Back to Journals » Clinical Epidemiology » Volume 10
Chronic diseases in the children of women with maternal thyroid dysfunction: a nationwide cohort study
Authors Jølving LR , Nielsen J, Kesmodel US , Nielsen RG, Nørgård BM , Beck-Nielsen SS
Received 3 March 2018
Accepted for publication 11 June 2018
Published 28 September 2018 Volume 2018:10 Pages 1381—1390
DOI https://doi.org/10.2147/CLEP.S167128
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Henrik Sørensen
Line Riis Jølving,1,2 Jan Nielsen,1,2 Ulrik Schiøler Kesmodel,3,4 Rasmus Gaardskær Nielsen,2,5 Bente Mertz Nørgård,1,2 Signe Sparre Beck-Nielsen6,7
1Center for Clinical Epidemiology, Odense University Hospital, 2Institute of Clinical Research, University of Southern Denmark, Odense C, 3Department of Obstetrics and Gynaecology, Herlev University Hospital, Herlev, 4Institute for Clinical Medicine, University of Copenhagen, København, 5Hans Christian Andersen Children’s Hospital, Odense University Hospital, Odense C, 6Department of Pediatrics Kolding, Hospital Lillebaelt, Kolding, 7Department of Regional Health Research, University of Southern Denmark, Odense C, Denmark
Objective: Maternal thyroid disease (TD) during pregnancy is associated with adverse birth outcomes, but little is known on its long-term outcomes. We aimed to examine if children born to mothers with TD have increased disease risk during childhood and adolescence.
Patients and methods: A register-based cohort study was conducted on all live born children in Denmark from 1989 to 2013, including the association between maternal TD during pregnancy and somatic and psychiatric diseases in the children. Cox proportional hazards models were used to compute hazard ratios (HRs) according to the type of maternal TD, Graves’ disease, and Hashimoto’s thyroiditis.
Results: A total of 2,618 children were born to women with Graves’ disease, 760 to women with Hashimoto’s thyroiditis (exposed), and 1,557,577 to women without any TD (unexposed). The median follow-up time for children born to mothers with Graves’ disease was 9.3 years (25/75 percentile 5.4/13.9 years) and with Hashimoto’s thyroiditis was 4.8 years (25/75 percentile 2.5/8.2 years). In children exposed to maternal Graves’ disease in utero, the adjusted HR of TD was 12.83 (95% CI, 9.74–16.90), Graves’ disease was 34.3 (95% CI, 20.23–58.35), and type 1 was diabetes 2.47 (95% CI, 1.46–4.18). In children exposed to maternal Hashimoto’s thyroiditis, the adjusted HR of Hashimoto’s thyroiditis was 24.04 (95% CI, 5.89–97.94).
Conclusion: Our data suggest that children born to women with Graves’ disease and Hashimoto’s thyroiditis have excess long-term morbidities in childhood and adolescence. We particularly found an increased risk of any TD and type 1 diabetes to be diagnosed in children exposed in utero to Graves’ disease. These novel findings are relevant for pediatricians, stressing the importance of history of maternal disease when evaluating children with suspected endocrine disorders.
Keywords: thyroid disease, Graves’ disease, Hashimoto’s thyroiditis, reproduction, child morbidity, clinical epidemiology
Introduction
Thyroid diseases (TDs) are among the most prevalent chronic diseases in pregnant women and the prevalence has increased during the last decades.1–3 In Denmark, the two most frequent TDs are Graves’ disease with a prevalence of 4.51 cases per 1,000 inhabitants and Hashimoto’s thyroiditis with a prevalence of 0.58 cases per 1,000 inhabitants.4 These autoimmune diseases primarily affect women, with a male:female ratio for Graves’ disease of 1:5.6 and for Hashimoto’s thyroiditis of 1:8.0.5 Graves’ disease is caused by the presence of activating thyrotropin receptor stimulating IgG antibodies (TRAbs) acting on the thyrotropin receptor. TRAb stimulates the thyroid hormone production in an unregulated manner, incapacitating the hypothalamic–pituitary axis and resulting in hyperthyroidism.6 In Hashimoto’s thyroiditis, the thyroid peroxidase antibodies (TPOabs) are cytotoxic to the thyrocytes, leading to destruction of the thyroid gland and hypothyroidism.7 Graves’ disease often goes into remission after the first treatment years restoring a normal thyroid function and absence of autoantibodies, whereas Hashimoto’s thyroiditis is a chronic condition due to destruction of the thyroid tissue, resulting in a state of irreversible hypothyroidism requiring lifelong substitution with thyroid hormones. The TPOab level usually decreases over time. Therefore, even though the prevalence of Graves’ diagnosis codes is higher compared to Hashimoto’s, the number of patients being affected by the disease and attending long-term medical services in the community is significantly lower for Graves’ disease compared to Hashimoto’s thyroiditis.
When active autoimmune TD is present during pregnancy, the fetus will be exposed to circulating antibodies as both TRAb and TPOab pass the placenta from the mother to the fetus.8 In Graves’ disease, the presence of TRAb rarely causes intrauterine thyrotoxicosis and goiter of the fetus as pregnancy usually suppresses Graves’ disease.9,10 Furthermore, antithyroid medication has been found to pass the placenta in small amounts.11 In Hashimoto’s thyroiditis, the TPOabs have not been linked to fetal or neonatal TD.8
Maternal hyperthyroidism has been linked to miscarriage, stillbirth, preterm birth, intrauterine growth retardation, low birth weight, preeclampsia, and fetal TD.12,13 If the mother with Graves’ disease experiences overt hyperthyroidism during pregnancy, the Danish Endocrine Society recommends treatment with the antithyroid medication propylthiouracil during the first trimester, and later changing the medication to methimazole during the second and third trimesters.14 Both of these medications have been associated with congenital malformations, especially methimazole with malformations arising during the first trimester.13,15 In addition, the long-term effects of maternal hyperthyroidism on the offspring are linked to an increased risk of neurologic or psychiatric diseases including seizure, attention-deficit hyper activity disorders, and other psychiatric disorders.16–18
Maternal hypothyroidism, as Hashimoto’s thyroiditis, is associated with several adverse pregnancy and child outcomes such as spontaneous abortion, intrauterine growth retardation, preeclampsia, and preterm birth.12,19 In a long-term perspective, impaired neurodevelopment in the offspring has been suggested to be a result of maternal hypothyroxinemia during pregnancy.20–22 In one study, children of mothers with untreated hypothyroidism during pregnancy showed a significantly lower IQ than their peers.23 In addition, it has recently been observed that children born to mothers with hypothyroidism have an increased risk of hypertension, neurologic and psychiatric diseases later in life.18,21,24
To our knowledge, no reports of a wider range of chronic somatic conditions as outcomes in the children of mothers with TD are available. We, therefore, aimed to examine the association between in utero exposure of the most prevalent diagnoses of maternal TD (Graves’ disease and Hashimoto’s thyroiditis) and the long-term risk of several chronic diseases in the offspring during childhood and adolescence. We hypothesized that active maternal TD during pregnancy has an impact on the developing fetus in a long-term perspective due to the intrauterine environment, antibodies, genetics, medication, or other unknown factors. Our study was based on a nationwide cohort of all live births during a 25-year period in Denmark with up to 25 years of follow-up.
Patients and methods
Setting and data sources
In Denmark, all citizens have free and equal access to a tax-financed health care system and its uniform organization enabled us to use a population-based study design. The Danish Civil Registration System was established in 1968 and contains information on date of birth and death, sex, place of residence, and dates of emigration and immigration, with daily updates, and all residents have a personal identification number in The Danish Civil Registration System (the DCRS number). The DCRS number is a unique digit identifier used in all Danish health registries, and the DCRS number ensures valid linkage between the registries.25 A national cohort was established by linking the registries including information from the DCRS, the Danish Medical Birth Registry (DMBR) – a register that has information on all births in Denmark established in 1973,26,27 and the Danish National Registry of Patients (DNRP). The DNRP contains diagnoses of all inpatients admitted to a non-psychiatric public hospital in Denmark since 1977 and emergency and outpatient contacts since 1995. The diagnosis codes in the DNRP were assigned according to the eighth revision of the World Health Organization’s International Classification of Diseases (ICD-8) until the end of 1993, and according to the tenth revision (ICD-10) thereafter.28 From the Danish Psychiatric Central Register (DPCR), we extracted information on psychiatric outcomes. The DPCR was established as a computerized nationwide register in 1969, and the register includes information on all psychiatric hospitalizations, including outpatient visits since 1995.
Study population
The study population comprised all live born children, born between January 1, 1989 and December 31, 2013 according to the DMBR.
Children born to women with autoimmune TD, exposed cohorts
To ensure active autoimmune maternal TD, data on maternal TD were obtained within a period of 1 year before childbirth. The child was allocated to the exposed cohort if the mother was assigned with a diagnosis of autoimmune hyperthyroidism/Graves’ disease (ICD-8 codes: 242.00, 242.01, 242.08, 242.09; ICD-10 code: E05.0) or autoimmune hypothyroidism/Hashimoto’s thyroiditis (ICD-8 code: 244.01; ICD-10 codes: E06.3, E06.3A+E06.3B) within 1 year before childbirth. We constructed two exposed cohorts: one cohort of children exposed in utero to maternal autoimmune hyperthyroidism/Graves’ disease and another cohort of children exposed in utero to maternal autoimmune hypothyroidism/Hashimoto’s thyroiditis. Mothers registered with both diseases (Graves’ disease and Hashimoto’s thyroiditis) within 1 year before childbirth were allocated to the Graves’ disease group (21 mothers giving birth to 22 children). At the start of hyperthyroidism in Graves’ disease, TPOabs are present in many cases along with TRAbs,29 which may lead to the assignment of diagnoses of both Graves’ disease and Hashimoto’s thyroiditis. As TRAbs are specific for Graves’ disease in the state of hyperthyreosis, the presence of TPOab in this case is not assigned any pathological effect.6 In addition, TPOabs were found to be present in ~13% of Danish women in fertile age without any TD, indicating the low specificity of TPOabs.30
Children born to women without autoimmune TD, unexposed cohort
All live born children from the study population, born to women with no registered diagnoses of any TD within 1 year before childbirth, were allocated to the unexposed cohort.
Long-term child morbidity outcome assessment
In order to report on chronic diseases during childhood and adolescence in children born to women with TD, the authors went through the entire ICD classification of diseases and reached consensus on 15 disease groups covering major non-malignant somatic and psychiatric disease categories. These 15 included disease categories were as follows: 1) chronic lung disease, including asthma, 2) TD, 2a) Graves’ disease, 2b) Hashimoto’s thyroiditis, 3) parathyroid diseases, 4) type 1 diabetes, 5) type 2 diabetes, 6) polycystic ovary syndrome (PCOS), 7) epilepsy, 8) multiple sclerosis (MS), 9) rheumatoid arthritis, 10) systemic lupus erythematosus (SLE), 11) inflammatory bowel disease (IBD), 12) coagulation disorders, 13) anxiety and personality disorders, 14), affective mood disorders, and 15) schizophrenia and other paranoid psychoses. The included ICD codes are provided in Table S1.
Statistical analysis and confounders
Initially, contingency tables were constructed for the main study variables of the exposed and unexposed cohorts. We used multivariate Cox proportional hazard regression models to compute the associations between maternal TD (stratified on Graves’ disease and Hashimoto’s thyroiditis) and offspring morbidity, in a cluster analysis accounting for multiple children to the same mother.
Crude hazard ratio (HR) and adjusted hazard ratios (aHR) and the associated 95% CIs were reported. Follow-up started at the date of birth, but for the occurrence of child and adolescence outcomes of chronic lung disease and asthma, and the psychiatric disorders, the follow-up period started from the age of 5 years. These diseases are in general not assigned to children aged <5 years of age, and the presence of the diagnoses before that age could be due to misclassification. Children in the cohorts were followed until the date of event (disease), death, or end of follow-up by December 31, 2014. Potential confounders were identified a priori based on the currently available scientific literature and applied to the statistical model.31 We extracted the following information related to childbirth from the DMBR: sex of the child, year of birth (in five calendar periods: 1989–1993, 1994–1998, 1999–2003, 2004–2008, and 2009–2013), maternal age at birth (<20, 20–29, 30–39, and >40 years), preterm birth defined as <37 completed weeks of gestation (no/yes), small for gestational age defined as birth weight below –2 SD of the sex-specific expected birth weight (no/yes),32 mode of delivery (vaginal/ cesarean section), parity (1/≥2), multiple birth (no/yes), maternal smoking during pregnancy (no/yes; information on maternal smoking was collected in the registries from 1991), and maternal pre-pregnancy body mass index (<18.5, 18.5–24, 25–29, and >30; information on body mass index was available in the registries from 2003). Furthermore, we collected data on maternal comorbidity from the DNRP and the DPCR in the exposed and the unexposed cohorts. Adjustment for other maternal underlying chronic diseases was carried out and, for instance, when assessing the risk of type 1 diabetes in the offspring, we adjusted for the effect of underlying maternal type 1 diabetes. The same approach was used when we estimated the risk of other child diseases (except in the analyses for Graves’ disease and Hashimoto’s thyroiditis where these diseases constituted the exposure). We only performed the adjusted analyses in disease groups with ≥10 childhood outcomes in the exposed groups, or a significant result in the crude analysis, and this was in order to ensure solid statistical precision.
All statistical analyses were performed using Stata Software (v 14.0; StataCorp LP, College Station, TX, USA) and we chose a 5% level of significance.
The study was approved by The Danish Data Protection Agency (Journal no. 2013-41-2092) and followed the rules of security and privacy. According to Danish law, approval by the ethical review board or patient consent is not required for register-based studies.
Results
From 1989 to 2013, we included 1,560,955 live born children. A total of 3,378 of these children were exposed to maternal TD in terms of Graves’ disease and Hashimoto’s thyroiditis during pregnancy, of whom 2,618 were born to mothers with active autoimmune Graves’ disease and 760 children were born to mothers with active autoimmune Hashimoto’s thyroiditis. Thus, a total of 1,557,577 children born to women without any kind of TD were allocated to the unexposed cohort. In children born to mothers with Graves’ disease, the median follow-up time was 9.3 years (25/75 percentile 5.4/13.9 years). In children born to women with Hashimoto’s thyroiditis, the median follow-up time was 4.8 years (25/75 percentile 2.5/8.2 years).
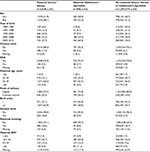
The basic characteristics of the exposed cohort of children born to women with Graves’ disease, the cohort of children born to women with Hashimoto’s thyroiditis, and the unexposed cohort are given in Table 1. The percentage of children born to women with Graves’ disease remained relatively constant from 2004 and onward (~30%). The percentage of children born to women with Hashimoto’s thyroiditis increased with increasing calendar time, and 60.9% of the children born to women with Hashimoto’s thyroiditis were thus born in the latest calendar period between 2009 and 2013. The frequency of cesarean section in women with Graves’ disease and Hashimoto’s thyroiditis (24.3% and 26.2%, respectively) was significantly higher than the frequency of cesarean section among unexposed women (15.0%). In addition, mothers with Graves’ disease were more likely to experience a multiple birth (10.0%) compared to mothers with Hashimoto’s thyroiditis or with no TD (5.0% and 3.7%, respectively). Women with Graves’ disease were more likely to smoke during pregnancy (18.6%) than those with Hashimoto’s thyroiditis (6.8%). The proportion of smokers in the unexposed cohort was by comparison 18.7%.
Long-term child morbidity when exposed to maternal Graves’ disease
We found a total of 246 childhood disease outcomes in children exposed in utero to Graves’ disease (n=2,618). There were no outcomes for childhood diseases of MS and SLE among the exposed children. We found that in utero exposure to Graves’ disease was associated with an increased risk of the development of selected chronic diseases in the outcome. The risk of TD was aHR 12.83 (95% CI, 9.74–16.90), Graves’ disease was aHR 34.33 (95% CI, 20.23–58.25), and type 1 diabetes was aHR 2.47 (95% CI, 1.46–4.18). The crude HR of Hashimoto’s thyroiditis was 4.79 (95% CI, 1.54–14.90) and the crude HR for IBD was 2.31 (95% CI, 1.16–4.63); however, the results were not statistically significant after adjustment. The HRs and aHRs for childhood disease outcome groups are shown in Figure 1.
Long-term child morbidity when exposed to maternal Hashimoto’s thyroiditis
We found a total of 33 childhood disease outcomes in children exposed in utero to Hashimoto’s thyroiditis (n=760). These few outcomes were distributed across many different childhood disease groups, and we could therefore only perform a few adjusted analyses. There were no outcomes of parathyroid diseases, type 2 diabetes, PCOS, SLE, MS, IBD, coagulation disorders, and affective mood disorders. The crude HRs were increased for the TD diagnoses (including the subgroups of Graves’ disease and Hashimoto’s thyroiditis) and in the disease group of schizophrenia and other paranoid psychoses. After adjustment, the results remained significant for TD with aHR of 11.12 (95% CI, 5.53–22.34), in the subgroup of Hashimoto’s thyroiditis with the aHR 24.02 (95% CI, 5.89–97.94), and in schizophrenia and other paranoid psychoses with the aHR 10.64 (95% CI, 2.80–40.41). The HRs and aHRs for childhood disease outcome groups are shown in Figure 2.
Discussion
To the best of our knowledge, this is the first study to examine the long-term consequences in children exposed to active, autoimmune maternal TD in utero in a wider perspective. In this large nationwide study on one of the most prevalent chronic disease group during pregnancy,3 we found that children were more often exposed in utero to Graves’ disease compared to Hashimoto’s disease. We also found a relatively larger disease burden in children born to mothers with Graves’ disease than in those born to women with Hashimoto’s disease. For several of the diagnostic groups examined, the point estimates indicated excess long-term morbidity in children exposed to active maternal TD. In general, the diseases with significantly increased risks in the children of the mothers with TD were identified within the group of autoimmune diseases, that is, TD, Graves’ disease, Hashimoto’s thyroiditis, and type 1 diabetes. Our findings are in accordance with familial association studies in which an aggregation of different autoimmune diseases has been reported.33
We found an increased risk of being diagnosed with TD (including the subgroup of Graves’ disease), and type 1 diabetes when the offspring was exposed to active maternal Graves’ disease in utero. In addition, we found that children exposed to active maternal Hashimoto’s thyroiditis in utero had an increased risk of being diagnosed with TD (including the subgroup of Hashimoto’s thyroiditis), whereas the significantly increased crude HR for being diagnosed with Graves’ disease disappeared after the confounder adjustment. The increased risk of being diagnosed with type 1 diabetes in children exposed to Graves’ disease in utero is, to our knowledge, novel. In contrary, it is well established that type 1 diabetes is associated with a higher risk of developing other autoimmune diseases, where 15%–30% have autoimmune TD (especially, Hashimoto’s thyroiditis and rarely, Graves’ disease) and 4%–9% have celiac disease, suggesting that these diseases share genetic susceptibility.34–36 The development of hyperthyroidism in type 1 diabetes has been linked to specific human leukocyte antigen haplotypes.36 The association of increased aHR for developing type 1 diabetes when exposed to Graves’ disease in utero may thus, in part, be explained by an inherited common genetic decisive factor for the two diseases.
It is suggested that the offspring of a parent suffering from a specific autoimmune disease carries a higher susceptibility for developing the same autoimmune disease as their parent, compared to the background population.37 This association was also present in this study as the children exposed to Graves’ disease had a significantly increased risk of developing Graves’ disease and the children exposed to Hashimoto’s thyroiditis in utero had a significantly increased risk for developing Hashimoto’s thyroiditis themselves later in childhood and adolescence. This points toward the possibility of an inherited causative genetic mutation for the diseases, which was investigated by Medici et al by a genome-wide association study meta-analysis, revealing new genetic loci associated with the presence of TPOab and the risk of developing clinical TD including both hypo- and hyperthyroidism.38 Twin studies show that ~75% of the phenotypic variance in autoimmune TDs is due to genetic effects.39 In our study, the identified higher risk in the offspring may be driven by the child inheriting a genetic mutation in this case stratified to their mother or by epigenetic modulations occurring during in utero when exposed to autoantibodies. Hypo-/hyperthyroidism or thyroid medications or even influence by pre-/postnatal environmental factors may also be contributing factors. Unfortunately, the current dataset derived from registers does not allow any conclusion regarding the effect of genetic, epigenetic, or other pre-/postnatal environmental exposures contributing to the increased risk of developing autoimmune disease in the offspring.
The validity of the captured information in studies based on the use of national registries is a core issue.40 We were able to identify maternal autoimmune TD by hospital diagnosis from the DNPR. The validity of the diagnosis of autoimmune TD registered in the DNPR is high, showing only misclassification in 2% of the cases according to a study based on biomarkers.2 Only women with active autoimmune TD, who had a hospital contact with the relevant diagnoses within 1 year before childbirth, were considered part of the exposed cohort in our study. For both Hashimoto’s thyroiditis and Graves’ disease, we found that a higher proportion of the women were diagnosed during the years 2004–2013, compared to the periods before 2004. This may be due to a larger proportion being diagnosed with increasing awareness of these diseases during pregnancy – noting that the inclusion criterion was a diagnosis within 1 year of child birth. It could also be related to a genuine increase in the incidence of the two diseases during the last part of our study period or could be related to the registration of diseases in the registries. Unfortunately, the registers available do not comprise patients assigned with the diagnoses by the general practitioner (GP). When pregnant, patients with especially newly diagnosed TD are expected to be referred from the GP to the hospital for treatment. Our study has several methodological strengths including the size of the study population and the population-based approach using valid and complete registries. We have complete follow-up of all children in our cohorts, and the examined outcomes in the registers can be assessed independently of the exposure status, thereby reducing both selection and information bias. Even so, some information bias cannot be ruled out if physicians are more prone to diagnose diseases in the offspring of mothers with TD compared to children born to mothers without TD and detection bias would thus be induced. Furthermore, we were able to take important potential confounders into account (eg, calendar time of birth, preterm birth, and maternal comorbidity) that were based on valid information from the DMBR and the DNRP. In the exposed cohort, we only included women who were diagnosed with TD within 1 year before childbirth in order to ensure that maternal autoimmune TD was actively present during pregnancy. In addition, it is an important strength of the study that we examined the risk of a large number of different chronic diseases in childhood and adolescence and had not selected only a single or a few disease categories in the offspring.
Our study has some limitations. Firstly, we cannot rule out that some of the diagnoses on the child outcomes are misclassified. Secondly, the results on schizophrenia and other paranoid psychoses that were based on only two child outcomes should be interpreted carefully. Thirdly, for some of the disease categories with only a few outcomes (eg, Graves’ disease and Hashimoto’s thyroiditis in children born to mothers with Hashimoto’s thyroiditis during pregnancy and Hashimoto’s disease in children born to mothers with Graves’ disease during pregnancy), we had low statistical precision. Furthermore, when examining the health effect in the offspring, it is valuable to have as many years of follow-up as possible; but the length of follow-up in this study is limited by the onset of the nationwide registries, although we used as long follow-up periods as possible based on data from the registries. For some of our outcomes (eg, psychiatric disorders, PCOS, and asthma), it would have been especially valuable to have longer follow-up time in the children, as these diseases are diagnosed in late childhood/adolescence. We did not have outcome data on children only diagnosed by the GP, but as we are examining serious chronic diseases in the children, virtually all children suspected for these diseases will be referred for exact diagnosis and treatment in a hospital setting.
Conclusion
Our data suggest that children born to women with active Graves’ disease and Hashimoto’s thyroiditis have excess long-term morbidities in childhood and adolescence. We particularly found an increased risk of TD and type 1 diabetes in children exposed in utero to Graves’ disease. Whether these findings reflect the TD exposure in early life, exposure to thyroid antibodies, the medical treatment of the maternal disease, genetics, epigenetics, or environmental aspects still needs to be clarified in future studies.
In the pediatric setting, children with TDs are frequently diagnosed late in disease progression, when suffering from growth delay, and concentration, learning, and attention difficulties. Based on our results, we find it relevant to consider especially TD in children who are exposed to active maternal TD during pregnancy, and attention to type 1 diabetes should also be paid to children exposed in utero to Graves’ disease. Hopefully, an increased awareness of both GPs and pediatricians on this association between the exposure to active maternal TD during fetal life and later development of disease in the offspring may result in early diagnosis and, thereby, prevention of severe developmental delay in the diseased child.
Disclosure
The authors report no conflicts of interest in this work.
References
Cignini P, Cafà EV, Giorlandino C, Capriglione S, Spata A, Dugo N. Thyroid physiology and common diseases in pregnancy: review of literature. J Prenat Med. 2012;6(4):64–71. | ||
Carlé A, Pedersen IB, Knudsen N, et al. Epidemiology of subtypes of hyperthyroidism in Denmark: a population-based study. Eur J Endocrinol. 2011;164(5):801–809. | ||
Andersen SL, Olsen J, Laurberg P. Maternal thyroid disease in the Danish National Birth Cohort: prevalence and risk factors. Eur J Endocrinol. 2016;174(2):203–212. | ||
Eaton WW, Pedersen MG, Atladóttir HO, Gregory PE, Rose NR, Mortensen PB. The prevalence of 30 ICD-10 autoimmune diseases in Denmark. Immunol Res. 2010;47(1-3):228–231. | ||
Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. J Autoimmun. 2007;29(1):1–9. | ||
Smith TJ, Hegedüs L. Graves’ disease. N Engl J Med. 2016;375(16):1552–1565. | ||
Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348(26):2646–2655. | ||
Dallas JS. Autoimmune thyroid disease and pregnancy: relevance for the child. Autoimmunity. 2003;36(6–7):339–350. | ||
Marx H, Amin P, Lazarus JH. Hyperthyroidism and pregnancy. BMJ. 2008;336(7645):663–667. | ||
Balucan FS, Morshed SA, Davies TF. Thyroid autoantibodies in pregnancy: their role, regulation and clinical relevance. J Thyroid Res. 2013;2013:1–15. | ||
Laurberg P, Bournaud C, Karmisholt J, Orgiazzi J. Management of Graves’ hyperthyroidism in pregnancy: focus on both maternal and foetal thyroid function, and caution against surgical thyroidectomy in pregnancy. Eur J Endocrinol. 2009;160(1):1–8. | ||
Nazarpour S, Ramezani Tehrani F, Simbar M, Azizi F. Thyroid dysfunction and pregnancy outcomes. Iran J Reprod Med. 2015;13(7):387–396. | ||
Cooper DS, Laurberg P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013;1(3):238–249. | ||
Danish Endocrine Society [webpage on the Internet]. NBV: Thyroideasygdom ved graviditet og infertiliet. Available from: http://www.endocrinology.dk/index.php/nbvhovedmenu/2-thyroidea-sygdomme/5-thyroideasygdom-ved-graviditet-og-infertilitet. Accessed February, 2015. | ||
Pearce EN. Thyroid disorders during pregnancy and postpartum. Best Pract Res Clin Obstet Gynaecol. 2015;29(5):700–706. | ||
Andersen SL, Olsen J, Laurberg P. Foetal programming by maternal thyroid disease. Clin Endocrinol. 2015;83(6):751–758. | ||
Andersen SL, Laurberg P, Wu CS, Olsen J. Maternal thyroid dysfunction and risk of seizure in the child: a Danish nationwide cohort study. J Pregnancy. 2013;2013:10. | ||
Andersen SL, Laurberg P, Wu CS, Olsen J. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG. 2014;121(11):1365–1374. | ||
Ajmani SN, Aggarwal D, Bhatia P, Sharma M, Sarabhai V, Paul M. Prevalence of overt and subclinical thyroid dysfunction among pregnant women and its effect on maternal and fetal outcome. J Obstet Gynaecol India. 2014;64(2):105–110. | ||
Henrichs J, Ghassabian A, Peeters RP, Tiemeier H. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol. 2013;79(2):152–162. | ||
Rytter D, Andersen SL, Bech BH, et al. Maternal thyroid function in pregnancy may program offspring blood pressure, but not adiposity at 20 y of age. Pediatr Res. 2016;80(1):7–13. | ||
Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68–100. | ||
Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105(2):239–245. | ||
Andersen SL, Olsen J, Wu CS, Laurberg P. Psychiatric disease in late adolescence and young adulthood. Foetal programming by maternal hypothyroidism? Clin Endocrinol. 2014;81(1):126–133. | ||
Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–25. | ||
Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45(3):320–323. | ||
Kristensen J, Langhoff-Roos J, Skovgaard LT, Kristensen FB. Validation of the Danish Birth Registration. J Clin Epidemiol. 1996;49(8):893–897. | ||
Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30–33. | ||
Ogawa T, Sakata S, Nakamura S, et al. Thyroid hormone autoantibodies in patients with Graves’ disease: effect of anti-thyroid drug treatment. Clin Chim Acta. 1994;228(2):113–122. | ||
Pedersen IB, Knudsen N, Jørgensen T, Perrild H, Ovesen L, Laurberg P. Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency. Clin Endocrinol. 2003;58(1):36–42. | ||
Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol. 2013;122(5):957–965. | ||
Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843–848. | ||
Hemminki K, Li X, Sundquist J, Sundquist K. Familial association between type 1 diabetes and other autoimmune and related diseases. Diabetologia. 2009;52(9):1820–1828. | ||
Kahaly GJ, Hansen MP. Type 1 diabetes associated autoimmunity. Autoimmun Rev. 2016;15(7):644–648. | ||
Kakleas K, Soldatou A, Karachaliou F, Karavanaki K. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM). Autoimmun Rev. 2015;14(9):781–797. | ||
Barker JM. Clinical review: type 1 diabetes-associated autoimmunity: natural history, genetic associations, and screening. J Clin Endocrinol Metab. 2006;91(4):1210–1217. | ||
Cutolo M. Autoimmune polyendocrine syndromes. Autoimmun Rev. 2014;13(2):85–89. | ||
Medici M, Porcu E, Pistis G, et al. Identification of novel genetic loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 2014;10(2):e1004123. | ||
Brix TH, Hegedüs L. Twins as a tool for evaluating the influence of genetic susceptibility in thyroid autoimmunity. Ann Endocrinol (Paris). 2011;72(2):103–107. | ||
Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. |
Supplementary material
  | Table S1 ICD-8 and ICD-10 classification diagnostic codes used Abbreviation: ICD, International Classification of Diseases. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.