Back to Journals » Breast Cancer: Targets and Therapy » Volume 14
Chloride Intracellular Channel 1 is a Potential Biomarker for Breast Cancer
Authors Xia J, Wang Q, Ju F, Luo X, Wang F, Zhou Y, Huang H, Wang H, Bao X
Received 29 March 2022
Accepted for publication 8 July 2022
Published 2 September 2022 Volume 2022:14 Pages 247—258
DOI https://doi.org/10.2147/BCTT.S367519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jinwen Xia,1,2 Quhui Wang,1 Fei Ju,3 Xiang Luo,3 Feng Wang,4 Youlang Zhou,3 Hua Huang,5 Hua Wang,1,2 Xingli Bao6
1Department of Breast and Thyroid Surgery, Affiliated Hospital of Nantong University, Nantong, People’s Republic of China; 2Clinical Medicine, Medical College, Nantong University, Nantong, People’s Republic of China; 3Research Center of Clinical Medicine, Affiliated Hospital of Nantong University, Nantong, People’s Republic of China; 4Department of Laboratory Medicine, Affiliated Hospital of Nantong University, Nantong, People’s Republic of China; 5Department of Pathology, Affiliated Hospital of Nantong University, Nantong, People’s Republic of China; 6Department of Medical Equipment, Affiliated Hospital of Nantong University, Nantong, People’s Republic of China
Correspondence: Hua Wang, Department of Breast and Thyroid Surgery, Affiliated Hospital of Nantong University, No. 20, Xisi Road, Chongchuan District, Nantong City, Jiangsu Province, 226001, People’s Republic of China, Tel +86 137 062 92250, Email [email protected]
Purpose: Multiple reports have demonstrated that highly expressed chloride intracellular channel 1 (CLIC1) exists in a range of malignant tumors and is involved in proliferation, invasion, and migration of cancer cells. There are few studies on CLIC1 and breast cancer (BC). The purpose of this research was to evaluate the expression level of CLIC1 in BC and its impact on prognosis of BC patients.
Patients and Methods: Differences in CLIC1 expression levels in 25 pairs of BC and corresponding paracancerous specimens were tested by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot (WB). Immunohistochemistry (IHC) was performed to discuss the relevance between CLIC1 expression in BC tissue chips and clinicopathological parameters of BC patients. The effect of CLIC1 expression on patient prognosis was evaluated by Kaplan–Meier survival curve and Cox regression analysis. Receiver operating characteristic (ROC) curve assessed the diagnostic performance of CLIC1 for BC.
Results: The experimental results of qRT-PCR and WB demonstrated that CLIC1 was highly expressed in BC tissues. IHC results showed that overexpression of CLIC1 was strictly correlated with tumor size, TNM classification, pathological grade, lymph node metastasis and Ki67. Patients with lower CLIC1 expression had longer overall survival (OS) and progression-free survival (PFS). Cox regression analysis and ROC curve confirmed that CLIC1 could independently influence the prognosis of BC patients and might have diagnostic efficiency.
Conclusion: Overexpressed CLIC1 is closely related to the progression of BC and the poor prognosis of the patients, suggesting that it may act as a potential biological diagnostic index for BC.
Keywords: CLIC1, clinical pathological parameter, prognosis, diagnosis
Introduction
The latest global cancer burden data released by the International Agency For Research on Cancer (IARC) of the World Health Organization showed that in 2020, about 2.26 million women developed breast cancer (BC), of which 680 thousand died. The incidence of BC continues to increase at a rate of about 0.5% per year. The rate of new cases is so fast that BC replaces lung cancer as the number one cancer in the world.1 In recent years, through surgery, preoperative or postoperative chemotherapy, radiotherapy, targeted therapy and endocrine therapy, the five-year survival rate of BC patients has showed significantly improved,2 nevertheless, the current clinical practice still taking breast-removed modified radical mastectomy as the main surgical method and there are many limitations of the application of breast-sparing radical mastectomy, coupled with the side effects caused by postoperative chemotherapy and radiotherapy, the life quality of BC patients has been greatly influenced to varying degrees due to these various factors.3,4 In addition, highly aggressive triple-negative breast cancer is prone to distant metastasis in the short term. Clinically, there is still a lack of effective chemotherapy or targeted therapy. Compared with other types of BC patients, this type of patients have a poorer prognosis.5,6 Therefore, in order to better manage and diagnose patients with more aggressive BC and patients with metastatic disease, it is necessary to constantly search for new biomarkers and therapeutic targets. Chloride intracellular channel 1 (CLIC1), as one of the newly discovered chloride channel protein family, regulates the invasion, migration and apoptosis of a variety of cancer cells by participating in various signaling pathways, such as MAPK/ERK,7 ROS/ERK8 and MAPK/AKT9 etc. It has been found that CLIC1 can promote the adhesion function of platelets and endothelial cells, which is very important for vascular repair and angiogenesis.10 In mouse experiments, a large number of destruction of CLIC1 gene could lead to mild platelet disorder and impaired acidification of phagocytes of macrophages and dendritic cells, suggesting that CLIC1 plays a certain role in regulating immune cell function.11 In addition, it has been demonstrated that CLIC1 is a receptor of cell oxidation and participates in inflammatory response.12 According to the report, CLIC1 is not only expressed in BC tissues, but also in the blood vessels distributed in and around the tumor area.13 Another study found that CLIC1, associated with MRRPE1 and SERPINA, significantly influenced the overall survival rate of triple-negative BC patients.14 However, no relevant literature has proposed the correlation between CLIC1 and clinicopathological parameters of BC patients. It is uncertain whether CLIC1 is an independent risk factor affecting the prognosis of BC patients.
Materials and Methods
Clinical Sample Preparation
BC tissues and paracancerous tissues of 25 pairs of female patients with invasive BC who underwent modified radical mastectomy in Nantong University Affiliated Hospital were collected. None of the patients received any antitumor therapy before surgery. After collection, the tissues were rapidly transferred to −80℃ Store in refrigerator. In addition, the Department of Pathology of Nantong University Affiliated Hospital collected cancer tissue blocks and paracancerous tissue blocks from 116 paraffin-embedded BC patients between 2010 and 2013 and made pathological chips for retrospective research. The 116 patients who provided tissues for the chip were all women and the age distribution at surgery was 29–90 years, with a median age of 54 years. All patients were diagnosed with invasive BC without preoperative radiotherapy, neoadjuvant therapy, endocrine therapy and targeted drug therapy. According to the diagnosis and treatment guidelines of BC in 2021 Chinese Society of Clinical Oncology (CSCO), breast cancer was divided into four kinds of molecular subtypes: Luminal A-like, Luminal B-like, HER2-enriched, and triple-negative type. Of the 116 BC cases, 23 were Luminal A-like, 55 were Luminal B-like, 24 were HER2-enriched, and 14 were triple-negative type. The patients were followed up every 3 months by outpatient clinic and telephone, and the follow-up was stopped 5 years after the operation or after the death of the patient. This study was approved by the Ethics Committee of the Affiliated Hospital of Nantong University (protocol code 2020-L125). After being informed of the significance and procedure of the experiment, all patients agreed to participate in the study and signed the informed consent form. According to the Declaration of Helsinki, identifiable private information of all patients was encrypted.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
1 mL of TRIzol (Life Technologies Ltd, Paisley, UK) was added to approximately 50 mg of minced fresh tissue. The tissue gradually turned white after being thoroughly ground. RNA in the grinding fluid was extracted according to the instructions and its concentration was measured by a spectrophotometer (Thermo Fisher, USA). Complementary DNA then was synthesized by reverse transcription in accordance with the reaction system of the PrimeScript RT reagent kit with gDNA Eraser (Takara, Dalian, Liaoning, China). The steps of PCR were as follows: 95℃ for 2 min, followed by 95℃ for 5S and 60℃ for 10s as a cycle. After 40 cycles, 95℃ and 60℃ were maintained for 15 seconds respectively. 95℃ was gradually rised within 20 minutes and was continued to maintain for 15 seconds. At this point, the reaction was over. ACTIN and 2−ΔΔCT were used as an internal reference and to evaluate the CLIC1 mRNA, respectively. The primer sequences used were as follows: β-ACTIN forward: TAGTTGCGTTACACCCTTTCTTG, and reverse: GCTGTCACCTTCACCGTTCC), CLIC1 forward: CTCGCCTATGAGCAAGTGG, and reverse: ATTGGGTAGCAATGTGGAAAC. All primers were from Shanghai Ruimian Biotechnology Co., Ltd.
Western Blot (WB)
Twenty-five pairs of BC tissues and paired adjacent normal tissues were also used for WB analysis. Approximately 100ul of phenylmethylsulfonyl fluoride (PMSF, Life Technologies Ltd, Paisley, UK)-added radio immunoprecipitation assay (RIPA, Life Technologies Ltd, Paisley, UK) lysis buffer was used to dissociate protein from 10 mg of minced tissue (RIPA: PMSF=100:1). The extract was then spun at high speed for 20 minutes in a 12,000 rpm centrifuge precooled at 4℃. The supernatant was collected in clean EP tube. The protein content was qualified of each sample with a BCA kit (Beyotime Institute of Biotechnology, Nantong, China). Dissociated proteins were separated by 10% polyacrylamide gel electrophoresis and then transferred to formaldehyde-activated 0.45 μm polyvinylidene fluoride (PVDF, Merck Millipore, GER) membranes. PVDF membranes were sealed with 5% non-fat milk at room temperature without light for 2 hours. TBST-diluted rabbit polyclonal anti-CLIC1 (1:1000, Proteintech, USA) was added to the incubation box with PVDF membranes. Then the incubation box was placed on a shaker at 4℃ in the dark. Membranes were washed three times with TBST after overnight incubation and then were incubated with rabbit secondary antibody (1:10,000) diluted in TBST for an additional 2 hours at room temperature without light. After being thoroughly washed with TBST, PVDF membranes were scanned by Odyssey gel imager (LI-COR biosciences, USA). The gray value of the ACTIN band was used as a control.
Immunohistochemistry (IHC) and Scoring
The paraffin-embedded BC tissues’ histopathological chip was first dewaxed in a 65℃ oven for 1 hour, and then soaked in xylene solution for dewaxing again. The chip was then dehydrated with graded ethanol (5 minutes each time, each dehydrated 2 times) and rinsed with phosphate-buffered saline (PBS). The chip was put into the boiling alkaline repair solution (pH9.0), and was boiled for 30 min. After cooling at room temperature, the chip was rinsed 3 times with PBS. 3% H2O2 was dropped on the chip for 15 min in the dark to block endogenous peroxidase activity, followed by rinsing thrice in PBS. PBS-diluted rabbit polyclonal anti-CLIC1 (1:200) was dropped on the chip and then incubated overnight at 4℃. The chip was rinsed three times with PBS and then incubated with rabbit secondary antibody (1:500) for 2h. Chips were washed with PBS and then stained with DAB (1:49, Abcam, UK) and rinsed with tap water, followed by hematoxylin counterstaining for 3–5s. After dehydration with graded ethanol, the chip was placed in xylene for 16 h, and sealed with neutral resin. The staining of the chip was observed under a microscope (Olympus, Japan). CLIC1 staining was assessed by two pathologists with no knowledge of patient information. CLIC1 was expressed in the cytoplasm. When brown yellow staining was observed in the cytoplasm, the cell was considered as an immunoreactive cell. CLIC1 expression levels were assessed based on the percentage of immunoreactive cells and staining intensity. The scoring criteria for the percentage of immunoreactive cells was 1 point for 1~25%, 2 points for 26%~50%, 3 points for 51%~75%, and 4 points for 76%~100%. The classification of staining intensity was as follows: 0 point, negative; 1 point, weak; 2 points, moderately positive; 3 points, strong positive. The staining intensity score was multiplied by the immunoreactive cell percentage score and the results were used to assess the expression of CLIC1, with ≥3 as positive.
Statistical Processing
SPSS 20.0 (IBM Corp., Armonk, NY) was applied to process and analyze the data results of this study. The pictures were generated by Graphpad Prism 8 (GraphPad Software Inc., San Diego, CA). Measurement data were expressed as mean ± standard deviation. Paired t-test and χ2 test were used for between-group analysis and comparison of enumeration data, respectively. Survival analysis of BC patients was performed by Kaplan–Meier test. Univariate and multivariate Cox proportional hazards models were used to evaluate possible independent risk elements that may influence the outcome of BC patients. When P<0.05, the results were considered statistically significant.
Results
The Expression of CLIC1 is Significantly Up-Regulated in BC Tissues
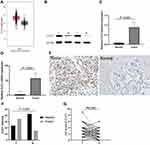
The expression of CLIC1 in breast was searched in GEPIA database (https://gepia.cancer-pku.cn/index.html). The website showed that CLIC1 expression was higher in BC tissues compared with paracancerous tissues (Figure 1A). In WB, using ACTIN as a reference, the results demonstrated that compared with paired noncancerous breast tissues, CLIC1 protein expression was evidently up-regulated in BC tissues, which was statistically significant (P<0.001, Figure 1B and C). qRT-PCR results proved that CLIC1 mRNA expression in BC tissues was increased, which was higher than that in adjacent normal tissues. There was statistical difference between two groups (P < 0.001, Figure 1D). By analyzing the results of IHC of 116 BC tissues in the chip, it could be observed that CLIC1 protein was expressed in the cytoplasm and the cytoplasm was brown (Figure 1E). In BC tissues, 69 cases were positive for CLIC1 protein expression (59.5%), and the remaining 47 cases were negative. In the adjacent normal tissues, 31 cases of CLIC1 protein expression were positive (26.7%), and the remaining 85 cases were negative. The difference was statistically significant (P < 0.001, Figure 1F). Statistical analysis was performed on the IHC scores of cancer tissues and paracancerous tissues. The results revealed that the score of cancer tissues was higher than that of paracancerous tissues, which was statistically significant (P = 0.003, Figure 1G). The experimental results mentioned above were consistent with the database result.
Expression of CLIC1 Correlates with Clinicopathological Parameters of BC
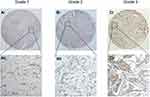
For the purpose of exploring the correlation between CLIC1 expression and the pathological parameters of BC patients, the IHC scores of BC tissue chips were statistically analyzed in our research. The results proved that CLIC1-positive expression was markedly associated with tumor size (P = 0.013), TNM stage (P = 0.006), pathological grade (P < 0.001), lymph node metastasis (P = 0.025) and ki67 (P = 0.011). Meanwhile, there was no significant difference in the association between its positive expression and age, ER, PR, HER2, molecular typing and vascular or neural invasion (Table 1). The expression of CLIC1 protein was significantly different in different pathological grades and TNM stages (P = 0.006, P = 0.003, respectively. Figures 2 and 3).
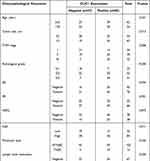 |
Table 1 The Relationship Between CLIC1 Expression and Clinicopathological Parameters in Patients with BC |
Connection Between CLIC1 Expression and Prognosis in BC Patients
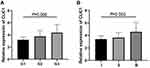
Comprehensive and up-to-date data and graphs related to CLIC1 and BC were obtained from https://kmplot.com/analysis/. As could be seen from Figure 4A, in BC patients, patients with high CLIC1 expression showed a lower overall survival (OS) rate than those with low CLIC1 expression (P < 0.05). Furthermore, Kaplan–Meier method was performed to analyze the relationship between CLIC1 expression in BC tissue chips and survival outcome of BC patients. The results confirmed that compared with CLIC1-positive patients, CLIC1-negative patients had longer OS time (P < 0.001) and progression-free survival time (PFS, P<0.001. Figure 4B and C), which were consistent with the website result. This study also assessed independent risk factors affecting the prognosis of BC patients by Cox proportional hazards model. Univariate Cox regression analysis showed that CLIC1-positive expression, pathological grade, TNM stage, TNBC, ER receptor positive expression, PR receptor positive expression, Ki67 level, lymph node metastasis and vascular or nerve invasion were associated with shorter OS time and PFS time. Multivariate Cox regression analysis further confirmed that CLIC1-positive expression, pathological grade, TNBC and lymph node metastasis were independent risk factors affecting the OS time of BC patients. Pathological grade, lymph node metastasis and CLIC1 positive expression were independent risk factors for shorter PFS time in BC patients (Tables 2 and 3). The ROC curve was drawn using the IHC score results, and the curve was analyzed. It could be seen that the area under the curve was 0.793, the 95% confidence interval (CI) was 0.701–0.885. P value was lower than 0.001, which means the difference was statistically significant. The sensitivity was 0.742 and the specificity was 0.765. The ROC curve proved that the expression of CLIC1 has diagnostic efficacy, and the positive expression of CLIC1 has diagnostic significance for BC (Figure 5).
 |
Table 2 Evaluation of Risk Factors Affecting Overall Survival in BC Patients by Univariate and Multivariate Cox Regression Analysis |
 |
Table 3 Evaluation of Risk Factors Affecting Progression-Free Survival in BC Patients by Univariate and Multivariate Cox Regression Analysis |
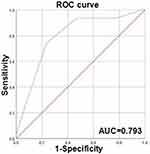 |
Figure 5 The diagnostic performance of CLIC1 for BC by ROC curve. |
Discussion
Studies have shown that the incidence of BC is on the rise in a total of 16 countries. Among these 16 countries, China is one of the countries with the fastest increase in incidences, and the rising trend of incidence of BC in patients older than 50 years is significantly accelerated. The incidence of BC also increased in patients younger than 50 years old.15 When BC metastasizes, the mortality rate of patients is greatly increased. This is because it is difficult to perform related palliative surgery on patients after cancer metastasis, and there is no targeted and definite drug that can cure patients. After a diagnosis of BC, the most immediate challenge for the diagnosis and treatment team in the overall management of the patient is to determine the most appropriate adjuvant therapy and to determine its prognosis. Usually, the prognosis of patients is judged based on a series of histopathological and immunohistochemical results, such as the expression of ER, PR, HER2, the presence or absence of lymph node metastasis and the number of metastatic lymph nodes, tumor size, TNM stage and pathological grade. Although all of these factors provide independent prognostic information for newly diagnosed BC, it is generally accepted that these factors alone are not sufficient for optimal management, especially now that we are moving towards an era of precision therapy. Therefore, the molecular heterogeneity of breast cancer, the different treatment response to chemotherapy drugs, the development of drug resistance after hormone therapy or HER2 targeted therapy, metastatic breast cancer and long-term unpredictable prognosis suggest that new diagnostic factors, prognostic markers and new therapeutic targets that can predict potential metastatic risks need to be continuously explored and verified. In recent years, a great quantity of studies focused on the discovery and validation of BC molecular markers. So far, there are few studies related to CLIC1 and BC.
CLICs have been widely studied in tumors and cancers, and show differences in expression and localization during cancer cell metastasis. One of its core members, CLIC1, has been confirmed to be up-regulated in various tumors, such as gallbladder cancer,16 colorectal cancer,17 nasopharyngeal cancer,18 ovarian cancer,19 hepatocellular carcinoma20 and high-grade glioma,21 etc. In a study regarding cell cycle, a CLICs blocker called IAA-94 was reported to be capable of arresting the merisis and development of CLICs cells in the G2-M phase.22 CLIC1 affects not only cell cycle progression, but also cell migration and metastasis. Malignant tumors exhibit a rapid and often uncontrolled process of angiogenesis, which provides blood supply to tumor tissue. The consequence of this unregulated angiogenesis is a persistent hypoxia-reoxygenation state that results in increased reactive oxygen species (ROS) production. A research found that blocking the production of CLICs protein with IAA-94 can reduce the production of ROS, which could block the invasion and migration of colon cancer cells.17,23 The findings of this report provide a new idea for studying the molecular mechanism of CLIC1 promoting tumor invasion and migration. Another study reported that in gastric cancer, CLIC1 could also promote the metastasis of gastric cancer cells by down-regulating the expression of AMOT-p130.24 Xu et al detected highly expressed CLIC1 protein in the blood of patients with oral squamous cell carcinoma. After the patients were treated with surgery and chemotherapy, the expression level of CLIC1 protein was significantly reduced,25 which suggested that CLIC1 may become a tumor therapeutic target and a biomarker for judging the therapeutic effect. In addition, CLIC1 significantly affected the prognosis of ovarian cancer patients.26 Besides, CLIC1 could be regulated by mircroRNA and hsamiR372 to later predict poor prognosis in gallbladder cancer patients.16,27 Another research speculated that the high expression of CLIC1 may lead to drug resistance in patients with gastric cancer and choriocarcinoma.28,29 These rich research results all suggest that CLIC1 may become a new tumor marker and potential research therapeutic direction.
In our research, GEPIA database analysis, qRT-PCR, and WB were used. The results confirmed that CLIC1 expression in BC tissues was distinctly increased. IHC was performed on BC tissue chips and the results were analyzed. It was proved that there were statistical differences in the expression of CLIC1 in different pathological grade and TNM stages. CLIC1-positive expression was also relevant to the overexpression of ki67, lymph node metastasis and vascular and neural invasion, suggesting that CLIC1 may be involved in the metastasis and invasion of BC. Kaplan–Meier survival curve analysis showed that CLIC1-positive expression was closely related to shorter OS and PFS in BC patients. Finally, by analyzing the ROC curve, it was proved that CLIC1 has possible value in the diagnosis and treatment of BC patients. All the above results indicate that CLIC1 is expected to be an independent prognostic and metastatic index for BC and a new potential biomarker.
Meanwhile, there are some shortcomings in this research. First of all, the research subjects are relatively limited. The objects of this study were all invasive BC patients, while patients with other BC pathological types such as carcinoma in situ and patients with metastatic BC were not included in the study. Secondly, the surgical methods of patients are single, but there are also many clinically qualified patients who choose radical mastectomy, sentinel lymph node biopsy and simple mastectomy. There are also many patients with breast reconstruction. Various conditions including the above reasons limited the research objects of this study, resulting in a small sample size. Differences in treatment regimens between patients may also lead to possible prognostic effects. The experimental methods were also relatively simple. The level of CLIC1 in the blood of BC patients before and after treatment were not detected. Cell experiments and animal experiments were not performed to study the molecular mechanism of CLIC1 promoting the occurrence, development and metastasis of BC. The results of this study showed that CLIC1-positive expression was not significantly correlated with the expression of ER, PR and HER2. The expression of CLIC1 did not show a statistically significant difference between TNBC and NTNBC, which may be due to the limitations of the simple methods and small sample size of this experiment. It may also suggest that when CLIC1 acts as an independent predictor without combining other molecules, its function of stratifying BC of different molecular types needs to be studied and discussion.
Conclusion
In conclusion, this study explored CLIC1 expression in BC and its relevance to patient prognosis. The expression of CLIC1 was significantly up-regulated in BC tissues. Positive expression of CLIC1 was evidently associated with lymph node metastasis, ki67, and vascular and neural invasion, which suggests that CLIC1 may play a role in the metastasis and invasion of BC. BC patients with high CLIC1 expression performed a poorer prognosis. The above results imply that CLIC1 has the potential to become a possible biomarker of BC in the future and it is worthy of research and exploration of its molecular mechanism affecting the occurrence and development of BC.
Acknowledgments
This study was supported by the Jiangsu Province Maternal and Child Health Research Project (F201682).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
3. Choi EK, Kim IR, Chang O, et al. Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psychooncology. 2014;23(10):1103–1110. doi:10.1002/pon.3531
4. Rautalin M, Jahkola T, Roine RP. Surgery and health-related quality of life - a prospective follow up study on breast cancer patients in Finland. Eur J Surg Oncol. 2021;47(7):00089. doi:10.1016/j.ejso.2021.02.006
5. Rodríguez-Pinilla SM, Sarrió D, Honrado E, et al. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res. 2006;12(5):1533–1539. doi:10.1158/1078-0432.CCR-05-2281
6. de Ruijter TC, Veeck J, de Hoon JP, van Engeland M, Tjan-Heijnen VC. Characteristics of triple-negative breast cancer. J Cancer Res Clin Oncol. 2011;137(2):183–192. doi:10.1007/s00432-010-0957-x
7. Tian Y, Guan Y, Jia Y, Meng Q, Yang J. Chloride intracellular channel 1 regulates prostate cancer cell proliferation and migration through the MAPK/ERK pathway. Cancer Biother Radiopharm. 2014;29(8):339–344. doi:10.1089/cbr.2014.1666
8. Wang P, Zeng Y, Liu T, et al. Chloride intracellular channel 1 regulates colon cancer cell migration and invasion through ROS/ERK pathway. World J Gastroenterol. 2014;20(8):2071–2078. doi:10.3748/wjg.v20.i8.2071
9. Li BP, Mao YT, Wang Z, et al. CLIC1 promotes the progression of gastric cancer by regulating the MAPK/AKT pathways. Cell Physiol Biochem. 2018;46(3):907–924. doi:10.1159/000488822
10. Knowles LM, Ampofo E, Menger MD, et al. CLIC1 cooperates with integrins to promote thrombus formation. Blood. 2018;132:2419. doi:10.1182/blood-2018-99-119290
11. Qiu MR, Jiang L, Matthaei KI, et al. Generation and characterization of mice with null mutation of the chloride intracellular channel 1 gene. Genesis. 2010;48(2):127–136. doi:10.1002/dvg.20590
12. Averaimo S, Milton RH, Duchen MR, Mazzanti M. Chloride intracellular channel 1 (CLIC1): sensor and effector during oxidative stress. FEBS Lett. 2010;584(10):2076–2084. doi:10.1016/j.febslet.2010.02.073
13. Raica M, Ceausu AR, Cimpean AM, Comşa Ş, Sarb S. Chloride Intracellular Channel Protein 1 (CLIC1), E-cadherin and P-cadherin define distinct subclasses of HER2, luminal B and triple-negative breast cancer. Anticancer Res. 2021;41(2):795–802. doi:10.21873/anticanres.14831
14. Katayama H, Tsou P, Kobayashi M, et al. A plasma protein derived TGFβ signature is a prognostic indicator in triple negative breast cancer. NPJ Precis Oncol. 2019;2(3):10. doi:10.1038/s41698-019-0082-5
15. Huang J, Chan PS, Lok V, et al. Global incidence and mortality of breast cancer: a trend analysis. Aging. 2021;13(4):5748–5803. doi:10.18632/aging.202502
16. He YM, Zhang ZL, Liu QY, et al. Effect of CLIC1 gene silencing on proliferation, migration, invasion and apoptosis of human gallbladder cancer cells. J Cell Mol Med. 2018;22(5):2569–2579. doi:10.1111/jcmm.13499
17. Wang P, Zhang C, Yu P, et al. Regulation of colon cancer cell migration and invasion by CLIC1-mediated RVD. Mol Cell Biochem. 2012;365(1–2):313–321. doi:10.1007/s11010-012-1271-5
18. Chang YH, Wu CC, Chang KP, et al. Cell secretome analysis using hollow fiber culture system leads to the discovery of CLIC1 protein as a novel plasma marker for nasopharyngeal carcinoma. J Proteome Res. 2009;8(12):5465–5474. doi:10.1021/pr900454e
19. Tang HY, Beer LA, Chang-Wong T, et al. A xenograft mouse model coupled with in-depth plasma proteome analysis facilitates identification of novel serum biomarkers for human ovarian cancer. J Proteome Res. 2012;11(2):678–691. doi:10.1021/pr200603h
20. Jiang X, Liu Y, Wang G, et al. Up-regulation of CLIC1 activates MYC signaling and forms a positive feedback regulatory loop with MYC in Hepatocellular carcinoma. Am J Cancer Res. 2020;10(8):2355–2370.
21. Wang L, He S, Tu Y, et al. Elevated expression of chloride intracellular channel 1 is correlated with poor prognosis in human gliomas. J Exp Clin Cancer Res. 2012;31(1):44. doi:10.1186/1756-9966-31-44
22. Valenzuela SM, Mazzanti M, Tonini R, et al. The nuclear chloride ion channel NCC27 is involved in regulation of the cell cycle. J Physiol. 2000;529(Pt3):541–552. doi:10.1111/j.1469-7793.2000.00541.x
23. Ponnalagu D, Singh H. Anion channels of mitochondria. Handb Exp Pharmacol. 2017;240:71–101.
24. Qiu Y, Mao YT, Zhu JH, et al. CLIC1 knockout inhibits invasion and migration of gastric cancer by upregulating AMOT-p130 expression. Clin Transl Oncol. 2021;23(3):514–525. doi:10.1007/s12094-020-02445-0
25. Xu Y, Xu J, Feng J, et al. Expression of CLIC1 as a potential biomarker for oral squamous cell carcinoma: a preliminary study. Onco Targets Ther. 2018;11:8073–8081. doi:10.2147/OTT.S181936
26. Ye Y, Yin M, Huang B, Wang Y, Li X, Lou G. CLIC1 a novel biomarker of intraperitoneal metastasis in serous epithelial ovarian cancer. Tumour Biol. 2015;36(6):4175–4179. doi:10.1007/s13277-015-3052-8
27. Zhou N, Cheng W, Peng C, Liu Y, Jiang B. Decreased expression of hsa-miR-372 predicts poor prognosis in patients with gallbladder cancer by affecting chloride intracellular channel 1. Mol Med Rep. 2017;16(5):7848–7854. doi:10.3892/mmr.2017.7520
28. Zhao K, Wang Z, Li X, Liu JL, Tian L, Chen JQ. Exosome-mediated transfer of CLIC1 contributes to the vincristine-resistance in gastric cancer. Mol Cell Biochem. 2019;462(1–2):97–105. doi:10.1007/s11010-019-03613-9
29. Wu J, Wang D. CLIC1 induces drug resistance in human choriocarcinoma through positive regulation of MRP1. Oncol Res. 2017;25(6):863–887. doi:10.3727/096504016X14772315906527
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.