Back to Journals » Clinical Epidemiology » Volume 13
Chronic Inflammatory Diseases – Diabetes Mellitus, Rheumatoid Arthritis, Coeliac Disease, Crohn’s Disease, and Ulcerative Colitis Among the Offspring of Affected Parents: A Danish Population-Based Registry Study
Authors Andersen V , Pedersen AK, Möller S , Green A
Received 14 October 2020
Accepted for publication 17 December 2020
Published 7 January 2021 Volume 2021:13 Pages 13—20
DOI https://doi.org/10.2147/CLEP.S286623
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Henrik Sørensen
Vibeke Andersen,1– 3 Andreas Kristian Pedersen,1 Sören Möller,4 Anders Green4
1Focused Research Unit for Molecular Diagnostic and Clinical Research, University Hospital of Southern Denmark, Åbenrå, Denmark; 2Institute of Regional Research (IRS-Center Sonderjylland), University of Southern Denmark, Odense, Denmark; 3Institute of Molecular Medicine, University of Southern Denmark, Odense, Denmark; 4Open Patient Data Explorative Network (OPEN), Department of Clinical Research, Odense University Hospital and University of Southern Denmark, Odense, Denmark
Correspondence: Vibeke Andersen
Focused Research Unit for Molecular Diagnostic and Clinical Research, University Hospital of Southern Denmark, Aabenraa, Kresten Philipsens Vej 15, Åbenrå DK-6200, Denmark
Email [email protected]
Background: Chronic inflammatory diseases (CIDs) may share aetiological factors across diseases. We used registry data to evaluate the risk of developing five common childhood CIDs dependent on the parents’ disease status.
Methods: We performed a national population-based registry study by linking data from the national Danish health registers from January 1973 to March 2016 to evaluate any potential associations between parents’ disease and development of CIDs among the offspring. Results were adjusted for parental age at birth, the decade of birth, gender of the child, and type of birth. A cohort of 2,699,449 liveborn children was established for investigating the primary outcome measures: diabetes mellitus (DM), rheumatoid arthritis (RA), coeliac disease, Crohn’s disease (CD), and ulcerative colitis (UC) and all diseases combined (CID).
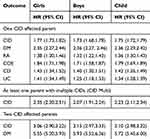
Results: Children with one CID affected parent (Hazard ratio (HR), 95% confidence interval (95% CI)=1.75 (1.72– 1.79, p< 0.001)), one multiple CID affected parent (HR=2.23 (2.11– 2.34), p< 0.001), and both parents affected (HR=3.10 (2.98– 3.22), p< 0.001) were at higher risk than children without CID affected parents. Children with DM, RA, and COE affected parents were at increased risk of three specific diseases (DM, RA and COE), whereas children with CD and UC affected parents were at increased risk of two specific diseases (CD and UC).
Conclusion: Children with CID affected parents were at increased risk of the same CID as their parents as well as other specific CIDs dependent on the parents’ CID. Future studies should address the aetiology underlying these findings to support the development of new strategies for prevention, treatment, and cure.
Keywords: parents’ disease, population study, chronic inflammatory disease, inflammatory bowel diseases, rheumatoid arthritis, coeliac disease, diabetes mellitus
Plain Language Summary
The authors investigated the sharing of diseases between parents and children to achieve a better understanding of disease causality. The studied diseases were five diseases commonly occurring in children, ie, diabetes mellitus, rheumatoid arthritis, coeliac disease, Crohn’s disease, and ulcerative colitis. Therefore, the authors assessed the risk of disease among children to parents with the same diseases.
We found that:
Children with one parent with one disease, one parent with multiple diseases, and two diseased parents were at progressive increasing risk for getting a disease compared to children with no diseased parents.
Children were at an increased risk of developing the same disease as their parents.
In addition, children were at increased risk of developing a different disease from their parents. First, children with parents with diabetes mellitus, rheumatoid arthritis, or coeliac disease were at increased risk of these three specific diseases. Next, children with parents with Crohn’s disease, or ulcerative colitis were at increased risk of these two specific diseases.
In conclusion, the children were at increased risk of the same disease as their parents. The children were also at increased risk of one or more other diseases, which were dependent on their parent’s disease. Further, the diseases were divided into two groups with shared risk, diabetes mellitus, rheumatoid arthritis, and coeliac disease on one hand, and Crohn’s disease, and ulcerative colitis on the other.
Introduction
Chronic inflammatory diseases (CIDs) include such disparate diseases as diabetes mellitus (DM),1 rheumatoid arthritis (RA),2 coeliac disease (COE),3 Crohn’s disease (CD),4 and ulcerative colitis (UC).5 The occurrence of these diseases increased dramatically during the recent decades in westernized countries,6–10 suggesting shared disease mechanisms and etiologies across the diseases. Indeed, CIDs share environmental risk factors11–14 including births by caesarian section15 and genetic susceptibility factors.16–18 In line with this, patients with one CID are at higher risk of developing other CIDs.19–22 Also, offspring to patients with CID had an increased relative risk of 4–32 for developing the same disease as their parents.23 A few studies also suggest that individuals with a family history of one CID may be at risk of developing other CID,24,25 including one study investigating the morbidity among the offspring of parents with RA.26
Since all CIDs involve immune-mediated etiological mechanisms,27 the identification of shared as well as unique risk factors of various CIDs may improve the understanding of disease causation. Studying recurrence risks could help understand the complicated relationship between these diseases by pinpointing possible shared and individual disease mechanisms. Such information might help to explain the mechanisms and aetiologies responsible for the increase in the incidence of these disorders. The hypothesis for the present study is that CIDs share a common etiological component associated with having a parent with CID, ie, that the risk of developing a CID is higher among children with an affected parent than among children with unaffected parents. The present study aimed to evaluate the risk of developing a CID dependent on the parents having a CID or not. In this study, we use the phrase “occurrence” to indicate that the child of a parent with CID develops CID regardless of the aetiology (genetics or environmental). This study took advantage of the National Danish registries completeness, size, and long follow-up.28–31 Thus, this study investigated the occurrence of five common childhood CID among children treated at Danish hospitals.
Method
Study Cohort
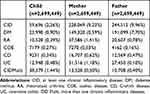
The cohort contained all Danish live born births from January 1973 to March 2016 (N=2701 408) and was extracted from the Danish Medical Birth Register and the Danish National Patient Register. Linking the different datasets from the Danish Medical Birth Register and the Danish National Patient Register as well as linking children to parents was done by the unique personal identity number assigned to every inhabitant in Denmark at birth or immigration. We excluded 1929 children (0.07%) due to missing date of birth and 30 children (0.001%) with lacking information about both parents’ disease resulting in a study cohort of 2,699,449 births. Due to the extended follow-up of the cohort, we allowed persons to be included in the study both as children and as parents of their own children.
CID in Offspring
We defined the outcome events as the first diagnosis of the child of each type of CID, as well as the first diagnosis of any CID (outcome “any CID”). Outcomes were identified in the Danish National Patient Register by ICD codes (Supplemental Table S1).
CID Among Parents
We defined CID in parents as exposure variables, separately for each child’s mother and father as dichotomous variables. A parent was classified as affected with one of the CIDs described in this study if there was registration in the Danish National Patient Register with an ICD code corresponding to the specific CID. A parent was classified as affected by any CID if there was a registration with any ICD code corresponding to the CIDs described in this study. A parent affected with multiple CIDs was classified if there were 2 or more registrations in the register with ICD codes corresponding to distinct CIDs included in this study. When evaluating the occurrence of DM among parents we did hot distinguish between the different types of DM.
Statistical Analyses
We investigated the risk of developing each of the CID as well as any of the CIDs as a separate outcome. We define the risk as the probability of a given event occurring. Because the risk changes over time, we estimate this probability by way of hazard ratios, where a hazard is defined as the number of cases at a given time point divided by the number of people which are not cases yet at time t. Kaplan Meier plots and Cox proportional hazards model with time from birth as the time scale were utilized to compare the risk of each the offspring acquiring CID depending on the parents’ different CIDs combined as well as stratified by gender of the child. To evaluate the proportional hazard assumption Schöenfeld residuals were investigated. We carried out three Cox proportional hazard models, an unadjusted, a partial, and a fully adjusted model. The unadjusted model contained the exposure variables which corresponded to the specific CID. The partial model was adjusted by the year of birth and the age of the mother and father at birth. The fully adjusted model included these variables as well as whether the child was born by caesarean section. We thus included as a covariate if the child was delivered by way of caesarean section or not by data from the Danish National Patient Register (from 1973), respectively, the Danish Medical Birth Register (before 1973).29,30 The number of affected children related to the same parents was not investigated, as these data are not accessible. The statistical analysis was repeated with exposure corresponding to multiple CIDs or any CID. Stata IC was used to conduct the statistical analysis for the study.
Patient and Public Involvement
Patients and public were not involved in the study design or execution. The patients’ associations (The Diabetes Association, The Danish Coeliac Disease Association, Danish Rheumatism Association, The Psoriasis Association, and The Danish Colitis-Crohn Association) will be invited to take part in the discussion and dissemination of the results. No public health policy recommendations are derived from this work.
Results
Of the 2,699,449 liveborn children between January 1973 and March 2016, 8.4% (228 069) had a mother with CID, and 9.1% (244,512), had a father with CID. Table 1 shows the characteristics of this cohort. In total, 32.9% of offspring from at least one affected CID parent developed CID (19,641) compared to 15.9% of the children with no CID affected parents (419 175) (Supplemental Table S2).
Relative Risk of CID Among Girls and Boys with at Least One Parent Affected by CID
Table 2 shows the relative risk of developing a specific CID or any CIDs among the girls, boys and children (girls and boys combined) that had a parent with a CID compared to children with no CID affected parent as the reference group. Children with one CID affected parent (Hazard ratio (HR), 95% confidence interval (95% CI)=1.75 (1.72–1.79, p<0.001)), one multiple CID affected parent (HR=2.23 (2.11–2.34), p<0.001), and both parents affected (HR=3.10 (2.98–3.22), p<0.001) were at higher risk than children without CID affected parents. Figure 1 and Supplemental Table S3 show the relative risk of developing a specific CID or any CID among the children that had a parent with a specific CID compared to children with no CID affected parents. Children with a parent with DM, RA, or COE were at increased risk of DM and COE. Children with a parent with DM and RA were at increased risk of RA. Children with CD or UC affected parents were at risk of CD and UC. Furthermore, girls with RA or CD affected parents were at increased risk of RA and CD. The relative risk of developing COE was 23.3 (95% CI 21.0–26.0) among girls, and 14.9 (95% CI 12.7–17.5) among boys with a COE affected parent. Children with CD and UC affected parents, and girls with RA affected parents were at increased risk of CD and UC.
Relative Risk of CID Dependent on the Sex of the CID Affected Parent
Supplemental Table S4 and Figure 2 show the fully adjusted relative risk for children with a CID affected parent dependent on the sex of the parent. The relative risk of developing COE was 22.9 (95% CI 20.7–25.4) if the mother had a COE diagnosis versus 13.1 (95% CI 11.0–15.6) if the father had a COE diagnosis. Similarly, for the other diseases, the risk was generally higher if the mother was affected by CID compared to the father, but the differences for disease other than COE were relatively small.
Relative Risk of CID Dependent on the Age and Sex of the Offspring
Supplemental Table S5 shows the full, partial and unadjusted relative risk for the association between parental CID and the incidence of CID among the offspring separated by gender and stratified according to the age of the child when the CID was diagnosed (0–9, 10–19, 20–29, 30–39 and over 40 years). Overall, although the risk of developing a CID was increased in both genders when having a parent with a CID, the risk seemed to affect the girls for a longer period compared to the boys, ie, the girls appeared to be at increased risk for an extended timeframe compared to the boys.
Discussion
This register-based national cohort study of 2699 449 children investigated the risk of developing five common childhood diseases – DM, RA, COE, CD, and UC – according to the parents’ CID status. Children without CID affected parents were used as the reference. Children with one CID affected parent, one multiple CID affected parent, and two CID affected parents were at progressively increased risk of CID. In addition to being at an increased risk of developing the same disease as their parents, children of a CID affect parent were at increased risk of developing a different CID from their parents dependent specifically on the parents’ disease (Figure 1). The results showed two patterns of increased cross-disease occurrence. The first pattern was between DM, RA, and COE and the second increased cross-disease pattern was between CD and UC. Thus, children with DM, RA, or COE affected parents were at increased risk of these three specific diseases, and children with CD or UC affected parents were at increased risk of these two specific diseases. Moreover, girls with RA or CD affected parents were at increased risk of RA and CD. Furthermore, girls with a CID affected parent were at higher risk than boys, and children with a CID affected mother were at higher risk than those with a CID affected father. Apart from COE, these differences were small. The increased risk was detectable for more than 40 years, and in general, girls were at an increased risk for an extended period of time compared to boys.
The strengths and weaknesses of this cohort have been discussed previously.15 The main strength of this observational study is the inclusion of more than 2.5 million nationwide births with complete ascertainment. The most common CIDs among children treated at hospitals in Denmark were chosen with an overall rate of CID among the parents of 8.4–9.1%. This information and a follow-up period of more than 40 years have provided sufficient power to analyse the associations between parental disease and risk of CIDs among the offspring. An additional strength includes the adjustment of known or suspected confounding factors such as caesarean section. The sensitivity analysis found that the hazard ratios (HR) increased from the unadjusted to the partially adjusted, which suggests that possible confounding variables were adequately taken into consideration. The association of parents’ disease with the risk of CIDs in children varied by the age of the child, hence the reported overall HR should be interpreted as average risk increase.32 The main weakness of this study is the observational design, which cannot infer causality from positive associations. One might expect that the suspicion of a CID could have been high among children with CID affected parents leading to a higher diagnostic rate and/or to earlier diagnosis. Although risks were increased for some CID, the risk of other CID was not increased, thus supporting the validity of the results. Still, we cannot exclude that having a CID affected parent may add to an earlier diagnosis of CID compared to children without CID affected parents. Furthermore, even though we have attempted to adjust for relevant confounders, we cannot exclude residual confounding in the analysis. Whereas it may be considered a strength to use already existing register data, produced for other purposes than that of the present study, the validity of the data may be affected by missing registrations and coding errors.33 Such errors few in numbers and have occurred without specific relation to the present study. Despite these limitations, this data does provide epidemiologic evidence for the concept of identifying shared pathways between different diseases.
This study is the first in-depth investigation of disease-specific and cross-disease-specific occurrence of one of five common childhood diseases according to whether the parent(s) had been diagnosed with one of these CID (Figure 1). The results support the idea of these diseases sharing common aetiological component(s) associated with having a parent with CID. The findings of our study support other literature.23,26 One reported an increased risk of developing DM among offspring to RA parents.26 The other study reported an increased risk of CD and UC among children to UC and CD affected parents and a higher risk to the offspring of developing the same CID as the parent.23 However, these studies investigated, in part, the same cohort as the present study.
This study is unique in finding shared occurrence of some CIDs but not others. Understanding the biology behind the pattern of occurrence of common CIDs is vital for the future treatment of these diseases. This study generates more hypotheses on specific and shared disease mechanisms in CID, which can be tested in the future. Future studies should explore the causal pathways underlying the disease risk conferred by a parents’ disease since this knowledge may support the development of prevention and optimised treatment strategies in CID.
Transparency Statement
VA affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Abbreviations
CI, confidence intervals; CID, chronic inflammatory disease; CD, Crohn’s disease; HR, hazard ratio; IBD, inflammatory bowel disease; RA, rheumatoid arthritis; DM, diabetes mellitus; UC, ulcerative colitis.
Data Sharing Statement
Data is stored at Open Patient data Explorative Network (OPEN). Bonafide researchers can apply to use the dataset by applying to [email protected].
Ethics Approval
This study does not require approval from the ethics committee or an institutional review board according to Danish law, which states that studies on data registries, not including biological material, should not be notified to the ethics committee or an institutional review boards in Denmark. All data accessed complied with relevant data protection and privacy regulations.
Funding
This study was funded by The Danish Rheumatism Association (Gigtforeningen R104-A2195-B760, V Andersen). The funders did not influence the conduct of the study, analysis, interpretation of the data, the writing of this report, or the decision to publish, and have no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; nor other relationships or activities that could appear to have influenced the submitted work.
Disclosure
The authors have no conflicts of interest to declare that are relevant to the content of this article.
References
1. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. doi:10.1016/S0140-6736(13)62154-6
2. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi:10.1016/S0140-6736(16)30173-8
3. Leonard MM, Sapone A, Catassi C, Fasano A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA. 2017;318(7):647–656. doi:10.1001/jama.2017.9730
4. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380(9853):1590–1605. doi:10.1016/S0140-6736(12)60026-9
5. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. doi:10.1016/S0140-6736(16)32126-2
6. Namatovu F, Sandstrom O, Olsson C, Lindkvist M, Ivarsson A. Celiac disease risk varies between birth cohorts, generating hypotheses about causality: evidence from 36 years of population-based follow-up. BMC Gastroenterol. 2014;14:59. doi:10.1186/1471-230X-14-59
7. Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol. 2014;2(11):867–874. doi:10.1016/S2213-8587(14)70161-5
8. Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–1322. doi:10.1136/annrheumdis-2013-204627
9. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. doi:10.1038/nrgastro.2015.150
10. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390(10114):2769–2778. doi:10.1016/S0140-6736(17)32448-0
11. van der Sloot KWJ, Amini M, Peters V, Dijkstra G, Alizadeh BZ. Inflammatory bowel diseases: review of known environmental protective and risk factors involved. Inflamm Bowel Dis. 2017;23(9):1499–1509. doi:10.1097/MIB.0000000000001217
12. Myleus A, Hernell O, Gothefors L, et al. Early infections are associated with increased risk for celiac disease: an incident case-referent study. BMC Pediatr. 2012;12:194. doi:10.1186/1471-2431-12-194
13. Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory infections in early life and the development of islet autoimmunity in children at increased type 1 diabetes risk: evidence from the BABYDIET study. JAMA Pediatr. 2013;167(9):800–807. doi:10.1001/jamapediatrics.2013.158
14. Kemppainen KM, Vehik K, Lynch KF, et al. Association between early-life antibiotic use and the risk of islet or celiac disease autoimmunity. JAMA Pediatr. 2017;171(12):1217–1225. doi:10.1001/jamapediatrics.2017.2905
15. Andersen V, Moller S, Jensen PB, Moller FT, Green A. Caesarean delivery and risk of chronic inflammatory diseases (inflammatory bowel disease, rheumatoid arthritis, coeliac disease, and diabetes mellitus): a population based registry study of 2,699,479 births in Denmark during 1973–2016. Clin Epidemiol. 2020;12:287–293. doi:10.2147/clep.S229056
16. Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60(12):1739–1753. doi:10.1136/gut.2009.199679
17. Ellinghaus D, Jostins L, Spain SL, et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet. 2016;48(5):510–518. doi:10.1038/ng.3528
18. Lettre G, Rioux JD. Autoimmune diseases: insights from genome-wide association studies. Hum Mol Genet. 2008;17(R2):R116–R121. doi:10.1093/hmg/ddn246
19. Halling ML, Kjeldsen J, Knudsen T, Nielsen J, Hansen LK. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23(33):6137–6146. doi:10.3748/wjg.v23.i33.6137
20. Elfstrom P, Sundstrom J, Ludvigsson JF. Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther. 2014;40(10):1123–1132. doi:10.1111/apt.12973
21. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: a systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33(1):115–121.
22. Bao YK, Weide LG, Ganesan VC, et al. High prevalence of comorbid autoimmune diseases in adults with type 1 diabetes from the HealthFacts database. J Diabetes. 2019;11(4):273–279. doi:10.1111/1753-0407.12856
23. Moller FT, Andersen V, Wohlfahrt J, Jess T. Familial risk of inflammatory bowel disease: a population-based cohort study 1977–2011. Am J Gastroenterol. 2015;110(4):564–571. doi:10.1038/ajg.2015.50
24. Kuo CF, Chou IJ, Grainge MJ, et al. Familial aggregation and heritability of type 1 diabetes mellitus and coaggregation of chronic diseases in affected families. Clin Epidemiol. 2018;10:1447–1455. doi:10.2147/CLEP.S172207
25. Kuo CF, Grainge MJ, Valdes AM, et al. Familial aggregation of rheumatoid arthritis and co-aggregation of autoimmune diseases in affected families: a nationwide population-based study. Rheumatology (Oxford, England). 2017;56(6):928–933. doi:10.1093/rheumatology/kew500
26. Rom AL, Wu CS, Olsen J, et al. Parental rheumatoid arthritis and long-term child morbidity: a nationwide cohort study. Ann Rheum Dis. 2016;75(10):1831–1837. doi:10.1136/annrheumdis-2015-208072
27. Schultze JL, Rosenstiel P. Systems medicine in chronic inflammatory diseases. Immunity. 2018;48(4):608–613. doi:10.1016/j.immuni.2018.03.022
28. Andersen JS, Olivarius Nde F, Krasnik A. The Danish national health Service register. Scand J Public Health. 2011;39(7 Suppl):34–37. doi:10.1177/1403494810394718
29. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39(7 Suppl):30–33. doi:10.1177/1403494811401482
30. Bliddal M, Broe A, Pottegard A, Olsen J, Langhoff-Roos J. The Danish medical birth register. Eur J Epidemiol. 2018;33(1):27–36. doi:10.1007/s10654-018-0356-1
31. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22–25. doi:10.1177/1403494810387965
32. Stensrud MJ, Hernán MA. Why test for proportional hazards? JAMA. 2020;323:1401. doi:10.1001/jama.2020.1267
33. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S91125
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.