Back to Journals » International Journal of Nanomedicine » Volume 13 » T-NANO 2014 Abstracts
Cholic acid-modified polyethylenimine: in vitro and in vivo studies
Authors Dube B, Pandey A, Joshi G, Mulherkar R, Sawant K
Received 14 October 2016
Accepted for publication 2 January 2017
Published 15 March 2018 Volume 2018:13(T-NANO 2014 Abstracts) Pages 83—85
DOI https://doi.org/10.2147/IJN.S124693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Brahmanand Dube,1,2 Abhijeet Pandey,1 Ganesh Joshi,3 Rita Mulherkar,3 Krutika Sawant1
1Pharmacy Department, Faculty of Pharmacy, The Maharaja Sayajirao University of Baroda, Vadodara, 2Wockhardt Research Centre, Wockhardt Ltd, Aurangabad, India; 3Genetic Engineering Laboratory, ACTREC Tata Memorial Centre, Kharghar, Navi Mumbai
Abstract: Low-molecular-weight polyethylenimine has lower cytotoxicity than high molecular weight polyethylenimine, but it is not an efficient transfection agent because of limitations of DNA delivery into the cytoplasm. Therefore, in the present study, the hydrophobic modification of low-molecular-weight polyethylenimine (PEI 2 kDa [PEI2]) by cholic acid (ChA) was performed to form PEI2-ChA, and in vitro and in vivo studies were performed. Results indicate that the nanoplexes of PEI2-ChA with gWIZ-GFP have greater transfection efficiency (27%) in NT8e cell lines as evaluated by flow cytometry and also observed by fluorescence imaging. The present study concluded that the transferrin-containing nanoplexes of PEI2-ChA conjugates with plasmid p53 warrant clinical trials in humans after exhaustive animal studies for use as a novel gene delivery system.
Keywords: polyethylenimine, biodistribution, tumor regression
Introduction
The high-molecular-weight polyethylenimine (PEI) has not been clinically used because of its nonspecific cytotoxicity displayed against different cell types.1 On the other hand, low-molecular-weight PEIs are much less cytotoxic, but they are not efficient transfection agents because of limitations of DNA delivery into the cytoplasm.2 Hence, the hydrophobic modification of low-molecular-weight PEI 2 kDa (PEI2) by cholic acid (ChA) was performed as previously reported3 to form PEI2-ChA, and in vitro and in vivo studies were performed.
Materials and methods
Materials
PEI2 was purchased from Sigma-Aldrich (St Louis, MO, USA). Plasmid p53 (p53-expressing plasmid) was a generous gift from Dr Bert Vogelstein Lab, Johns Hopkins University (Baltimore, MD, USA). Plasmid gWIZ-GFP (GFP-expressing reporter plasmid) was obtained from Aldevron (Fargo, Canada). Primary antibody p53 (F-8; # sc-374087) and secondary antibody IgG-HRP (# sc-2005) were obtained from Santa Cruz Biotechnology (New Delhi, India). Animal imaging was carried out using IVIS Lumina II (Caliper Life Sciences, Cheshire, UK). The NT8e cells were used as available (isolated and developed in Dr Rita Mulherkar’s Genetic Engineering Laboratory at Advanced Centre for Treatment, Research and Education in Cancer [ACTREC], Kharghar, Mumbai, India).
Methods
The NT8e cells were transfected with nanoplexes (polymer:pDNA weight ratio of 10)4 with 1.8 μg of gWIZ-GFP in 150 mM saline. After 48 h of transfection, cells were analyzed for transfection efficiency by flow cytometry and observed under fluorescence microscopy. For in vitro protein expression studies, the NT8e cells were transfected with 60 μL of nanoplexes containing 3.6 μg of gWIZ-GFP, and GFP expression was evaluated by Western blotting.5 Biodistribution of nanoplexes was evaluated by injecting animals with 100 μL of either pDNA in saline or nanoplexes without transferrin or nanoplexes incorporated with transferrin through tail vein of Swiss bare mice. For tumor regression studies, animals were treated with 8 μg of plasmid p53 in transferrin-containing PEI2-ChA nanoplexes and evaluated for tumor volume after 48 days of treatment.6 All mice were cared for and maintained in accordance with Animal Welfare Regulations under an approved protocol by the Institutional Animal Care and Use Committee of ACTREC Tata Memorial Centre, Kharghar, Navi Mumbai, India, and Animal Research Committee guidelines (CPCSEA reg number 65/1999, March 11, 1999) as per Animal Study Proposal permission from Institutional Animal Ethics Committee (IAEC). The use of NT8e cells used were also approved by the institutional ethical review board approval from ACTREC.
Results and discussion
The nanoplexes of PEI2-ChA with gWIZ-GFP were shown to increase the transfection efficiency (~27%) in NT8e cell lines (Figure 1) as evaluated by flow cytometry and also observed from fluorescence images (Figure 2). The cell cycle analysis of NT8e cells (p53 mutant) treated with transferrin-containing nanoplexes of PEI2-ChA with plasmid p53 showed increased apoptosis of cells, which appeared to be due to restored p53 function. In vitro protein expression study using Western blot analysis revealed the expression of exogenous p53 protein (Figure 3). In vivo imaging of mice treated with nanoplexes of PEI2-ChA-containing transferrin demonstrated localized signal for GFP protein in the brain region. Tumor regression is an improvement or cure from a disease that appears to be progressing in its severity.3 Treatment with wild-type plasmid p53 induced regression of ectopic solid tumors. The tumors of mice treated with transferrin-containing nanoplexes of PEI2-ChA were approximately five times smaller in size than the tumors of untreated animals.
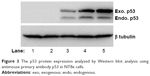  | Figure 3 The p53 protein expression analyzed by Western blot analysis using antimouse primary antibody p53 in NT8e cells. |
Conclusion
Transferrin-containing nanoplexes of PEI2-ChA conjugate with plasmid p53 demonstrated in vitro p53 protein expression and showed significant apoptosis, indicating their ability to restore the normal p53 function in NT8e cells. The transferrin-containing nanoplexes further demonstrated brain targeting efficiency in vivo. The nanoplexes of PEI2-ChA also showed significant tumor regression in xenograft model of nude mice with NT8e cells.
Thus, based on the results obtained in the present research work, it can be concluded that the transferrin-containing nanoplexes of PEI2-ChA conjugates with plasmid p53 warrant clinical trials in humans after exhaustive animal studies for use as a novel gene delivery system.
Disclosure
The authors report no conflicts of interest in this work.
References
Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114(1):100–109. | ||
Xue W, Zender L, Miething C, et al. Senescence and tumor clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–660. | ||
Dube B, Rose L, Sawant K, Uludag H. Cholic acid modified 2 kDa polyethylenimine as efficient transfection agent. Biotechnol Prog. 2013;29(5):1337–1341. | ||
Sarkar K, Meka SRK, Madras G, Chatterjee K. A self-assembling polycationic nanocarrier that exhibits exceptional gene transfection efficiency. RSC Adv. 2015;5:91619–91632. | ||
Taylor SC, Berkelman T, Yadav G, Hammond M. A defined methodology for reliable quantification of Western blot data. Mol Biotechnol. 2013;55(3):217–226. | ||
Chen SH, Shine HD, Goodman JC, Grossman RG, Woo SL. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci U S A. 1994;91(8):3054–3057. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


