Back to Journals » International Journal of Women's Health » Volume 11
Chlamydia trachomatis infection in primary fallopian tube and high-grade serous ovarian cancers: a pilot study
Authors Laban M , Ibrahim EA , Hassanin AS , Nasreldin MA, Mansour A, Khalaf WM , Bahaa-Eldin AM , Hussain SH , Elsafty MS , Hasanien AS
Received 30 September 2018
Accepted for publication 1 February 2019
Published 22 March 2019 Volume 2019:11 Pages 199—205
DOI https://doi.org/10.2147/IJWH.S188938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Mohamed Laban,1 Eman A Ibrahim,2 Alaa S Hassanin,3 Magda A Nasreldin,4 Amal Mansour,5 Waleed M Khalaf,3 Ahmed M Bahaa Eldin,3 Sherif H Hussain,3 Mohammed S Elsafty,1 Ahmad S Hasanien6
1Gynecologic Oncology Unit, Obstetrics and Gynecology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 2Pathology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 3Obstetrics and Gynecology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 4Pathology Department, Early Cancer Detection Unit of Ain Shams Maternity Hospital, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 5Biochemistry and Molecular Biology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 6Family Medicine Department, Royal Australian College of General Practitioners, Sydney, Australia
Background: The aim of this study was to evaluate the association of Chlamydia trachomatis (CT) infection with primary tubal and high-grade serous ovarian cancers.
Methods: This is a cross-sectional, retrospective study conducted at Ain Shams University Maternity Hospital, Egypt, from February 2008 to October 2017. Sixty-seven paraffin archival blocks specimens were retrieved from cases who underwent staging laparotomy due to high-grade serous ovarian cancer (30 cases), primary tubal serous cancer (25 cases), and control specimens of (12) tubal specimens from cases of benign gynecological conditions. All samples were examined for CT DNA using semiquantitative qRT-PCR.
Results: CT DNA was detected in 84% of high-grade tubal serous cancer, 16.7% of high-grade serous ovarian cancer, and 13.3% in controls (P<0.0005). Mean CT DNA relative quantity was significantly high (256) in tubal carcinoma, in comparison to that in high-grade serous ovarian cancer and controls (13.5 and 0.28, respectively; P<0.0005).
Conclusion: To the best of our knowledge, this is the first report on relation of CT to the tubal serous cancer, so the responsibility of CT tubal infection in the pathogenesis of primary tubal cancer needs to be considered.
Keywords: Chlamydia, DNA, PCR, tubal cancer, ovarian cancer, PFTC
Introduction
Following the revolutionary discovery of cervical cancer, human papilloma virus (HPV) link and the transformation of the status of cervical cancer from highly morbid and mortal to preventable in 90% of cases,1 the quest for a comparable infectious link in the development of ovarian cancer began.
Several studies reinforce the hypothesis that the genital infection by Chlamydia trachomatis (CT) is a principal risk factor for invasive cervical cancer, possibly through the instigation of chronic irritation and inflammation with the release of molecules of the innate immune response – such as selectins, cytokines, chemokines, prostaglandins, and their receptors – with decreased apoptosis, and of the local immune surveillance. These conditions seem to favor the HPV persistence, as well as the expression of their carcinogenic potential.2
On the other hand, it has also been noted that CT infection induces centrosome amplification, multipolar spindles, and early anaphase onset, which give rise to multinucleation in replicating cells, in addition to inducing anchorage independence in 3T3 fibroblasts. It has been proven that such cellular changes occur even in the absence of expressions of the E6 and E7 HPV genes, or of any other specific oncogene. Thus, it is possible that the presence of these defects within infected dividing cells is a likely mechanism for explaining the role of CT as a cofactor of HPV in the development of cervical lesions, including cancer.3
Characterized by its insidious onset, ovarian cancer is the most lethal gynecologic malignancy, with 80% of patients diagnosed in advanced disease stages. Hereditary genetic mutations account for only 10% of ovarian carcinomas, which implies that no identified etiology characterizes the remaining 90% of ovarian cancer patients.4
According to Shih and Kurman, surface epithelial tumors can be classified into two groups, type I and type II, thus reflecting two different pathways of carcinogenesis.5 The more prevalent type II tumors are rapidly growing, aggressive, high-grade neoplasms with de novo development, (ie, no identifiable morphologically recognizable precursor). They include high-grade serous carcinomas, carcinosarcomas, and undifferentiated carcinomas.6 Most ovarian carcinomas seen in BRCA-positive women fall within this group.7 Very limited data exist regarding the molecular alterations associated with type II tumors. It is suggested that most type II tumors originate from the tubal fimbria8 and are associated with tubal intraepithelial carcinoma and p53 signatures.9
Evidence supporting the tubal origin of ovarian cancer originates from the histopathological studies of the salpingo-oophorectomy specimens of patients with inherited BRCA gene mutations. Almost all tumor foci found in asymptomatic BRCA-positive patients in risk-reducing salpingo-oophorectomy specimens were classified as tubal.10
Functionally, the fimbria represents the junction between two different epithelia: the well-differentiated, ciliated epithelium of the endosalpinx, and the peritoneal mesothelium. In analogy to the cervical squamo-columnar junction and the esophagogastric junction – which represent a well-known, high-risk zone for carcinogenesis – mostly triggered by chronic inflammation in response to HPV and H. pylori infections in the cervix and stomach epithelia, respectively,11 the endosalpinx–peritoneal junction is believed to be a potential site of ovarian carcinogenesis.
CT infection is one of the most significant causes of chronic uterine tube inflammation via its direct cytotoxic effect, as well as the ensuing loss of microvilli and disruption of cellular junctions, and ultimately ending in epithelial cell rupture, possibly through the pathogenic immune response to 60 kDa chlamydial heat shock protein (CHSP60).12,13 Hence, this series of observations raises the possibility of CT’s susceptibility in causing ovarian carcinogenesis, particularly type II carcinomas.14
Primary fallopian tube carcinoma (PFTC) is a rare gynecological tumor that accounts for 0.14%–1.8% of genital malignancies. The most common age of occurrence is between 40 and 65 years, with a mean age of 55 years. Overall, survival rates for patients with PFTC are strikingly low, with the indicators ranging between 22% and 57%. Preoperative diagnosis is rare. In fact, PFTC is usually confirmed postoperatively on pathological examination. However, earlier diagnosis improves the prognosis significantly.15
The incidence of PFTC has risen over the past decades. The factors that contribute to its development are not well known. Parity and previous sterilization provide protection against its occurrence. However, there are no firm data to hold infections by CT or HPV as possible promoters of PFTC.16
The aim of the present work was to study the presence of CT DNA and its levels in cases of primary serous tubal and high-grade serous ovarian cancers in comparison to fallopian tubes retrieved from patients who underwent hysterectomy with bilateral salpingo-oophorectomy for benign gynecological conditions to find a possible role of CT in the pathogenesis of tubal serous cancer. It is worth noting that there are no other clinical studies to date that have attempted to examine the aforementioned topic.
Materials and methods
Specimens and data collection
A cross-sectional retrospective study was conducted at Ain Shams University Maternity Hospital, Cairo, Egypt. The study included archival paraffin blocks of tumor specimens from cases who underwent staging laparotomy due to high-grade serous ovarian cancer or primary fallopian tubal serous cancer. Furthermore, control specimens comprised tubal biopsies from cases who underwent simple hysterectomy due to benign gynecological conditions, such as dysfunctional uterine bleeding and uterine leiomyomas. All of the above mentioned cases were collected from May 2008 till October 2017 (due to the rarity of primary tubal serous cancer). The archival specimens were retrieved from the Early Cancer Detection Unit, Ain Shams Maternity Hospital, and the PCR procedure was done in the Biochemistry Department at Oncology Diagnostic Unit, Faculty of Medicine, Ain Shams University.
The study protocol was registered in ClinicalTrials.gov on January 2015 under the number of NCT02343510. All the data and specimens retrieved were collected in an anonymous fashion to avoid revealing the patients’ identities. The study was approved by the Ain Shams Maternity Hospital Board at Faculty of Medicine, Ain Shams University.
Representative H&E stained sections from each case were selected and revised independently by two expert pathologists. The paraffin blocks were then retrieved for further processing through PCR technique.
Sample processing
In control cases and uniform malignant lesions, the microtome was used to collect eight sections (each around 10 μm in thickness) from each representative paraffin block, while in nonuniform malignant specimens, the paraffin block was melted in a 60°C water bath. All samples were stored in PBS at 4°C until extraction was performed. Primary fallopian tube cancer was diagnosed according to the criteria proposed by Hu et al.17
Relative expression of chlamydia using qRT-PCR
Xylol (Sigma-Aldrich, Hamburg, Germany) was used to dissolve the paraffin and 98%–100% ethanol (Sigma-Aldrich) was used to remove the xylol and to reconstitute the buffers. The samples were incubated at 90°C after proteinase K digestion reversed formalin cross-linking. DNA bound to the membrane, and contaminants flew through. Residual contaminants were washed away, and then pure concentrated DNA was eluted from the membrane. DNA was detected using QIAamp® DNA FFPE Tissue Kit (Catalog no 56404, Qiagen, Hilden, Germany), according to manufacturers’ protocol. The concentration of the extracted pure DNA was measured spectrophotometrically (Ultraspec® 1000, Amersham Pharmacia Biotech, Cambridge, England) at 260 nm, and the DNA samples were stored at −80°C until the time in which quantitative PCR was administered. The sequence of the forward primer of chlamydia was 5′-GCC GCT TTG AGT TCT GCT TCC TC-3′ and the reverse primer was 5′-CCA AGT GGT GCA AGG ATC GCA-3′. Quantitative PCR was carried out using QuantiTect SYBR® Green PCR Kit (Applied Biosystems, Foster City, CA, USA) according to manufacturer’s protocol with β-actin (sense primer: CTACGTCGCCCTGGACTTCGAGC and antisense primer: GATGGAGCCGCCGATCCACACGG) used as the internal control to normalize the data. The volume of the first-strand reaction was brought to 20 μL with DNAase free water and template DNA (1 μg/reaction) were amplified on an iCycler (Bio-Rad) using 10 μL 2× QuantiTect SYBR Green PCR Master Mix and 2 μL of the gene-specific oligonucleotide primers. All PCRs were done by initial activation step at 95°C for 15 minutes followed by 45 cycles of 15, 30, and 30 seconds at 95°C, 50°C, and 72°C, respectively. Bio-Rad software was used to calculate threshold cycle (Ct) values for all target and β-actin genes. Relative expression levels of the Chlamydia genome were measured using 2−ΔΔCt method (relative to β-actin of the same sample).18 A positive quality control of Chlamydia (artus C. trachomatis plus RG PCR, and Qiagen) and a negative control were done with each run of RT-PCR.
Transposition errors and cross contamination were unlikely due to the automated extraction of the DNA in a closed system. The quantity is the number of genome copies of CT DNA per reaction.
Statistical analysis
Data were tabulated and analyzed using SPSS for Windows (software version 18, Chicago, IL, USA). ANOVA test was used to assess the difference of parametric data between multiple groups. Chi-square test was used to compare the qualitative data. Nonparametric test (Kruskal–Wallis test) was used to compare the mean and SD of the three groups, while Mann–Whitney U test was used to compare between two groups. P-value <0.05 was considered significant. Receiver operating characteristic (ROC) curve determined the best value that gave optimum sensitivity and specificity.
Results
Both qualitative and quantitative CT PCR tests were performed for 71 specimens (34 specimens of ovarian high-grade serous adenocarcinoma, 25 specimens of tubal serous cancer, and 12 control cases). Four specimens of high-grade serous ovarian cancer were excluded from the study due to the presence of excessive tumor necrosis. The total number of remaining specimens was 67: 30 specimens of ovarian high-grade serous adenocarcinoma (25 stage III, 3 stage II, and 2 cases were stage I), 25 specimens of primary tubal papillary serous cancer (19 cases were stage III, 3 cases with stage II, and the remaining 3 cases with stage I; Figures S1 and S2), and 12 control cases (8 cases of dysfunctional uterine bleeding and 4 cases of uterine leiomyomas).
Statistically significant differences were found between tubal serous cancer, ovarian carcinoma, and controls with respect to the presence of CT DNA. Qualitative PCR was found to be positive in 4 (13.3%), 21 (84%), and 2 (16.7%) of ovarian carcinoma, tubal serous cancer, and control specimens, respectively (P<0.0005; Table 1).
Furthermore, quantitative PCR values showed a statistically significant difference between the three groups. For tubal serous cancer, the mean value was 256.69 genome copies/reaction (95% CI; 58.63–454.76) compared with 13.54 genome copies/reaction (95% CI; −13.15–40.23) and 0.28 genome copies/reaction (95% CI; −0.13–0.68) for ovarian carcinoma and controls, respectively (P<0.0005; Table 2). ROC curve analysis was performed for tubal and ovarian tissue relative quantity of CT in tubal vs ovarian and control samples. The best cutoff value was 15.1. Sensitivity was 80%. Specificity was 93.7%. Accuracy was 88.46%. Positive predictive value (PPV) was 77.42% and negative predictive value (NPV) was 94.7%. Area under the curve was 0.8 (P=0.01) as shown in Figure 1.
Discussion
In the current study, 67 specimens were examined for the presence of CT DNA using both qualitative and quantitative PCR. CT DNA was found to be positive in 4 (13.3%), 21 (84%), and 2 (16.7%) in ovarian high-grade serous carcinoma, tubal serous cancer, and control specimens, respectively (P<0.0005). Quantitative measurement of DNA load found that the highest level existed among specimens of tubal serous cancer 256.69 (95% CI; 58.63–454.76) compared with 13.54 (95% CI; −13.15–40.23) and 0.28 (95% CI; −0.13–0.68) for ovarian carcinoma and controls, respectively (P<0.0005).
Roberta et al19 measured antibodies to CT, to CHSP60, and to CHSP10, in 117 women with ovarian cancer and in 171 age- and ethnicity-matched population-based controls. They found the probability of having ovarian cancer was 90% greater in women with the highest, compared with the lowest (OD: 0.40 vs 0.10), levels of chlamydia-elementary bodies antibodies (P<0.05). The current study differs in that it assesses the presence of CT DNA, as opposed to using serology in both ovarian and tubal carcinomas.
Ness et al20 used serologic measurements of CT antibodies as a surrogate marker of CT pelvic inflammatory disease. Five hundred twenty-one women with ovarian cancer and 766 population-based controls were tested. They concluded that the odds of having ovarian cancer among women with the highest titers (OD units ≥0.40) were 0.6 (95% CI 0.4–0.9). These results contradict with those of the current study. However, it ought to be mentioned that the study in question used serology only for ovarian cancer rather than using PCR for both ovarian and tubal carcinomas.
Idhal et al21 examined frozen ovarian tissue from 186 women with benign conditions, borderline tumors, and epithelial ovarian cancer, as well as tissue from the contralateral ovaries of 126 of these women, for the presence of CT and N. gonorrhoeae (transcription-mediated amplification), M. genitalium (real-time PCR), HPV (PCR), and BKV and JCV (PCR). They found all the tissue samples studied negative for the microorganisms analyzed. One year later, the same authors22 found an association between upper genital tract infections and ovarian tumor development, since they found chlamydial HSP60-1 IgG antibodies were associated with type II ovarian cancer (P=0.002). These results bolster our results regarding the link between CT infection and ovarian cancer.
Riska et al23 examined 79 postoperative serum samples from patients treated for PFTC in 1985–2000. For each case, two controls were selected. Seropositivity in serum antibody levels did not differ between PFTC patients and controls. The lack of association between antichlamydial antibodies and PFTC suggests that past chlamydial infection does not play a role in the etiopathogenesis of PFTC. However, because CT infection is common at young age and PFTC develops decades later, they could not definitively exclude the possibility that CT contributes to the development of PFTC. The current study found that CT DNA was not only found, but also highly expressed, in specimens of tubal cancer, and that this could be explained by both the lack of treatment of asymptomatic infections and the persistence of this CT infection burden untreated for long time.
Furthermore, Jonsson et al24 studied 69 patients with suspected ovarian pathology detecting the presence of CT DNA and CHSP60 in the ovarian specimens and antichlamydial IgG in plasma, comparing them to different subtypes of epithelial ovarian cancer. Eight patients had CT DNA in their ovarian specimens and all of these patients had invasive epithelial cancers. They concluded that CT may have a role in the pathogenesis of these tumors.
It may be too early to draw a solid conclusion regarding this hypothesis depending solely on this study’s preliminary data, and more studies are needed to confirm our findings. In addition, we believe that more research in the field of molecular biology is needed to discern how tubal infection with CT manifest with alteration in the mechanisms that may initiate or prevent carcinogenesis in the fallopian tube or even in high-grade serous ovarian cancers.
Another point that may need further clarification is whether there exists any relationship between the severity of the CT infection and the likelihood of future occurrence of malignant transformation given that our current study demonstrated that CT DNA is highly expressed in primary tubal cancer compared with high-grade serous ovarian tumors.
Implementing our data, if validated, in clinical practice, as well as in further studies, may be feasible to prevent aggressive tumors as primary tubal cancer by practicing prevention, early diagnosis, and aggressive treatment for all cases of CT in infected women. The adoption of this approach may very well contribute to a significant decrease in the cases of primary tubal cancer in the near future. Detection of CT DNA relative quantity by real-time PCR in tubal carcinoma group vs ovarian carcinoma and control groups showed the best sensitivity (80%), specificity (93.7%), PPV (77.42%), NPV (94.7%), and accuracy (88.46%).
Based on the available results, to the best of our knowledge, this is the first study to accurately measure the quantity of CT DNA in both tubal and high-grade serous ovarian cancers, which is a point of strength. However, one of the limitations of this study is the absence of an oncogenic study, which would have served to link the presence of CT DNA and its level to a specific molecular alteration that may be responsible for the initiation and progression of these tumors. This could serve as a point for future research in this field as it is beyond the scope of the current study.
Conclusion
CT DNA is highly expressed in tubal serous cancer compared with high-grade serous ovarian cancer and controls. The responsibility of CT tubal infection in the pathogenesis of primary tubal cancer needs to be considered.
Data sharing statement
Relevant clinical and pathological data of participants are available and can be shared by the corresponding author for the 6 months following publication.
Disclosure
The authors report no conflicts of interest in this work.
References
Center of Disease Control. Vital signs. 2014. Available from: https://www.cdc.gov/vitalsigns/pdf/2014-11-vitalsigns.pdf. Accessed 14 May, 2017. | ||
Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki AB. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26(3):222–232. | ||
Knowlton AE, Fowler LJ, Patel RK, Wallet SM, Grieshaber SS. Chlamydia induces anchorage independence in 3T3 cells and detrimental cytological defects in an infection model. PLoS One. 2013;8(1):e54022. | ||
Yehia M, Mansour A, Mekawy S. Human epididymis protein 4 (HE4) mRNA as a prognostic marker in ovarian tumors in relation to RMI and CA125. Int J Cancer Res. 2015;11(4):175–185. | ||
Shih LEM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164(5):1511–1518. | ||
Dehari R, Kurman RJ, Logani S, Shih le-M. The development of high-grade serous carcinoma from atypical proliferative (borderline) serous tumors and low-grade micropapillary serous carcinoma: a morphologic and molecular genetic analysis. Am J Surg Pathol. 2007;31(7):1007–1012. | ||
Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5(1):35–44. | ||
Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19(1):3–9. | ||
Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211(1):26–35. | ||
Carcangiu ML, Peissel B, Pasini B, et al. Incidental carcinomas in prophylactic specimens in BRCA1 and BRCA2 germ-line mutation carriers, with emphasis on fallopian tube lesions: report of six cases and review of the literature. Am J Surg Pathol. 2006;30(10):1222–1230. | ||
Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. | ||
Eckert LO, Hawes SE, Wölner-Hanssen P, et al. Prevalence and correlates of antibody to chlamydial heat shock protein in women attending sexually transmitted disease clinics and women with confirmed pelvic inflammatory disease. J Infect Dis. 1997;175(6):1453–1458. | ||
Peeling RW, Kimani J, Plummer F, et al. Antibody to chlamydial HSP60 predicts an increased risk for chlamydial pelvic inflammatory disease. J Infect Dis. 1997;175(5):1153–1158. | ||
Quirk JT, Kupinski JM. Chronic infection, inflammation, and epithelial ovarian cancer. Med Hypotheses. 2001;57(4):426–428. | ||
Kalampokas E, Kalampokas T, Tourountous I. Primary fallopian tube carcinoma. Eur J Obstet Gynecol Reprod Biol. 2013;169(2):155–161. | ||
Riska A, Leminen A. Determinants of incidence of primary fallopian tube carcinoma (PFTC). Methods Mol Biol. 2009;472:387–396. | ||
Hu CY, Taymor ML, Hertig AT. Primary carcinoma of the fallopian tube. Am J Obstet Gynecol. 1950;59:58–67. | ||
Samir L, Mansour A, Shaban E, et al. MCM7 gene expression versus risk of malignancy index (RMI) for prediction of ovarian malignancy. Austin J Obstet Gynecol. 2017;4(3):1081–1086. | ||
Ness RB, Goodman MT, Shen C, Brunham RC. Serologic evidence of past infection with Chlamydia trachomatis, in relation to ovarian cancer. J Infect Dis. 2003;187(7):1147–1152. | ||
Ness RB, Shen C, Bass D, et al. Chlamydia trachomatis serology in women with and without ovarian cancer. Infect Dis Obstet Gynecol. 2008;21:9672. | ||
Idahl A, Lundin E, Elgh F, et al. Chlamydia trachomatis, Mycoplasma genitalium, Neisseria gonorrhoeae, human papillomavirus, and polyomavirus are not detectable in human tissue with epithelial ovarian cancer, borderline tumor, or benign conditions. Am J Obstet Gynecol. 2010;202(1):71.e1–e6. | ||
Idahl A, Lundin E, Jurstrand M, Kumlin U. Chlamydia trachomatis and Mycoplasma genitalium plasma antibodies in relation to epithelial ovarian tumors. Infect Dis Obstet Gynecol. 2011;2011:824627. | ||
Riska A, Finne P, Alfthan H, et al. Past chlamydial infection is not associated with primary fallopian tube carcinoma. Eur J Cancer. 2006;42(12):1835–1838. | ||
Jonsson S, Oda H, Lundin E, Olsson J, Idahl A. Chlamydia trachomatis, chlamydial heat shock protein 60 and anti-chlamydial antibodies in women with epithelial ovarian tumors. Transl Oncol. 2018;11(2):546–551. |
Supplementary materials
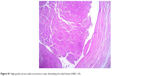  | Figure S1 High-grade serous tubal carcinoma is seen distending the tubal lumen (H&E ×10). |
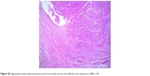  | Figure S2 High-grade serous tubal carcinoma arises from tubal mucosa and infiltrates the submucosa (H&E ×10). |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



