Back to Journals » Nature and Science of Sleep » Volume 12
Chronic Insomnia Is Associated with Higher Circulating Interleukin-8 in Patients with Atherosclerotic Cerebral Small Vessel Disease
Authors Wang J, Chen X, Men X, Chen M, Tao J, Lu Z
Received 18 November 2019
Accepted for publication 23 January 2020
Published 11 February 2020 Volume 2020:12 Pages 93—99
DOI https://doi.org/10.2147/NSS.S239030
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven A Shea
Jihui Wang,1 Xiaodong Chen,2 Xuejiao Men,2 Minhua Chen,1 Jiong Tao,1 Zhengqi Lu2
1Department of Psychiatry, the Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou 510630, People’s Republic of China; 2Department of Neurology, the Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou 510630, People’s Republic of China
Correspondence: Jiong Tao
Department of Psychiatry, The Third Affiliated Hospital, Sun Yat-sen University, No.600 Tianhe Road, Tianhe District, Guangzhou, Guangdong 510630, People’s Republic of China
Tel +8613600071635
Email [email protected]
Zhengqi Lu
Department of Neurology, The Third Affiliated Hospital, Sun Yat-sen University, No.600 Tianhe Road, Tianhe District, Guangzhou, Guangdong 510630, People’s Republic of China
Tel +8618922102767
Email [email protected]
Objective: Chronic inflammatory responses and leukocyte infiltration are classical pathological features of cerebral small vessel disease (CSVD). To date, limited evidence of a relationship between chronic insomnia and inflammatory responses in patients with CSVD has been uncovered. The purpose of the present study was to investigate the potential relationship between chronic insomnia and pro-inflammatory cytokine levels in patients with atherosclerotic CSVD (A-CSVD).
Methods: In total, 76 A-CSVD patients with or without chronic insomnia (CI) confirmed using magnetic resonance (MR) were prospectively recruited. Overnight polysomnography (PSG) was performed and serum levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, IL-17A, IL-8, and IL-12 assessed. Cytokine levels were compared between CSVD+CI (study group) and CSVD without CI (control group) patients, and the correlations between PSG parameters and cytokine levels were explored in all patients via multiple linear regression analyses.
Results: The serum IL-8 level of the study group (12.3± 4.4 pg/mL) was significantly higher than that of the control group (7.5± 2.2 pg/mL; P< 0.05). PSG measurements showed that patients in the study group had significantly higher sleep onset latency (SOL), arousal index (ArI) and wake after sleep onset (WASO) as well as lower total sleep time (TST), sleep efficiency (SE) and stage 3 NREM sleep (N-3) ratio, compared with the control group (P< 0.05). Multiple linear regression analyses led to the identification of ArI (β=0.026, P< 0.05) and TST (β=− 0.054, P< 0.05) as significant positive and negative predictors of the IL-8 level, respectively.
Conclusion: Chronic insomnia, in particular, sleep fragmentation and short sleep duration, may be involved in promotion of serum IL-8 expression in patients with atherosclerotic CSVD.
Keywords: cerebral vascular disease, sleep, polysomnography, fragmentation, markers
Introduction
The term “cerebral small vessel disease” (CSVD) represents a group of pathological processes that affect the small arteries, arterioles, venules, and capillaries of the brain.1 CSVD causes up to 45% of dementia and accounts for 20% of all stroke cases worldwide.1 The disease has multifarious etiology and pathogenesis, the most common form being arteriolosclerotic CSVD (A-CSVD), which is also known as age- and vascular risk-factor-related CSVD.2 Recurrent minor strokes, blood–brain barrier (BBB) impairment, inflammatory responses and leukocyte infiltration are classical pathological features of CSVD.3 The inflammatory reaction is triggered both at the onset of ischemic and hemorrhagic stroke (attack phase)4 and chronic stages (remitting phase) of “silent CSVD”.5
Sleep and the circadian system exert a strong regulatory influence on immune functions. Prolonged or severe sleep disturbance could trigger inflammatory responses through activation of stress response pathways, such as the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous activity (SNS).6 Several studies suggest that diagnosis of insomnia is associated with immune dysregulation, ranging from increased levels and diurnal rhythm changes of inflammatory cytokines7,8 to decreased lymphocyte subsets.9
Previous research by our group showed an increased proportion of chronic insomnia (54%) in CSVD patients,10 which was markedly higher than that of older adults (12–20%).11 To our knowledge, the relationship between insomnia and chronic inflammatory response in A-CSVD patients is yet to be established. In the present investigation, objective sleep characteristics and pro-inflammatory cytokines were evaluated in A-CSVD patients with or without chronic insomnia. Our collective findings indicate that chronic insomnia is associated with increased activation of pro-inflammatory cytokines in A-CSVD patients.
Methods
Study Design
This single-center, cross-sectional, patient assessor-blinded clinical study was conducted in the Third Affiliated Hospital of Sun Yat-sen University. The ethics committee of the above institution approved this study, which was conducted in accordance with the Declaration of Helsinki. Written informed consent was required for all patients.
Participants
Patients admitted to the outpatient clinics of the neurology and psychiatry departments because of CSVD-related symptoms were sequentially screened and enrolled from August 2017 to December 2018. The symptoms of CSVD included nonembolic lacunar stroke (unilateral motor/sensory signs involving the whole of at least two of three body parts (face, arm, and leg)), cognitive, motor (gait), dysphagia, dysuria, and mood disturbances. Subjects who were eligible due to an acute cerebrovascular event were included only ≧3 months after the episode to avoid significant effects on the outcomes. Prior to enrollment, all participants underwent extensive baseline evaluation comprising standard questionnaires, structured clinical interview for DSM-IV Axis-I Disorders (SCID-I),12 cognitive function assessment, and clinical laboratory testing.
Inclusion criteria were as follows: (1) subject ages of 50–75 years, (2) presence of one or more vascular risk factors, such as hypertension, atherosclerosis, diabetes mellitus, hyperhomocysteinemia, past or current smoking, (3) presence of typical radiological findings of CSVD,13 including lacunars (LN) of presumed vascular origin, white matter hyperintensities (WMH) of presumed vascular origin with Fazekas grade 2 (early confluent) or higher,14 visible perivascular spaces (PVS) and cerebral microbleeds (CMB), and (4) with or without chronic insomnia. The criteria of chronic insomnia are based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) and include:15 (1) predominant complaint of dissatisfaction with sleep quantity or quality, (2) sleep disturbance causing clinically significant distress or impairment, (3) sleep difficulty occurring at least three nights per week and lasting for at least 3 months, (4) sleep difficulty occurring despite adequate opportunity for sleep, (5) insomnia is not attributable to the physiological effects of a substance, and (6) coexisting mental disorders and medical conditions do not adequately explain the predominant complaint of insomnia. All patients signed the written informed consent form.
Exclusion criteria were as follows: (1) patients with large artery disease (carotid, vertebral or intracranial stenosis >50%), (2) patients with larger subcortical or cerebral watershed infarctions (>1.5 cm) on MR since these are often embolic, (3) patients with severe mental disorders, uncontrolled somatic or other inflammatory diseases that could affect the experiment, (4) patients with any sleep-wake disorder other than insomnia, such as obstructive sleep apnea hypopnea syndrome (OSAHS) or restless legs syndrome (RLS) based on structured interview and polysomnography, (5) apnea-hypopnea index ≥15 per hour of sleep during a screening laboratory polysomnogram, (6) patients with CSVD caused by other reasons, such as hereditary or inflammatory factors and amyloidosis, and (6) patients treated with sleeping pills within 2 weeks of initial screening.
Cytokine Measurements
Serum samples were collected and frozen at −80°C for experimental use. Serum levels of TNF-α, IL-1β, IL-6, IL-17A, IL-8, and IL-12P70 were quantified using a Magnetic Luminex® Assay multiplex kit (USA R&D Systems, Inc., Minneapolis, USA) on a Luminex 200 system (Luminex Corporation, Texas, USA). Each sample measurement was performed in duplicate and all samples run on the same assay. Cytokine measurements below the detection limit determined based on the standard curve were selected as the detection limits, established as follows for each cytokine: TNF-α (1.2 pg/mL), IL-1β (0.8 pg/mL), IL-6 (1.7 pg/mL), IL-17A (1.8 pg/mL), IL-8 (1.8 pg/mL), and IL-12 (1.1 pg/mL). Cytokines with detection rates of ≤50% were excluded from analysis.
Polysomnography (PSG)
For assessment of sleep quality, standard, multichannel, whole-night PSG was conducted using the wireless telemetry PSG system (Germany SOMNO Medics, product model: SOMNOscreen plus PSG +, analysis software “DOMINO”) in the sleep laboratory. Recording of PSG was initiated based on the subject’s usual sleeping habits and continued for at least 8 hrs. All PSG records were analyzed by a certified psychologist in a blinded model based on the American Academy of Sleep Medicine (AASM) Manual.16 The following PSG outcome variables were assessed: sleep onset latency (SOL, time it took for the participant to fall asleep after going to bed), total sleep time (TST, total sleep time during the PSG recording), sleep efficiency % (SE %; TST divided by time in bed), wake after sleep onset (WASO, time being awake after initial sleep onset until the last awakening), percentage of each sleep stage (N1, N2, N3 [slow wave sleep; SWS] and RNEM), apnea-hypopnea index (AHI, mean number of apneas and hypopneas per hour of sleep), arousal index (ArI; number of arousals per hour of sleep), oxygen desaturation index (ODI, mean number of oxygen desaturation ≥3% episodes per hour of sleep), respiratory effort-related arousal index (RERAI, the sum of respiratory effort-related arousals divided per hour of sleep, with RERA being scored as an increase in respiratory effort or flattening of the nasal pressure waveform with a duration of ≥10 s leading to arousal from sleep).
MR Acquisition
Imaging was performed on a General Electric (GE) 3 T superconducting MR scanner with eight channels and head-and-neck coils. MR acquisition sequences included 3D TOF-MRA, MRI T1Flair, T2WI, T2Flair and susceptibility-weighted imaging (SWI) for each patient. Images were analyzed by a certified and registered neuroradiologist masked to group assignment for the presence of four SVD markers (LN, WMH, PVS and CMB). The presence of each SVD marker was summed as an ordinal “SVD burden score” (range 0–4).17
The Chinese version of Pittsburgh Sleep Quality Index (PSQI), a self-reported questionnaire aimed at assessing quality of sleep over a month, was employed. The questionnaire contains seven domains, each scored using a scale of 0 to 3. The global PSQI score ranges from 0 to 21, whereby a higher score indicates poorer sleep quality.18
The Chinese version of Zung Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) was further used to assess anxiety and depression symptoms, respectively. SAS and SDS include 20 question items, with every item rated on a four-point scale: 1 (a little of the time) to 4 (most of the time). The total score is the summation of scores of 20 items and the standard score is the integer of the total score multiplied by 1.25.19
Statistical Methods
SPSS (SPSS, Inc., Chicago, IL) version 20.0 was applied for statistical analyses. Descriptive statistics were presented as means ± standard deviation (SD) for continuous normal variables and numbers or proportions for categorical variables. Key demographic variables and risk factors were compared between two groups using independent t-test, Mann–Whitney test and χ2 test, as appropriate. PSG parameters and cytokine levels were compared between the two groups using Student’s t-test. Multiple linear regression analysis was performed to determine the specific PSG variables that could be used to independently predict cytokine levels. Two-sided P-values ≤0.05 were considered statistically significant.
Results
Demographic and Clinical Characteristics of Participants
In total, 90 CSVD patients were prospectively screened for recruitment, among which 24 were excluded due to: (1) OSAHS (n=7) or RLS (n=1), (2) large artery disease (n=8), (3) major depression (n=4), (4) inflammatory diseases (n=2), (5) or refusal to participate in the study (n=2). The final participant count was a cohort of 76 patients, including 38 females and 38 males with an average age of 60.4 years. Among the 76 CSVD patients, 44 (57.9%) met the DSM-5 criteria for chronic insomnia (study group) and 32 (42.1%) patients that did not meet the criteria were taken as the control group. We observed no significant differences in demographic data, vascular risk factors and SVD burden scores between the two groups (P > 0.05), as shown in Table 1.
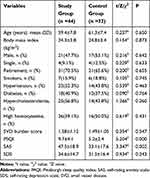 |
Table 1 Comparison of Demographic and Clinical Data Between the Two Groups |
The IL-8 level in the study group was significantly higher, compared with the control group (P<0.05), as shown in Table 2.
 |
Table 2 Comparison of Cytokine Levels Between the Two Groups (pg/mL) |
Comparison of PSG Parameters Between the Two Groups
Compared with the control group, the study group showed significantly lower TST, SE and N-3 ratio but markedly higher SOL, WASO and ArI (P<0.05; Table 3). The data indicate that patients in the study group had shorter sleep duration and more sleep fragmentation relative to the control group.
 |
Table 3 Comparison of PSG Measures Between the Two Groups |
Multiple Linear Regression Analysis of Factors Influencing the IL-8 Level
We applied multiple linear regression analyses to investigate the relationship between IL-8 level (outcome variable) and potential predictive variables, which included PSQI score, SAS anxiety score and a number of PSG parameters (SOL, TST, SE, WASO, ArI, N3 sleep, and ODI). ArI (β=0.026, P<0.05) was identified as a significant positive predictor and TST (β=−0.054, P<0.05) as a significant negative predictor of IL-8 level (Table 4), suggesting that short sleep duration and sleep fragmentation are related to increased IL-8 production.
 |
Table 4 Multiple Linear Regression Analysis of Factors Influencing the IL-8 Level |
Discussion
Several sleep disturbance conditions, including sleep apnea, periodic limb movement disorder and insomnia, are associated with increased risk of vascular disorders.20 OSAHS21,22 and RLS23 have been identified as common sleep pathologies related to CSVD. However, evidence of a relationship between insomnia and CSVD is lacking. A study by Del Brutto (2015) revealed a correlation between non-breathing-related poor sleep quality and WMH severity. However, only subjective sleep scales were employed to evaluate sleep in this study, which may have led to experimental bias since patients with breathing-related sleep disorders could not really be excluded.24 In the present study, objective methods were used to evaluate sleep and excluded sleep-wake disorders other than insomnia, which improved the homogeneity of patients recruited and reliability of results. The results showed that 48.9% (44/90) of the 90 CSVD patients initially screened had chronic insomnia, 7.8% (7/90) had OSAHS and 1.1% (1/90) had RLS, clearly indicating that insomnia is most common sleep complaint in CSVD patients.
According to PSG measurements, patients in the study group had decreased sleep duration (total sleep time) and slow wave sleep (SWS), increased wake time after sleep onset and sleep fragmentation. Short sleep duration and sleep fragmentation are related to sympathetic excitation,25 high blood pressure,26 vascular endothelial inflammation27 and endothelial injury,28 while decrease in SWS could lead to abnormal immune regulation in view of its importance in adaptive immune memory.29 Based on these results, it is reasonable to assume that changes in sleep characteristics of CSVD patients contribute to the abnormal inflammatory response.
In this study, we compared the serum concentrations of pro-inflammatory cytokines in A-CSVD patients with and without chronic insomnia. The results showed higher IL-8 levels in patients with insomnia, compared with those without insomnia. Moreover, the IL-8 concentration was correlated positively with ArI and negatively with sleep duration. IL-8, a member of the chemokine subclass of cytokines, is actively secreted by monocytes and macrophages as a result of multiple cellular stimuli, such as bacteria, viruses, pro-inflammatory cytokines,30 or oxidative stress.31 As the principal human chemoattractant for neutrophils, IL-8 plays a major role in the acute inflammatory response.32
Patients with OSAHS showed increased expression of IL-8. Moreover, the IL-8 level was correlated positively with AHI and negatively with mean oxygen saturation. The mechanisms underlying IL-8 elevation in OSAHS patients include recurrent episodes of upper airway collapse, oxyhemoglobin desaturation, sleep fragmentation due to brief arousals and sustained activation of the SNS.33 We observed no serious hypoxemia during sleep for all patients since OSAHS cases were excluded. However, patients in the study group had more serious sleep fragmentation due to insomnia than those in the control group. The results of multiple linear regression analyses consistently showed positive correlation of IL-8 level with ArI, suggesting that non-breathing-related sleep fragmentation contributes to increased IL-8 expression.
In addition to sleep fragmentation, sleep duration of the study group was shorter than that of the control group. Multiple linear regression analyses also showed negative correlation of IL-8 with total sleep time, suggesting that short sleep duration is related to IL-8 activation. Partial sleep deprivation (wakefulness from 11:00 pm to 3:00 am) is associated with upregulation of mRNA expression of toll-like receptors (TLRs).34 Activation of these TLRs could lead to stimulation of the NF-κB signaling cascade35,36 and induction of several pro-inflammatory cytokines,37 including IL8.38
In conclusion, our preliminary results clearly suggest that chronic insomnia, particularly sleep fragmentation and short sleep duration, is involved in elevation of serum IL-8 expression in patients with A-CSVD, supporting the theory that effective treatment of insomnia could aid in reducing the inflammatory response of A-CSVD.
Limitations
This was a cross-sectional study, which could not determine the causal relationship between insomnia and activation of IL-8. Further prospective studies are therefore required to clarify this issue. Due to the limited number of cases in this investigation, we could not subdivide patients based on types of insomnia or clarify the relationship between different types of insomnia and IL-8 expression.
Ethical Approval
Participants were informed about the requirements, benefits and potential risks of the study before providing written informed consent to participate. All procedures were performed in accordance with the ethical standards of the Institutional Review Board Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi:10.1016/S1474-4422(10)70104-6
2. Cuadrado-Godia E, Dwivedi P, Sharma S, et al. Cerebral small vessel disease: a review focusing on pathophysiology, biomarkers, and machine learning strategies. J Stroke. 2018;20(3):302–320. doi:10.5853/jos.2017.02922
3. Fu Y, Yan Y. Emerging role of immunity in cerebral small vessel disease. Front Immunol. 2018;9:67. doi:10.3389/fimmu.2018.00067
4. Chen S, Yang Q, Chen G, Zhang JH. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res. 2015;6(1):4–8. doi:10.1007/s12975-014-0384-4
5. Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43–62. doi:10.1007/s00281-011-0290-8
6. Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. doi:10.1146/annurev-psych-010213-115205
7. Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51(7):887–892. doi:10.1053/meta.2002.33357
8. Burgos I, Richter L, Klein T, et al. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006;20(3):246–253. doi:10.1016/j.bbi.2005.06.007
9. Savard J, Laroche L, Simard S, Ivers H, Morin CM. Chronic insomnia and immune functioning. Psychosom Med. 2003;65(2):211–221. doi:10.1097/01.psy.0000033126.22740.f3
10. Wang J, Chen X, Liao J, et al. The influence of non-breathing-related sleep fragmentation on cognitive function in patients with cerebral small vessel disease. Neuropsychiatr Dis Treat. 2019;15:1009–1014. doi:10.2147/NDT.S193869
11. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69(6):592–600. doi:10.1016/j.biopsych.2010.10.023
12. First MB, Spitzer RL, Gibbon M, Williams JB. The structured clinical interview for DSM-III-R personality disorders (SCID-II). Part I: description. J Pers Disord. 1995;9(2):83–91. doi:10.1521/pedi.1995.9.2.83
13. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi:10.1016/S1474-4422(13)70124-8
14. Fazekas F, Chawluk JB, Alavi A, HI H, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. doi:10.2214/ajr.149.2.351
15. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.5th Ed. Washington, DC: American Psychiatric Association; 2013:362–363.
16. Rosenberg RS, Van Hout S. The American Academy of Sleep Medicine inter-scorer reliability program: sleep stage scoring. J Clin Sleep Med. 2013;9(1):81–87. doi:10.5664/jcsm.2350
17. Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014;83(14):1228–1234. doi:10.1212/WNL.0000000000000837
18. Liu X, Tang M, Hu L, et al. Reliability and validity of the Pittsburgh sleep quality index. Chin J Psychiatry. 1996;29(2):103–107.
19. Zhang MY. Handbook of Rating Scales in Psychiatry. Changsha: Hunan Science Publishing House; 2003:147–153.
20. Plante GE. Sleep and vascular disorders. Metabolism. 2006;55(10 Suppl 2):S45–S49. doi:10.1016/j.metabol.2006.07.013
21. Kim H, Yun CH, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep. 2013;36(5):709B–715B. doi:10.5665/sleep.2632
22. Song TJ, Park JH, Choi KH, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med. 2017;30:36–42. doi:10.1016/j.sleep.2016.03.006
23. Ferri R, Cosentino FI, Moussouttas M, et al. Silent cerebral small vessel disease in restless legs syndrome. Sleep. 2016;39(7):1371–1377. doi:10.5665/sleep.5966
24. Del Brutto OH, Mera RM, Zambrano M, Lama J, Del Brutto VJ, Castillo PR. Poor sleep quality and silent markers of cerebral small vessel disease: a population-based study in community-dwelling older adults (The Atahualpa project). Sleep Med. 2015;16(3):428–431. doi:10.1016/j.sleep.2014
25. Taylor KS, Murai H, Millar PJ, et al. Arousal from sleep and sympathetic excitation during wakefulness. Hypertension. 2016;68(6):1467–1474. doi:10.1161/hypertensionaha.116.08212
26. Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39(5):1037–1045. doi:10.5665/sleep.5748
27. Aggarwal B, Makarem N, Shah R, et al. Effects of inadequate sleep on blood pressure and endothelial inflammation in women: findings from the american heart association go red for women strategically focused research network. J Am Heart Assoc. 2018;7(12):e008590. doi:10.1161/JAHA.118.008590
28. Hall MH, Mulukutla S, Kline CE, et al. Objective sleep duration is prospectively associated with endothelial health. Sleep. 2017;40:1. doi:10.1093/sleep/zsw003
29. Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463(1):121–137. doi:10.1007/s00424-011-1044-0
30. Russo RC, Garcia CC, Teixeira MM, Amaral FA. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10(5):593–619. doi:10.1586/1744666X.2014.894886
31. DeForge LE, Preston AM, Takeuchi E, Kenney J, Boxer LA, Remick DG. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993;268(34):25568–25576.
32. Remick DG. Interleukin-8. Crit Care Med. 2005;33(12 Suppl):S466–S467. doi:10.1097/01.ccm.0000186783.34908.18
33. Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9(10):1003–1012. doi:10.5664/jcsm.3070
34. Aho V, Ollila HM, Rantanen V, et al. Partial sleep restriction activates immune response-related gene expression pathways: experimental and epidemiological studies in humans. PLoS One. 2013;8(10):e77184. doi:10.1371/journal.pone.0077184
35. Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64(6):538–540. doi:10.1016/j.biopsych.2008.05.004
36. Doyle SL, O’Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72(9):1102–1113. doi:10.1016/j.bcp.2006.07.010
37. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi:10.1016/j.immuni.2011.05.006
38. Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2003;284(4):L566–L577. doi:10.1152/ajplung.00233.2002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
