Back to Journals » Infection and Drug Resistance » Volume 14
Chromone Derivatives CM3a Potently Eradicate Staphylococcus aureus Biofilms by Inhibiting Cell Adherence
Authors Zhan Q, Xu Y, Zhan L, Wang B, Guo Y, Wu X, Ai W, Song Z, Yu F
Received 13 January 2021
Accepted for publication 18 February 2021
Published 11 March 2021 Volume 2021:14 Pages 979—986
DOI https://doi.org/10.2147/IDR.S301483
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Qing Zhan,1 Yanlei Xu,1 Lingling Zhan,2 Bingjie Wang,3 Yinjuan Guo,3,4 Xiaocui Wu,3,4 Wenxiu Ai,5 Zengqiang Song,6 Fangyou Yu3,4
1Jiangxi Provincial Key Laboratory of Preventive Medicine, School of Public Health, Nanchang University, Nanchang, 330006, People’s Republic of China; 2Department of Laboratory Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 3Department of Clinical Laboratory Medicine, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, 200082, People’s Republic of China; 4Shanghai Key Laboratory of Tuberculosis, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, 200082, People’s Republic of China; 5Department of Respiratory Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 6School of Pharmaceutical Sciences, Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China
Correspondence: Fangyou Yu; Zengqiang Song Tel +86-021-65115006
; +86-577-86699396
Email [email protected]; [email protected]
Introduction: The ability of Staphylococcus aureus to form biofilms is associated with high mortality and treatment costs. Established biofilms cannot be eradicated by many conventional antibiotics due to the development of antibiotic tolerance by S. aureus. Here we report the synthesis and biological characterization of novel small-molecule compounds with antibiofilm activity. Chromone 5-maleimide substitution compounds (CM3a) showed favorable antibacterial activity against S. aureus.
Methods: CM3A with antibacterial activity was synthesized and screened. The minimum inhibitory concentration (MIC) of CM3a were determined by the broth microdilution method. Biofilm eradication assay and colony count methods were used to investigate the effect of CM3a on S. aureus biofilm disruption and killing. Changes in biofilm architecture when subjected to CM3a, were visualized using confocal laser scanning microscopy (CLSM). CCK-8 assay and survival rate of Galleria mellonella larvae were used to test the toxicity of CM3a.
Results: The minimum inhibitory concentration (MIC) of CM3a against S. aureus was about 26.4 μM. Biofilm staining and laser scanning confocal microscopy analysis showed that CM3a eradicated S. aureus biofilms by reducing the viability of the constituent bacterial cells. On the other hand, CM3a showed negligible toxicity against mouse alveolar epithelial cells and Galleria mellonella larvae.
Conclusion: Chromone derivatives CM3a has therapeutic potential as a safe and effective compound for the treatment of S. aureus infection.
Keywords: chromone derivative, maleimide, Staphylococcus aureus, biofilm, eradicate
Introduction
Staphylococcus aureus is a Gram-positive opportunistic human pathogen that causes many community-acquired, nosocomial, and biofilm-related infections worldwide.1 Implanted medical devices (eg, joint prostheses, fluid shunts, and pacemakers) and catheters (eg, intravenous, urinary, and dialysis catheters) have revolutionized modern healthcare and are widely used in clinical practice. However, they are often associated with chronic and recurrent infections caused by S. aureus biofilms that form on the device surface.2 Biofilms are produced upon switching the bacterial growth mode from planktonic to sessile, the process of its formation comprises 3 main stages: attachment, maturation, and detachment.3 In the detachment phase, cell clusters or a single cell separates from the biofilm, spreading to and colonizing other parts of the host.4,5 Bacterial biofilms are key players in the development of highly refractory to antibacterial agents because of the complex structure of the extracellular polymeric substances (EPS) matrix.6 Biofilm formation can lead to the persistence and recalcitrant of S. aureus and is a major clinical challenge.7 Currently employed antibiotics mainly target microbial growth mechanisms and cell division, while bacterial biofilms resist clearance by antibacterial agents and host defense molecules. Thus, bacterial biofilms increase the risks of morbidity and mortality in patients, as well as treatment costs.8,9
In several decades, although many non-growth-altering biofilm inhibitors and dispersal agents have been described, there are few biofilm-eradicating agents.10 It is impendency to develop new biofilm eradication agents and complementary antimicrobial strategies with multiple therapeutic applications to address persistent bacterial infections and reduce patient mortality and treatment costs.
Chromone is a natural compound present in the diet of humans and animals that has low toxicity to mammalian cells.11,12 Previous studies have also shown that chromone derivatives possess a broad range of biological activities13 depending on the substitution pattern of the chromone scaffold.14 Maleimide is an important structural parent nucleus of a class of bioactive molecules and functional materials from Marine natural alkaloids.15 In this study, we synthesized chromone 5-maleimide substitution compounds and assessed their therapeutic potential by characterizing their capacity to eradicate S. aureus biofilm.
Materials and Methods
Procedure for Synthesis
Chromone 1a (0.2 mmol, 1 equiv) and maleimide 2 (0.5 mmol, 2.5 equiv) were combined in a 12-mL screw-capped tube with 2 mL of 1.2-dichloroethane (0.1 M). [Ru(p-cymene) Cl2]2 (0.01 mmol, 0.05 equiv), AgNTf2 (0.04 mmol, 0.2 equiv), and AgOAc (0.6 mmol, 3 equiv) were added to the reaction mixture, which was heated to 120°C in a heating mantle with stirring for 2 h. After the reaction was completed, the reaction mixture was directly loaded onto a silica gel column and purified with a petroleum ether/EtOAc solution to obtain the 3a product (≥83% yield; >95% purity; Figure 1). The full name of CM3a is 3-(7-chloro-4-oxo-4H-chrome-5-yl)-1-ethyl-1H-pyrrole-2,5-dione.
 |
Figure 1 Procedure for synthesis of CM3a.1a: chromone. 2: maleimides. 3a: CM3a. |
Bacterial Strains and Cells
S. aureus strains JP21, ZSA01, ZSA02, ZSA03, ZSA04, and ZSA05 were provided by the First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China). S. aureus strain SA113 was a gift from the Department of Infectious Diseases and the Key Laboratory of Endogenous Infection, Shenzhen Nanshan People’s Hospital (Shenzhen, China). S. aureus isolates were identified using a VITEK-2 automated system (bioMérieux, Marcy l’Etoile, France) according to the manufacturer’s instructions. As all S. aureus strains used in this study are routinely encountered during laboratory procedures at the hospital, the Ethics Committee of Shanghai Pulmonary Hospital of Tongji University School of Medicine waived the requirement for ethics approval.
The mouse alveolar epithelial cells MALE-12 and human bronchial epithelial cell BEAS-2B was a gift from the Department of Anesthesiology, Shanghai Pulmonary Hospital, Tongji University School of Medicine (Shanghai, China).
Biofilm Formation
Overnight cultures of S. aureus strains JP21, SA113, ZSA01, ZSA02, ZSA03, ZSA04, and ZSA05 were diluted 1:200 in tryptic soy broth (TSB) containing 0.5% glucose (Sigma-Aldrich, St. Louis, MO, USA) and dispensed into 96-well microfilter plates (BD Biosciences, Franklin Lakes, NJ, USA). After 24 h of static culture at 37°C, the wells were washed 3 times with 50 mM phosphate-buffered saline (PBS [pH 7.2]) to remove unattached bacterial cells. Biofilms were stabilized by fixation with methanol (99.5%) for 15 min and stained with 1% crystal violet for 15 min. The wells were gently washed 3 times with distilled water to remove floating cells. After drying, 33% glacial acetic acid was added to the well to release the biofilms into the solution, and the optical density at 600 nm (OD600) was measured.16
Determination of Minimum Inhibitory Concentration (MIC)
CM3a MICs for S. aureus strains JP21, SA113, ZSA01, ZSA02, ZSA03, ZSA04, and ZSA05 were determined by the broth microdilution method. Samples (100 μL) were added to a 96-well microfilter plate containing CM3a (1–512 μg/mL) and 100 μL of cation-adjusted Mueller–Hinton broth (CAMHB). After 24 h of static cultivation at 37°C, OD600 was measured with a microplate reader (Bio-Rad, Hercules, CA, USA). The MIC was defined as the lowest concentration that completely inhibited S. aureus growth.
The MICs of vancomycin, telithromycin, daptomycin for SA113 were determined by the broth microdilution method, according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.17
Growth Assay
S. aureus strains were grown in MHB for 3 h, then diluted 1:200 with CAMHB; 100-μL aliquots were added to a 96-well microfilter plate. A 100-μL volume of CM3a was added to each well to obtain final concentrations of 1/32, 1/16, 1/8, and 1/4 MIC. The fully automated Bioscreen C microbial growth curve analyzer (Growth Curves USA, Piscataway, NJ, USA) was used to measure OD600 every 30 min for 24 h and a growth curve was generated from the measured values.
CM3a Eradicate the Established Biofilms of S. aureus
Overnight cultures of S. aureus were diluted 1:200 in 200 µL of TSB with 0.5% glucose (TSBG) and inoculated into a 96-well microfilter plate. After 24 h of static incubation at 37°C, mature biofilms formed and the supernatant was discarded. The plate was washed with PBS to remove floating cells, and fresh TSBG containing CM3a was added to the wells; TSBG without CM3a served as the control. After static incubation for 48 h with daily medium replacement, the remaining biofilms were stained with crystal violet. Vancomycin, telithromycin, and daptomycin were used as control antibacterial agents for SA113 biofilms.
Detecting the Adherent Cells in the Established Biofilms
S. aureus SA113 and JP21 were inoculated into 12 polystyrene microtiter plates with TSBG and mature biofilms formed after static incubation for 24 h. After discarding the supernatant and washing the plates 3 times, fresh TSBG containing CM3a was added to the wells; TSBG without CM3a served as an untreated control. After 48 h of static incubation with daily medium replacement, the adherent cells remaining in the established biofilms were collected by scratching the wall of the wells with a cell scraper after discarding the supernatant. Ultrasonic Bacteria Dispersion Counter (TB healthcare, Guangdong, China) was used to disperse bacteria of collected biofilms. The colony-forming units (CFU) of live bacteria were counted after diluting and plating the cells.18
Laser Scanning Confocal Microscopy
S. aureus strains were statically incubated in a 20-mm glass-bottomed cell culture dish (NEST, Wuxi, China) for 48 h. The intermediate steps were the same as those described in Section 2.6. SYTO-9 (300 μL, 0.02%; Thermo Fisher Scientific, Waltham, MA, USA) and propidium iodide (300 μL, 0.067%; Thermo Fisher Scientific) were added to the cell culture dish for 30 min in the dark to stain the cells. The samples were imaged by laser scanning confocal microscopy (TCS SP5; Leica, Wetzlar, Germany) using a 63×1.4-numerical aperture oil immersion objective lens. Images were reconstructed into a 3-dimensional (3D) model using Imaris v7.4.2 software (Bitplane, Belfast, UK).19
Assessment of CM3a Cytotoxicity
Cell Counting Kit (CCK)-8 (Beyotime, Shanghai, China) was used according to the manufacturer’s instructions to assess the viability of mouse alveolar epithelial cells MALE-12 and human bronchial epithelial cell BEAS-2B treated with CM3a. Briefly, the cells were seeded in a 96-well plate at 105, 104, and 103 cells/well. After incubation for 48 h in a serum-free medium containing CM3a or PBS, the cells were incubated with 20 μL CCK-8 solution for 1 h at 37°C. OD450 was measured with a Biotek Synergy 2 microplate reader (BioTek, Winooski, VT, USA).20
Evaluation of CM3a Toxicity to Galleria mellonella Larvae
As the larvae of the greater wax moth G. mellonella turn from white to black when they die, they are well-suited for testing drug toxicity. We injected the larvae with PBS or CM3a (16 and 32 μg/mL; n=~10 per group). The viability of the larvae was recorded after 48 h. The experiment was performed in triplicate.
Statistical Analysis
Data were analyzed with Student’s t-test and by 1-way factorial analysis of variance. Data analyses were performed using SPSS v19 software (IBM, Armonk, NY, USA). P values <0.05 were regarded as statistically significant.
Results
Characterization of Product CM3a
CM3a was a white solid (50.3 mg; >95% purity) after purification by chromatography (elution: 10% EtOAc in petroleum ether) with a melting point of 205°C–206°C. The compound was characterized by nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (HRMS), yielding the following values:1H NMR (400 MHz, CDCl3) δ 7.81 (d, J = 6.0 Hz, 1H), 7.59 (d, J = 1.9 Hz, 1H), 7.22 (d, J = 1.9 Hz, 1H), 6.46 (s, 1H), 6.28 (d, J = 6.0 Hz, 1H), 3.64 (q, J = 7.2 Hz, 2H), 1.25 (t, J = 7.2 Hz, 3H) ppm. 13C[1H] NMR (126 MHz, CDCl3) δ 176.2, 170.0, 168.8, 157.2, 154.8, 148.0, 139.2, 131.0, 127.4, 125.2, 122.0, 120.2, 114.0, 33.3, 13.9 ppm. HRMS (ESI-TOF) m/z: [M+H]+ calcd for C15H11ClNO4: 304.0377, found: 304.0370.
CM3a Inhibits the Growth of S. aureus
The MIC value of CM3a against all S. aureus strains tested in our study was 8 μg/mL (about 26.4 μM). Subinhibitory concentrations of CM3a—ie, 1/4 MIC (2 μg/mL), 1/8 MIC (1 μg/mL), 1/16 MIC (0.5 μg/mL), and 1/32 MIC (0.25 μg/mL)—had no effect on the growth of S. aureus SA113 and JP21. At 1/2 MIC (4 μg/mL), both strains grew more slowly than cells in the control group after 10 h (Figure 2).
CM3a Eradicating the Established Biofilms of S. aureus
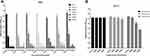
We selected 7 S. aureus strains that formed robust biofilms to evaluate the biofilm-inhibitory activity of CM3a. At 2×MIC (16 μg/mL), the OD600 of the biofilms decreased from ~3 to ~1. At CM3a concentrations of 4×MIC (32 μg/mL), 8×MIC (64 μg/mL), 16×MIC (128 μg/mL), and 32×MIC (256 μg/mL), biofilm formation was almost completely inhibited (OD600<0.3), showing statistically significant differences relative to the control group (Figure 3A).
The MIC Values of Vancomycin, telithromycin, and daptomycin against SA113 were 1, 2, and 0.25 separately. Vancomycin, telithromycin, and daptomycin were used as control drugs to treat the biofilm of SA113. Vancomycin and daptomycin have almost no effect on the biofilm of SA113, and telithromycin has a certain effect on the concentrations of 4×MIC and 8×MIC (Figure 3B).
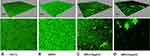
To confirm these findings, we examined the state of the biofilms after CM3a treatment with the LIVE/DEAD assay followed by laser scanning confocal microscopy. The biofilms were sparse and weak at 2× MIC (16 μg/mL) and almost disappeared at 4× MIC (32 μg/mL) (Figure 4).
CM3a Kills Adherent Cells in S. aureus Biofilms
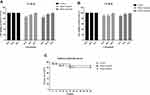
Adherent (live) cells remaining in S. aureus biofilms following CM3a treatment were counted. CM3a was highly effective in inhibiting the growth of adherent cells in the established biofilm. Due to the quite remarkable difference between the c. f. u. values, we used the log10(c.f.u. values) algorithm to draw a bar graph and compare the number of remaining live bacteria. At 2× MIC (16 μg/mL) of CM3a, the number of live bacteria (CFU/mL) was around 105 times lower than in the control sample; when the CM3a concentration was ≥4× MIC (32 μg/mL), the survival number of the remaining bacteria was about 108 times lower than in the control group, living bacteria are almost gone (Figure 5).
 |
Figure 5 The survival rate of adherent cells in biofilms after treatment with CM3a (16–128 μg/mL). Each experiment was repeated 3 times, and data represent mean±standard deviation. **P<0.01. |
CM3a Has Low Toxicity in Mammalian Cells and Insect Larvae
We assessed the cytotoxicity of CM3a in mouse alveolar epithelial cells MALE-12 and human bronchial epithelial cell BEAS-2B cultured with different concentrations of CM3a (16 and 32 μg/mL) with the CCK-8 assay. There were no differences in survival rates between the treatment groups (>80%) and the control group (Figure 6A and B). We also injected G. mellonella larvae with PBS or CM3a (16 and 32 μg/mL) and evaluated their viability after 48 h. Consistent with the results of the CCK-8 assay, there were no differences in survival rates between the treatment groups and the control group (Figure 6C).
Discussion
The ability of S. aureus to develop robust biofilm on implanted medical devices is a major barrier to successful treatment, as biofilm bacteria are 10–1000 times more recalcitrance than planktonic bacteria to conventional antibiotics.21 The presence of persister cells can disseminate and enter the circulation to colonize other organs and cause the recalcitrance and relapse of persistent bacterial infections.2,22 Many conventional antibiotics and new drugs in development stages can only inhibit the biofilm during the biofilm formation process but cannot eradicate the mature biofilm. Additionally, some antibiotics may thicken biofilms throughout treatment, making them more difficult to clear.23,24 Therefore, new drugs are needed that can eradicate difficult-to-treat biofilms.
Chromone has a unique structure that is suited to the synthesis of new drugs.25 Chromone has been recognized as a privileged structure for the invention and development of new drugs.26 Lots of research studies prove that the Medicinal properties exhibited by chromone derivatives are antibacterial, antifungal, antioxidant, antimalarial, neuroprotective, and HIV inhibitory potential.13,27 Maleimides are the base compounds for drug conjugation to antibodies, peptides, and other targeting units through their reaction with thiol groups, and have the advantages of rapid kinetics, quantitative conversion, and high specificity.15 In our study, the chromone 5-maleimide substitution compound CM3a showed strong antibacterial activity toward S. aureus, with a MIC of 26.4 μM. In the presence of 4 μg/mL CM3a, the growth of S. aureus was slowed by 10 h. CM3a effectively eradicated S. aureus biofilms and effectively killed the constituent bacteria: the survival rate of the remaining live bacteria was about 105 times lower than in the control sample at CM3a concentrations ≧32 μg/mL (about 106 μM). CM3a also promoted biofilm detachment and disintegration.
Chromone is known to have minimal toxicity to mammalian cells. We found that CM3a had low toxicity to mouse alveolar epithelial cells MALE-12 and human bronchial epithelial cell BEAS-2B, and did not cause the death of G. mellonella larvae. It is worth mentioning that we first synthesized the chromone derivatives without Cl, but the solubility was poor. After modification with Cl or Br, the solubility was greatly increased, and the 2 derivatives had similar antibacterial activity and capacity to eradicate S. aureus biofilms. Although further study is required to clarify the mechanism of action of CM3a in the destruction of biofilms, our findings show that CM3a is a promising new drug for eradicating mature S. aureus biofilms and thereby improving the outcomes of patients with implanted medical devices or catheters.
Ethical Approval and Consent to Participate
The Ethics Committee of the Shanghai Pulmonary Hospital of Tongji University School of Medicine approved our study. The use of all cell lines in this study was approved by the Ethics Committee of the Shanghai Pulmonary Hospital of Tongji University School of Medicine.
Acknowledgments
The authors would like to thank Yang Wu and Di Qu (Key Laboratory of Medical Molecular Virology of Ministries of Education and Health, School of Basic Medical Science and Institutes of Biomedical Sciences, Shanghai Medical College of Fudan University), Jingxin Zheng, Xiang Sun, and Zhong Chen (Department of Infectious Diseases and the Key Lab of Endogenous Infection, Shenzhen Nanshan People’s Hospital and The 6th Affiliated Hospital of Shenzhen University Health Science Center), Li Zheng (the Department of Anesthesiology, Shanghai Pulmonary Hospital, Tongji University School of Medicine) for their excellent technical support and kindly suggestions.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Natural Science Fund of China (81871704).
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Tong S, Davis JS, Eichenberger E, et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–661. doi:10.1128/CMR.00134-14
2. Del Pozo J. Biofilm-related disease. Expert Rev Anti Infect Ther. 2018;16(1):51–65. doi:10.1080/14787210.2018.1417036
3. Suresh M, Biswas R, Biswas L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int j Med Microbiol. 2019;309(1):1–12. doi:10.1016/j.ijmm.2018.11.002
4. Pinto R, Soares FA, Reis S, et al. Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Front Microbiol. 2020;11:952. doi:10.3389/fmicb.2020.00952
5. Lister J, Horswill A. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front Cell Infect Microbiol. 2014;4:178. doi:10.3389/fcimb.2014.00178
6. Grecka K, Xiong ZR, Chen H, et al. Effect of Ethanol Extracts of Propolis (EEPs) against staphylococcal biofilm-microscopic studies. Pathogens. 2020;9:8.
7. Del Pozo J, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther. 2007;82(2):204–209. doi:10.1038/sj.clpt.6100247
8. Conlon B. Staphylococcus aureus chronic and relapsing infections: evidence of a role for persister cells: an investigation of persister cells, their formation and their role in S. aureus disease. BioEssays: news and reviews in molecular. Cell Dev Biol. 2014;36(10):991–996.
9. Savage V, Chopra I, O’Neill A. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob Agents Chemother. 2013;57(4):1968–1970. doi:10.1128/AAC.02008-12
10. Hoque J, Konai MM, Gonuguntla S, et al. Membrane active small molecules show selective broad spectrum antibacterial activity with no detectable resistance and eradicate biofilms. J Med Chem. 2015;58(14):5486–5500. doi:10.1021/acs.jmedchem.5b00443
11. Mohsin N, Irfan M, Hassan SU. et al. Current strategies in development of new chromone derivatives with diversified pharmacological activities: a review. Pharm Chem j;2020. 1–17. doi:10.1007/s11094-020-02187-x
12. Legoabe L, Petzer A, Petzer J. Inhibition of monoamine oxidase by selected C6-substituted chromone derivatives. Eur J Med Chem. 2012;49:343–353. doi:10.1016/j.ejmech.2012.01.037
13. Yadav P, Parshad B, Manchanda P, et al. Chromones and their derivatives as radical scavengers: a remedy for cell impairment. Curr Top Med Chem. 2014;14(22):2552–2575. doi:10.2174/1568026614666141203141317
14. Matos M, Vazquez-Rodriguez S, Uriarte E, et al. Synthesis and pharmacological activities of non-flavonoid chromones: a patent review (from 2005 to 2015). Expert Opin Ther Pat. 2015;25(11):1285–1304. doi:10.1517/13543776.2015.1078790
15. Lahnsteiner M, Kastner A, Mayr J, et al. Improving the stability of maleimide-thiol conjugation for drug targeting. Chemistry. 2020;26(68):15867–15870. doi:10.1002/chem.202003951
16. Sun X, Lin Z-W, Hu -X-X, et al. Biofilm formation in erythromycin-resistant Staphylococcus aureus and the relationship with antimicrobial susceptibility and molecular characteristics. Microb Pathog. 2018;124:47–53. doi:10.1016/j.micpath.2018.08.021
17. CLSI. Performance Standards for Antimicrobial Susceptibility Testing.
18. Zheng J, Sun X, Lin ZW, et al. In vitro activities of daptomycin combined with fosfomycin or rifampin on planktonic and adherent linezolid-resistant isolates of Enterococcus faecalis. J Med Microbiol. 2019;68(3):493–502. doi:10.1099/jmm.0.000945
19. Bortolin M, Vecchi E, Romanò CL, et al. Antibiofilm agents against MDR bacterial strains: is bioactive glass BAG-S53P4 also effective? J Antimicrob Chemother. 2016;71(1):123–127. doi:10.1093/jac/dkv327
20. Li Y, Wang R, Xue L, et al. Astilbin protects against cerebral ischaemia/reperfusion injury by inhibiting cellular apoptosis and ROS-NLRP3 inflammasome axis activation. Int Immunopharmacol. 2020;84:106571.
21. Donlan R, Costerton J. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–193. doi:10.1128/CMR.15.2.167-193.2002
22. Fisher R, Gollan B, Helaine S. Persistent bacterial infections and persister cells. Nat Rev Microbiol. 2017;15(8):453–464. doi:10.1038/nrmicro.2017.42
23. Jin Y, Guo Y, Zhan Q, et al. Subinhibitory concentrations of mupirocin stimulate staphylococcus aureus biofilm formation by upregulating cidA. Antimicrob Agents Chemother. 2020;64(3):3. doi:10.1128/AAC.01912-19
24. Yu W, Hallinen K, Wood K. Interplay between antibiotic efficacy and drug-induced lysis underlies enhanced biofilm formation at subinhibitory drug concentrations. Antimicrob Agents Chemother. 2018;62:1.
25. Keri R, Budagumpi S, Pai RK, et al. Chromones as a privileged scaffold in drug discovery: a review. Eur J Med Chem. 2014;78:340–374. doi:10.1016/j.ejmech.2014.03.047
26. Reis J, Gaspar A, Milhazes N, et al. Chromone as a privileged scaffold in drug discovery: recent advances. J Med Chem. 2017;60(19):7941–7957. doi:10.1021/acs.jmedchem.6b01720
27. Park J, Lee SU, Kim SH, et al. Chromone and chromanone derivatives as strand transfer inhibitors of HIV-1 integrase. Arch Pharm Res. 2008;31(1):1–5. doi:10.1007/s12272-008-1111-z
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.