significantly. Variables associated with a P-value of <0.25 were further tested in the multivariate analysis logistic regression model. A two-tailed P-value of <0.05 was considered significant. QoL results calculated from the EORTC QLQ C 30 and QLQ CML-24 were compared between adherent and nonadherent respondents by using the Student’s t-test. All analyses were performed by using Stata version 14 (StataCorp LP, College Station, TX, USA).
Results
Demographics and diagnosis information
Demographics and diagnosis information are shown in Table 1. In total, 218 respondents (66%) were male, with a median age of 36 years (18–80 years); 314 respondents were covered by medical insurance (95%). Education levels of the participants varied. Median time since diagnosis for respondents was 4.7 years (0.2–37.3 years). These patients presented to the hospital due to symptoms (60%) or routine blood tests (40%) prior to the diagnosis of CML. The most commonly reported symptoms were fatigue (N=115), excessive sweating (N=71), weight loss (N=68), abdominal cramps (N=66), and fever (N=63). Other less common symptoms were dizziness (N=46), pain (N=31), lacked appetite (N=26), and bleeding (N=19). According to the Sokal scores, participants were allocated to low (62%), intermediate (27%), and high-risk groups (11%). On the contrary, most patients (82%) met criteria for the low-risk group, and fewer were allocated to the intermediate-risk (16%) and high-risk (2%) groups using the ELTS scores (Figure 1).
  | Table 1 Demographics of the respondents |
  | Figure 1 Prognosis scores of the respondents. |
Treatment and self-reported adverse reactions
At the time of the survey, 76% of the respondents were receiving first-generation TKIs, with 60% on imatinib (Glivec®), 15% on Chinese generic imatinib (Xinwei®), and 4% on Indian generic imatinib (Veenat®). The rest were treated with second-generation TKIs, with 15% on nilotinib (Tasigna®), 6% on dasatinib (Sprycel™). 94% (N=267) of them reported having side effects from imatinib, most commonly nausea and vomiting (N=104). Diarrhea (N=79), joint pains (N=70), edema (N=59), skin problems (N=54), muscle cramps (N=48), and conjunctivitis (N=34) have also been described. A comparison was performed on the adverse reactions due to imatinib and the Chinese generic imatinib (Table 2). Fever (3% vs 0%) and cough (7% vs 0%) were found to be more frequent in those who were using the Chinese generic imatinib. No significant difference was observed in the comparisons of other adverse events (P>0.05). Common adverse reactions of the second-generation TKIs (nilotinib vs dasatinib) were skin problems (51% vs 20%), joint pain (23% vs 20%), headache (14% vs 12%), and muscle cramp (14% vs 20%). A statistical test indicated that skin problems were more frequent in those taking nilotinib rather than dasatinib.
  | Table 2 Self-reported adverse events in imatinib |
Adherence and expense
Twenty-six percent (N=85) of the patients had attempted to reduce the dose of TKIs to various extents; 38% (N=124) of them forgot to take TKIs at some point in the past month. The adherence rate was 50% (N=163) among the respondents. According to the univariate analysis (Table 3), respondents who were males (OR=2.24, 95% CI=1.40–3.58), older (OR=1.03, 95% CI=1.01–1.05), or with heavier symptom burden (OR=0.87, 95% CI=0.80–0.95) were identified to be associated with good adherence. Educational level, treatment duration, financial difficulty, and out-of-pocket cost were not significantly associated with adherence (P<0.05). The multivariate analysis indicated that male patients were more likely to comply with the TKI prescription (OR=1.82, 95% CI=1.10–3.01, P=0.019).
Fifty-two percent and 70% of respondents reported achieving major molecular response at 12 months and at the time of the survey, respectively. The all-time median annual costs of out-of-pocket, medical insurance, and total were 16,328 CNY (Chinese Yuan), 81,188 CNY, and 97,563 CNY for the respondents, respectively. The out-of-pocket expenses decreased throughout the years (Figure 2). Annual costs were 19,860 CNY, 14,411 CNY, and 13,828 CNY in those patients diagnosed during the periods of earlier to 2010, 2011 to 2013 and 2014 to present, respectively.
  | Figure 2 Box plots of TKI expense in respondents. |
Quality of life
An evaluation on QoL including a survey on associated adverse symptoms were performed by using the EORTC QLQ C30. The results are shown in Table 4. Along with the survey, an additional assessment comprising six domains was done by using the QLQ CML 24. As shown in Table 4, the EORTC QLQ C30 scores on physical activity (92.5 vs 89.5, P=0.032) were better among adherent patients. From the assessment using the QLQ CML 24, nonadherence was associated with higher symptom burden (21.9 vs 17.1), mood impact (30.8 vs 25.7), and daily impact (35.9 vs 29.3). No significant difference was observed in the comparisons of other domains (P>0.05).
  | Table 4 Quality of life and adherence |
Preference data
Sixty six percent of the respondents were satisfied with the treatment, whereas 64% and 68% of the respondents were pleased with their medical insurance and patient assistance program, respectively. Respondents expressed the desire for more information about TKIs on effectiveness (N=193), medical insurance (N=187), new development (N=173), expense (N=173), side effects (N=165), and TFR (N=151). Treatment goals varied with 48% of the respondents wishing to be cured, 40% aiming for a normal life expectancy with good QoL, and 8% wanting a normal life expectancy, and 4% of the participants hoped to slow down the progression of disease.
Thirty-four percent of the patients were willing to attempt TFR positively; 27% of the patients were unsure but were willing to try if it was recommended by their physician (Figure 3). The dominant reason for discontinuing TKI therapy was due to poor QoL which is one of the side effects of TKI therapy (56%) followed by high cost (52%), inconvenience (42%), pregnancy need (41%), privacy need (15%), and pressure from family members (10%). Thirty-one percent of the respondents were unwilling to cease TKI therapy because of the risk of relapse in the future (91%), out of patient assistance programs (15%), pressure from family members (15%), and frequent monitoring (9%). According to the univariate analysis (Table 5), sex (OR=0.51, 95% CI=0.29–0.89), age (OR=0.95, 95% CI=0.93–0.98), disease duration (OR=0.88, 95% CI=0.81–0.96), and symptom burden were identified to be associated with TFR attitudes rather than education, TKI types, adherence, financial difficulty, or out-of-pocket cost. The multivariate analysis further indicated that younger patients (OR=0.96, 95% CI=0.94–0.99), patients with shorter disease duration (OR=0.90, 95% CI=0.82–0.98), and those with worse disease symptom burden (OR=1.08, 95% CI=0.98–1.21) were more likely to have a positive attitude about TFR.
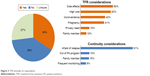  | Figure 3 TFR attitudes of respondents. |
Discussion
The current study has evaluated the characteristics of 329 CML patients and emphasized patient attitudes or preferences about TFR in China. The adherence rate was suboptimal and sex dependent, affecting the physical activity, symptom burden, mood impact, and daily impact of CML patients. One third of the patients were willing to attempt TFR positively, with side effects, high cost, inconvenience, and pregnancy need being the main reasons. We found that patients who were younger, with shorter disease duration and higher disease symptom burden, and were more likely positive about TFR. Our data may help promote the adherence of CML patients by adapting tailored strategies. In addition, our results are important for designing clinical trials and implementation of TFR in the regions where treatment cost of CML was well covered by medical insurances and patient assistance program.
Decreased compliance was associated with a reduced chance of cytogenetic and molecular remission, consequently, an elevated risk of progression and resistance.28 Optimal TKI compliance has played an important role in maximizing the benefits in achieving long-term survival for CML patients.29 However, daily adherence to TKI treatment is still a challenging factor for a significant proportion of patients. Sanford et al surveyed CML patients in the UK and found that treatment adherence of 100%, 75%, and 50% was reported by 76.8%, 19.6%, and 1.8% of participants, respectively.30 In another study in Italy by Breccia et al, good compliance was shown, but some of the participants had occasionally delayed a dose and more than a half had stopped treatment due to forgetfulness.31 These findings seem to be different to that of our study’s, where 26% of the respondents had once attempted to reduce the dose of TKIs, and 38% of them had forgotten to comply in the past month. As a result, the absolute adherence rate was 50% among all respondents, making the medication adherence in our patients’ group suboptimal. This adherence rate was also consistent with the result from a study conducted by Unnikrishnan et al in 2016.32
Furthermore, the interaction between contributors who can potentially improve adherence in CML patients is not well understood. Eliasson et al studied the intentional and unintentional motivations for noncompliance. They concluded that the factor that is associated with better compliance was the occurrence of adverse reactions.33 In this study, we investigated adherence behaviors and potential relationships with age, sex, educational level, treatment duration, symptoms burden, financial condition, and annual cost. Interestingly, our analysis indicated that male patients (123/218) were more likely to be in compliance with the TKI prescription compared with female patients (40/111; OR=1.81, P=0.042). This might be because females might take pregnancy or their body image into more careful consideration than men do regarding CML treatment.
We further explored how adherence had influenced the QoL of the respondents by using the EORTC QLQ C30 and CML 24 scores. As a result, physical activity, symptom burden, mood impact, and daily impact were found to be better among adherent patients. A similar conclusion was reported in a recent study in India that increased symptoms burden was associated with nonadherence to imatinib in patients with CML.32,34
Few patients in our study were willing to try TFR. They expressed several perceived noncost factors of TFR, most commonly including relief of medication side effects, pregnancy need, and convenience. This was different from the results of a recent survey conducted in some regions of China.25 We found that only 34% of patients were willing to attempt TFR positively, other 27% of them were unsure but were willing to try if it was recommended by their physicians. The dominant reason for wanting to discontinue TKI was poor QoL from side effects of TKI therapy (56%), followed by high cost (52%), inconvenience (42%), pregnancy need (41%), undisclosed reasons (15%), and pressure from family members (10%). According to the multivariate analysis, age, disease duration, and symptom burden of patients were identified to be associated with the TFR attitudes rather than the sex, education, TKI types, adherence, financial difficulty, and out-of-pocket cost. On the contrary, in the survey by Jiang et al, nearly half of the subjects reported TFR (48%) as their therapy goal, and most respondents (83%) indicated they preferred to discontinue TKI treatment in the future. The main reasons were the cost (78%), TKI adverse reactions (41%), long-term side effects (41%), pregnancy plan (14%), disruption of daily life (8%), and others (5%). Both younger age and higher out-of-pocket cost were associated with patients’ preference for stopping TKI therapy in their study.25
These contradictions can be explained by the heavy financial burden of TKI treatment in most regions of China, where the cost of TKI treatment is not covered by medical insurance.25 Another similar finding was identified in a Japanese study on CML. They reported that about one-third (31.7%) of respondents considered stopping TKI because of high cost and 22.1% because of side effects.35 Although their medical expenses were covered by medical insurance and did not change during the investigated period, their annual income fell by 13,000 USD due to the financial crisis.35 Differently in Shenzhen, the expenses of TKI treatments were well covered by medical insurances (90%). This Special Economic Zone was also one of the fastest-growing cities in the world during the 1990s and the 2000s. Our study results showed that adverse events rather than cost was the most common reason cited for preferring to cease TKI treatment. This is consistent with the data from the CML patients in other countries where expenses of TKI treatment were well paid by public medical insurance. From the study of Sanford et al in the UK (London), most patients with CML chose to continue with TKIs, and neither payment concerns nor occurrence of side effects significantly affected their willingness to try TFR.30 In Brazil (Rio De Janeiro), the most expressed anticipated influences of TFR were the relief of adverse reactions (75%), reduced cost (58%), convenience (43%), positive emotional impact (43%), and increased activity level (30%).36 Another study reported toxicity (n=26) and convenience (n=18) as the two most common motivations for TFR in Australia (Melbourne).37
Limitations should be considered in this study. This investigation had a limited number of respondents, potentially resulted in a lack of representation and statistical power. Another limitation is the possible biased answers caused by the interviewer-related factors. The accuracy of these responses could be determined as self-reported questionnaire might have important impacts on it. This study has not discussed the association between patient attitudes and adverse consequences of TKIs, including cardiotoxicity, proatherogenic changes, and increased arterial stiffness.38 These factors pose a high risk for future cardiovascular events, and such risk may influence the patients’ attitude toward treatment.
Conclusion
Patients who were younger with shorter disease duration and heavier disease symptom burden were more likely to try TFR. They expressed several perceived noncost factors of TFR, including relief of medication side effects, pregnancy need, and convenience. Our data may help promote the management of CML and the designing of clinical trials of TFR in some regions of China where the expenses of TKI treatment are well paid by medical insurance.
Acknowledgment
This work was supported by Special Support Funds of Shenzhen for Introduced High-Level Medical Team.
Disclosure
JL, JH, and ZW were listed as co-first authors of this publication. The authors report no conflicts of interest in this work.
References
O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. | ||
Cherrier-De Wilde S, Rack K, Vannuffel P, Delannoy A, Hagemeijer A. Philadelphia-negative acute lymphoblastic leukemia developing in a CML patient in imatinib mesylate-induced complete cytogenetic remission. Leukemia. 2003;17(10):2046–2048. | ||
Hantschel O, Grebien F, Superti-Furga G. The growing arsenal of ATP-competitive and allosteric inhibitors of BCR-ABL. Cancer Res. 2012;72(19):4890–4895. | ||
Gambacorti-Passerini C, Antolini L, Mahon FX, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103(7):553–561. | ||
Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851–2857. | ||
Bocchia M, Ippoliti M, Gozzetti A, et al. CD34+/Ph+ cells are still detectable in chronic myeloid leukemia patients with sustained and prolonged complete cytogenetic remission during treatment with imatinib mesylate. Leukemia. 2008;22(2):426–428. | ||
Chu S, McDonald T, Lin A, et al. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood. 2011;118(20):5565–5572. | ||
Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. | ||
Smith BD. Imatinib for chronic myeloid leukemia: the impact of its effectiveness and long-term side effects. J Natl Cancer Inst. 2011;103(7):527–529. | ||
Saussele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30(8):1638–1647. | ||
Merante S, Orlandi E, Bernasconi P, Calatroni S, Boni M, Lazzarino M. Outcome of four patients with chronic myeloid leukemia after imatinib mesylate discontinuation. Haematologica. 2005;90(7):979–981. | ||
Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109(1):58–60. | ||
Yhim HY, Lee NR, Song EK, et al. Imatinib mesylate discontinuation in patients with chronic myeloid leukemia who have received front-line imatinib mesylate therapy and achieved complete molecular response. Leuk Res. 2012;36(6):689–693. | ||
Breccia M, Alimena G. Discontinuation of tyrosine kinase inhibitors and new approaches to target leukemic stem cells: treatment-free remission as a new goal in chronic myeloid leukemia. Cancer Lett. 2014;347(1):22–28. | ||
Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035. | ||
Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515–522. | ||
Rousselot P, Charbonnier A, Cony-Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424–430. | ||
Cross NC, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29(5):999–1003. | ||
Lee SE, Choi SY, Song HY, et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016;101(6):717–723. | ||
Imagawa J, Tanaka H, Okada M, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2(12):e528–e535. | ||
Rea D, Nicolini FE, Tulliez M, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129(7):846–854. | ||
Hughes TP, Hochhaus A, Kantarjian HM, et al. Safety and efficacy of switching to nilotinib 400 mg twice daily for patients with chronic myeloid leukemia in chronic phase with suboptimal response or failure on front-line imatinib or nilotinib 300 mg twice daily. Haematologica. 2014;99(7):1204–1211. | ||
Hochhaus A, Masszi T, Giles FJ, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7):1525–1531. | ||
Vigna E, Gentile M, Giagnuolo G, et al. Long-term molecular remission in Philadelphia-positive acute lymphoblastic leukemia elderly patient after dasatinib discontinuation. Leuk Lymphoma. 2016;57(10):2445–2447. | ||
Jiang Q, Liu ZC, Zhang SX, Gale RP. Young age and high cost are associated with future preference for stopping tyrosine kinase inhibitor therapy in Chinese with chronic myeloid leukemia. J Cancer Res Clin Oncol. 2016;142(7):1539–1547. | ||
Pfirrmann M, Baccarani M, Saussele S, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30(1):48–56. | ||
Efficace F, Baccarani M, Breccia M, et al. International development of an EORTC questionnaire for assessing health-related quality of life in chronic myeloid leukemia patients: the EORTC QLQ-CML24. Qual Life Res. 2014;23(3):825–836. | ||
Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113(22):5401–5411. | ||
Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–2388. | ||
Sanford D, Kyle R, Lazo-Langner A, et al. Patient preferences for stopping tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Oncol. 2014;21(2):e241–e249. | ||
Breccia M, Efficace F, Sica S, et al. Adherence and future discontinuation of tyrosine kinase inhibitors in chronic phase chronic myeloid leukemia. A patient-based survey on 1133 patients. Leuk Res. 2015;39(10):1055–1059. | ||
Unnikrishnan R, Veeraiah S, Mani S, et al. Comprehensive evaluation of adherence to therapy, its associations, and its implications in patients with chronic myeloid leukemia receiving imatinib. Clin Lymphoma Myeloma Leuk. 2016;16(6):366.e363–371.e363. | ||
Eliasson L, Clifford S, Barber N, Marin D. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res. 2011;35(5):626–630. | ||
Cella D, Nowinski CJ, Frankfurt O. The impact of symptom burden on patient quality of life in chronic myeloid leukemia. Oncology. 2014;87(3):133–147. | ||
Kodama Y, Morozumi R, Matsumura T, et al. Increased financial burden among patients with chronic myelogenous leukaemia receiving imatinib in Japan: a retrospective survey. BMC Cancer. 2012;12:152. | ||
Boquimpani CM, Szczudlo T, Mendelson E, Benjamin K, Masszi T. Attitudes and perceptions of patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) toward treatment-free remission (TFR). Blood. 2014;124:abstract 4547. | ||
Villemagne Sanchez LA, O’Callaghan C, Gough K, et al. Patient perceptions of treatment-free remission in chronic myeloid leukemia. Leuk Lymphoma. 2018;59(2):406–415. | ||
Mozos I, Borzak G, Caraba A, Mihaescu R. Arterial stiffness in hematologic malignancies. Onco Targets Ther. 2017;10:1381–1388. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


