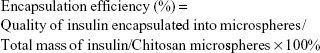Back to Journals » International Journal of Nanomedicine » Volume 13
Chitosan-microcapsulated insulin alleviates mesenteric microcirculation dysfunction via modulating COX-2 and VCAM-1 expression in rats with diabetes mellitus
Authors Xu J, Cao L, Suo Y, Xu X, Sun H, Xu S, Zhu X, Yu H, Cao W
Received 13 May 2018
Accepted for publication 2 October 2018
Published 25 October 2018 Volume 2018:13 Pages 6829—6837
DOI https://doi.org/10.2147/IJN.S174030
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Jun Xu, Lijun Cao, Yuan Suo, Xiaoqin Xu, Hui Sun, Songao Xu, Xiangyun Zhu, Huijie Yu, Weizhong Cao
Department of Emergency Medicine, The First Hospital of Jiaxing, Jiaxing 314001, Zhejiang Province, China
Background: The study of the experiment was to display the therapeutic function of insulin-loaded chitosan (insulin/chitosan) on mesenteric microcirculation via down-regulating cyclooxygenase-2 (COX-2) and vascular cell adhesion molecule (VCAM-1) expressions in rats with diabetes mellitus (DM) as compared to free insulin.
Methods: Diabetic rats were administrated with 24 U/kg insulin or 120 U/kg insulin/chitosan compounds. The blood and mesenteriums were collected, blood glucose levels, arteriole velocity, arteriole diameter, venular diameter, and hemodiapedesis were measured, and COX-2, VCAM-1 expressions were measured in mesenteriums tissues.
Results: Both insulin and insulin/chitosan administration decreased blood glucose and improved the state of mesenteric microcirculation through down-regulating COX-2 and VCAM-1 expressions as compared to DM groups, while insulin/chitosan remarkably augmented this functions.
Conclusion: Chitosan-microcapsulated insulin alleviates mesenteric microcirculation dysfunction via modulating COX-2 and VCAM-1 expressions in rats with DM.
Keywords: insulin, chitosan, COX-2, VCAM-1
Introduction
Microcirculation disorder is the important pathophysiological basis of diabetic complications. Studies have shown that microcirculatory disorders occur earlier in the development of diabetes.1 Abnormal microcirculation may be involved in the development of diabetes. Therefore, it is of great significance to study the changes in diabetic microcirculation in order to explore the pathogenesis of diabetes and guide the treatment of diabetes.
Cyclooxygenase (COX) is a key limiting enzyme in membrane phospholipid–arachidonic acid–prostaglandin metabolic loop; there are two isoenzymes of structural (COX-1) and inducible (COX-2) type. COX-1 is a structural enzyme participating in normal physiological functions and in regulating the blood flow of the kidney, protecting the gastric mucosa, and promoting the aggregation of the plates. In physiological state (animals without disease), COX-2 is not expressed or low in most tissues. Tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), nuclear factor-κB (NF-κB), and lipopolysaccharide can stimulate the high expression of COX-2, and many studies have corroborated that COX-2 is an important proinflammatory mediator and the key enzyme to initiate inflammatory reaction.2–4 In addition, COX-2 can promote vasopermeability and augment tissues’ injury.5 Some researches have shown that COX-2 inhibitors have protective effects on intestinal mucosal barrier.6–9 Several studies have affirmed that increased inflammatory mediators in diabetes can induce COX-2 activity and enhance inflammation injury.10,11
In diabetes mellitus (DM), the blood flow velocity in the microcirculation is slowed down, especially in the venules.12 After the blood flow slows down, granular or massive red blood cells can be seen in the microcirculation and sometimes the blood flow is interrupted, swayed, or even silted; at the same time, the blood flow of microcirculation in diabetic rats is slowed down, the white blood cells that rolled along the vascular wall and adhered to the wall increase significantly, and at this time, the expression of vascular cell adhesion molecule (VCAM-1) on the surface of microvascular endothelial cells is significantly increased.12 The adhesion and activation of leukocytes lead to the production of free radicals, which not only results in the injury of local vascular endothelial cells and its subsequent effects but also causes damage to the surrounding tissue.13
It has been shown that insulin plays an important role in normal intestinal physiology and promotes the absorption of nutrients. Insulin has a nutritional effect on the intestinal mucosa of newly born miniature pigs and accelerates the proliferation of intestinal mucosal cells in suckling pigs.14 Some reports have demonstrated that insulin improves intestinal microcirculation in sepsis and protects vascular endothelium, inhibits platelet aggregation, and ameliorates microcirculation dysfunction in stress state.15,16 In the present research, we try to explore the protective role of insulin on mesenteric microcirculation dysfunction in DM. As well known, the in vivo half-life of insulin is extremely short; therefore, controlling insulin dose clinically is extremely difficult with the risk of hypoglycemia.17,18 Here, the preparation of oral insulin microspheres using chitosan (structure as shown in Figure S1) as carrier is reported. Insulin is encapsulated in chitosan using the solvent evaporation technique. The loaded insulin showed sustained release, which could facilitate the investigation on the protective role of insulin. Insulin-encapsulating capacity and in vitro release were measured. In vivo researches were conducted through the oral administration of the insulin/chitosan complex to mesenteric microcirculation dysfunction in DM, followed by the measurement of blood glucose, arteriole velocity, arteriole diameter, venular diameter, and hemodiapedesis level and finally the expression of COX-2 and VCAM-1 in mesenteric tissues.
Materials and methods
Materials
Insulin (novolin intermediate-acting insulins, NPH) was bought from Sigma-Aldrich Co. (St Louis, MO, USA); chitosan (molecular weight, 50 kDa) was bought from Zhejiang Golden Shell Marine Life Co., Ltd. (Hangzhou, Zhejiang Province, China); and other reagents were bought from Sigma-Aldrich Co.
Methods
Preparation of insulin/chitosan microspheres
Chitosan (molecular weight, 50 kDa) was dissolved in dichlorotetrane, then, insulin was added to emulsify for 20 min via ultrasonic by solvent evaporation technique, the emulsion was added into polyvinyl alcohol dispersion medium and was stirred 12 h at 37°C, then the mixed solution was centrifuged to remove the supernatant, and the precipitate was washed by distilled water for several times to form homogeneous suspension (insulin/chitosan microspheres). The chitosan-loaded insulin was measured through particle size meter and transmission electron microscopy (TEM).
Encapsulation efficiency measurement
Appropriate amount of insulin/chitosan was weighted and was dissolved in hydrochloric acid solution (0.1 mol/L) in volumetric flask. At fixed time interval, 2 mL of solution was extracted to measure its absorbance at 276 nm and its concentration was calculated. Until the three times the insulin concentration did not change, it was thought that insulin had been released completely. Calculation formula of drug load measurement was as follows:
|
In vitro release of insulin from insulin/chitosan
Appropriate amount of insulin/chitosan was weighted and dissolved in hydrochloric acid solution (0.1 mol/L) in volumetric flask. At 0.5, 1, 2, 4, 8, 12, 24, 36, 48, 72, 96, and 120 h, 2 mL of solution was extracted and then added equal amount of hydrochloric acid solution (0.1 mol/L). The absorbance was measured at 276 nm, and the in vitro release capacity was calculated.
Hypoglycemic role
Male Sprague Dawley rats (180–200 g) were acquired from Jiaxing University Medical College in China. The procedures and care of the Sprague Dawley rats were approved by the Institutional Ethics Committee of Jiaxing University Medical College in China. The expedition conformed to the guidelines for the care and use of laboratory animals published by the US National Institutes of Health (NIH Publication updated in 2011).
Sprague Dawley rats were injected with streptozotocin (STZ) only once at a dose of 60 mg/kg by abdominal cavity. After 3 days, diabetes was estimated by detecting blood glucose contents using the glucose oxidase–peroxidase (GOD–POD) method.19,20 Animals with blood glucose contents >16.7 mmol/L were contained in this experiment. Forty diabetic rats were randomly divided into the following four groups (n=10 in each group): 1) control group: no medication was given; 2) insulin group (subcutaneous injection): 24 U/kg as a single abdominal subcutaneous injection; 3) insulin/chitosan group (subcutaneous injection): 120 U/kg as a single subcutaneous injection; and 4) insulin/chitosan group (oral administration): 120 U/kg as a single oral administration. The doses of insulin and insulin/chitosan compounds mainly referred to previous documents.19 At a certain time interval, blood glucose contents were detected using the GOD–POD method.20,21
Procedure of mesenteric microcirculation dysfunction in DM
The experimental procedures of Sprague Dawley rats were the same as above. Forty diabetic rats were randomly divided into the following four groups (n=10 in each group): 1) control group; 2) insulin group (subcutaneous injection): 24 U/kg as a single abdominal subcutaneous injection; 3) insulin/chitosan group (subcutaneous injection): 120 U/kg as a single subcutaneous injection; and 4) insulin/chitosan group (oral administration): 120 U/kg as a single oral administration. Blood sample was collected from the abdominal aorta and centrifuged at 3,600× g for 15 min to gain the sera. Mesenteriums were collected and stored at −80°C until further analysis.
Histopathological evaluation
The mesenteric tissues were fixed in formalin, paraffin-embedded, sliced into 4 μm sections, stained with H&E staining, and measured under an optical microscope. Briefly, 24 areas corresponding to the mesenteriums were graded for the degree of mesenteric injury based on the following parameters: mesenteric microvascular deformation, blood stasis, mesenteric microvascular crinkle, and swelling of microvascular endothelial cells. Specifically, one whole deep coronal section was measured under the microscope and graded according to the extent of injury, based on the percentage of mesenterium area affected. Higher scores represented more severe injury, with the maximum score being 4 (0, histopathological changes <10%) (1 [10%–25%], 2 [25%–50%], 3 [50%–75%], and 4 [75%–100%]). The mean score for each parameter was decided and subjected to statistical analysis.
Mesenteric microcirculation experiment
The mesentery of the small intestine near the cecum was extracted and laid flat in a small pool of plexiglass irrigation, mesentery was put into physiological saline to keep moist at 37°C and observe the dynamic changes in microcirculation under microcirculation microsystem. And microvascular diameter, arteriole diameter, arteriole velocity, and capillary blood exudation (the amount of blood exudation in 10 visual fields) were measured.
Western blot analysis
The Western blot methodology had been depicted previously.22,23 Briefly, mesenteric tissues were homogenized in protein lysate buffer. The homogenates were resolved on polyacrylamide SDS gels and electrophoretically transferred to polyvinylidene difluoride membranes. The membranes were blocked with 3% BSA and, then, incubated with primary Abs against active COX-2 or VCAM-1 and subsequently with alkaline phosphatase-conjugated secondary Abs. The membranes were developed with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium. Blots were probed with an anti-β-actin Ab, and the levels of proteins were normalized to β-actin expression.
Statistical analysis
All data were completed in triplicate unless otherwise noted. They were expressed as mean ± SD. Statistical analyses were executed by ANOVA through using post hoc testing, and data were analyzed using SPSS (Version 19.0; IBM Corporation, Armonk, NY, USA). A P-value of <0.01 was considered statistically significant.
Results
Encapsulating capacity
Insulin was observed to be efficiently loaded in chitosan. The loading efficiency of insulin was found to be 37%.
Characterization of insulin/chitosan
Insulin/chitosan was observed using TEM and particle size meter, and the images are shown in Figures S2 and S3. Insulin/chitosan showed an orbicular structure with the diameter of ~56 nm and the particle size of ~23 nm.
In vitro release
The release of insulin from chitosan was observed at 37°C, with 5 mL of insulin-encapsulated chitosan. The cumulative release rates of insulin from insulin/chitosan are shown in Figure 1. Approximately 15.27% of insulin was released from insulin/chitosan after 1 h, indicating an initial burst release of insulin. Approximately 94.57% of insulin was released after 120 h.
  | Figure 1 Cumulative release. |
Blood glucose
To measure the blood glucose content in a fixed time of diabetic rats administered insulin/chitosan (oral administration) and to compare with that of the insulin and insulin/chitosan (subcutaneous injection) groups, the results showed that diabetic rats injected with insulin maintained blood glucose contents in normal change for about 30 min, after which glycemia slowly raised; diabetic rats injected with insulin/chitosan (subcutaneous injection) maintained blood glucose contents in normal change for about 6 h, after which glycemia slowly raised. By contrast, insulin/chitosan (oral administration) lessened blood glucose contents to 6.3 mmol/L at 2 h after administration. Over the following 120 h, blood glucose content was augmented to 5.1 mmol/L and was then maintained at ~4.3–6.3 mmol/L for 120 h (Figure 2).
Histopathological evaluation
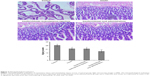
Mesenteric microvascular deformation, blood stasis, mesenteric microvascular crinkle, and swelling of microvascular endothelial cells were measured in histological specimens in the control group. Histological alterations were ameliorated in specimens from the insulin and insulin/chitosan (subcutaneous injection) groups compared to the control group. Histological alterations were significantly decreased in specimens from the insulin/chitosan (oral administration) group than those from the control group (Figure 3).
Changes in microcirculation
The levels of arteriole velocity in the control group were significantly lower than in the insulin and insulin/chitosan (subcutaneous injection) groups (P<0.01). The level of arteriole velocity was 53.4±4.32 V/μm/g in the control group rats. Administration of insulin and insulin/chitosan (subcutaneous injection) increased the arteriole velocity levels (70.3±3.28 and 74.7±3.77 V/μm/g, respectively), whereas administration of insulin/chitosan (oral administration) significantly increased the arteriole velocity level (87.6±3.89 V/μm/g) compared with the control group rats (P<0.01).
The levels of arteriole diameter in the control group were significantly lower than those in the insulin and insulin/chitosan (subcutaneous injection) groups (P<0.01). The level of arteriole diameter was 16.4±2.54 L/μm in the control group rats. Administration of insulin and insulin/chitosan (subcutaneous injection) increased the arteriole diameter levels (19.2±3.08 and 19.9±2.38 L/μm, respectively), whereas administration of insulin/chitosan (oral administration) significantly increased the arteriole diameter level (24.1±2.66 L/μm) compared with the control group rats (P<0.01).
The levels of venular diameter in the control group were significantly lower than those in insulin and insulin/chitosan (subcutaneous injection) groups (P<0.01). The level of venular diameter was 19.3±2.11 L/μm in the control group rats. Administration of insulin and insulin/chitosan (subcutaneous injection) increased the venular diameter levels (24.2±3.79 and 25.1±2.86 L/μm, respectively), whereas administration of insulin/chitosan (oral administration) significantly increased the venular diameter level (29.6±2.67 L/μm) compared with the control group rats (P<0.01).
The levels of hemodiapedesis in the control group were significantly higher than those in the insulin and insulin/chitosan (subcutaneous injection) groups (P<0.01). The level of hemodiapedesis was 5.4±0.91/visual field in the control group rats. Administration of insulin and insulin/chitosan (subcutaneous injection) reduced the hemodiapedesis levels (3.6±0.76 and 3.4±0.69/visual field, respectively), whereas administration of insulin/chitosan (oral administration) significantly reduced the hemodiapedesis level (2.3±0.53/visual field) compared with the control group rats (P<0.01) (Figure 4).
Expression of COX-2 and VCAM-1
Expression of COX-2 and VCAM-1 in mesaraic tissues were induced by the control group than by the insulin and insulin/chitosan (subcutaneous injection) groups (P<0.01). Administration of insulin and insulin/chitosan (subcutaneous injection) downregulated expression of COX-2 and VCAM-1 in mesaraic tissues compared with expression of COX-2 and VCAM-1 in the control group (P<0.01), whereas administration of insulin/chitosan (oral administration) significantly downregulated expression of COX-2 and VCAM-1 compared to the control group (P<0.01) (Figure 5).
Discussion
Microcirculatory disturbance was an important basis for the occurrence of diabetic complications.24 In this study, we observed that the arteriole velocity slowed down, the endothelial cells swelled, the arteriole diameter decreased, the venular diameter reduced, and the hemodiapedesis increased obviously. Therefore, there were several kinds of microvascular dysfunction and blood flow disorder in diabetic rats. Our study found that the blood flow in the blood vessels of diabetic rats slowed down and stagnated, which led to insufficient blood perfusion in tissues and organs, resulting in organ dysfunction, so blood glucose was controlled; it was of great significance to alleviate the symptoms of diabetes and reduce the occurrence of complications. Clinical study had also shown that glycemic control improved microcirculation in diabetics and protected organ function.25
In addition, diabetic patients showed high viscosity, high concentration, and high coagulation in hemorheology, which affected the perfusion of microcirculation and the decrease in oxygen supply in microcirculation. The exchange of blood materials in microvessels was blocked, and a large number of oxygen free radicals were produced during the formation of the end products of glycosylation. The adhesion of autocytes was enhanced, and fibrinogen was increased, which resulted in the injury of vascular endothelial cells and vascular wall and peripheral microcirculation network microthrombotic formation eventually caused by circulatory disorders.26 From the results of this study, the mesenteric microvessels of the diabetic rats became smaller, the blood flow rate slowed down, and the capillary blood exudation was obvious; the results suggest that microvascular disease and abnormal blood velocity were the main changes and causes of diabetic microcirculation disorder.27 In contrast, vascular endothelial cells might be activated and enhanced VCAM-1 expression by the stimulation of hyperglycemia, leading to the adhesion and activation of leukocytes that caused the production of free radicals.27 In addition, the increase in VCAM-1 promoted neutrophils’ adhesion, aggregation, and release of mediators in the intestinal tract of diabetic rats, which further led to the obstruction of intestinal microcirculation and the exacerbation of intestinal injury. Leukocyte migration to vascular wall space was a necessary step for tissue injury and inflammation. Leukocyte adhesion to vascular endothelium was the most important part of the process, and adhesion molecules such as VCAM-1 mediated this process. This not only resulted in the injury of local vascular endothelial cells and its subsequent effects but also damaged the surrounding tissues, which was one of the main mechanisms for the formation of diabetic microangiopathy.27 In our study, we found that the expression of VCAM-1 was increased in the control group and decreased in the treatment group. It suggested that insulin and insulin/chitosan could improve the mesenteric microcirculation dysfunction of diabetic rats by decreasing the expression of adhesion molecules.
Several lines of evidence obtained in experimental and clinical research studies have shown increased COX-2 expression in diabetic rats.28 And it was well documented that the enhanced inflammatory response in the early stages of diabetic rats was mediated by the expression of proinflammatory proteins such as COX-2.28 Many studies had demonstrated that COX-2 inhibitors have protective effects on intestinal mucosal barrier.6–9 Our findings confirmed that the expression of COX-2 was increased in the control group and lessened in the treatment group in the microvessels and the endothelial cells of these vessels. The results showed that COX-2 played an important role in intestinal microcirculation disorder in diabetic rats. It suggested that insulin and insulin/chitosan could alleviate the expression of COX-2 in intestinal microcirculation disorder in diabetic rats.
Studies had shown that insulin had protective effects on vascular endothelium and antiplatelet aggregation and played an important role in improving microcirculation disturbance, tissue ischemia, and hypoxia under stress.29 And the binding sites of insulin and insulin-like growth factor expressed by intestinal epithelial cells were the physiological basis of insulin action.30 Previous study had affirmed that insulin played an important role in improving microcirculation in sepsis and had important clinical significance in avoiding or alleviating ischemia and hypoxia in midgut of sepsis and protecting intestinal function of sepsis.31–33 However, the half-life of insulin was very short; therefore, controlling insulin dose clinically was very difficult with the risk of hypoglycemia.17 Here, chitosan was reported as a potential insulin nanocarrier. The loaded insulin showed sustained release, which could facilitate the investigation on the protective effect of insulin. Insulin-encapsulating capacity and in vitro release were measured. In vivo researches were conducted by the administration of the insulin/chitosan complex to mesenteric microcirculation dysfunction in DM. These results showed that insulin/chitosan (oral administration) could significantly reduced blood glucose and improved mesenteric microcirculation in DM.
In our study, we proved that insulin and chitosan-microcapsulated insulin alleviated mesenteric microcirculation dysfunction via modulating COX-2 and VCAM-1 expression in rats with DM. However, this mechanism was complex and we would continue to explore it in future experiments.
Conclusion
The synthesized insulin/chitosan complex can significantly improve the bioactivity and half-life of insulin. The measured protective role can be attributed to the hypoglycemic activity, microcirculation regulation, and modulation of COX-2 and VCAM-1 expression via insulin/chitosan (oral administration) in DM as compared to free insulin and insulin/chitosan (subcutaneous injection). More detailed researches are needed to explore the underlying mechanism of insulin/chitosan on mesenteric microcirculation dysfunction in (DM).
Acknowledgment
The authors appreciate the support from the Science and Technology Planning Project of Jiaxxing, Zhejiang Province (Jun Xu).
Disclosure
The authors report no conflicts of interest in this work.
References
Wiernsperger NF. In defense of microvascular constriction in diabetes. Clin Hemorheol Microcirc. 2001;25(2):55–62. | ||
Crofford LJ, Wilder RL, Ristimäki AP, et al. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. J Clin Invest. 1994;93(3):1095–1101. | ||
Weaver SA, Russo MP, Wright KL, et al. Regulatory role of phosphatidylinositol 3-kinase on TNF-alpha-induced cyclooxygenase 2 expression in colonic epithelial cells. Gastroenterology. 2001;120(5):1117–1127. | ||
Lim JW, Kim H, Kim KH. Nuclear factor-kappaB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab Invest. 2001;81(3):349–360. | ||
Xiao L, Dong JH, Jin S, et al. Hydrogen Sulfide Improves Endothelial Dysfunction via Downregulating BMP4/COX-2 Pathway in Rats with Hypertension. Oxid Med Cell Longev. 2016;2016:8128957–10. | ||
Cuzzocrea S, Mazzon E, Serraino I, et al. Celecoxib, a selective cyclo-oxygenase-2 inhibitor reduces the severity of experimental colitis induced by dinitrobenzene sulfonic acid in rats. Eur J Pharmacol. 2001;431(1):91–102. | ||
Martín AR, Villegas I, La Casa C, Alarcón de La Lastra C. The cyclo-oxygenase-2 inhibitor, rofecoxib, attenuates mucosal damage due to colitis induced by trinitrobenzene sulphonic acid in rats. Eur J Pharmacol. 2003;481(2–3):281–291. | ||
Moses T, Wagner L, Fleming SD. TLR4-mediated Cox-2 expression increases intestinal ischemia/reperfusion-induced damage. J Leukoc Biol. 2009;86(4):971–980. | ||
Zamuner SR, Warrier N, Buret AG, Macnaughton WK, Wallace JL. Cyclooxygenase 2 mediates post-inflammatory colonic secretory and barrier dysfunction. Gut. 2003;52(12):1714–1720. | ||
Linton MF, Fazio S. Cyclooxygenase-2 and inflammation in atherosclerosis. Curr Opin Pharmacol. 2004;4:116–123. | ||
St-Onge M, Flamand N, Biarc J, et al. Characterization of prostaglandin E2 generation through the cyclooxygenase (COX)-2 pathway in human neutrophils. Biochim Biophys Acta. 1771;2007:1235–1245. | ||
Qian R, Xu C, Jin H. Changes of mesentery microcirculation in type 2 diabetic rats and its clinical significance. J FuDan University (Medical Sciences). 2006;33:776–780. | ||
Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42(6):1075–1081. | ||
Shulman RJ. Oral insulin increases small intestinal mass and disaccharidase activity in the newborn miniature pig. Pediatr Res. 1990;28(2):171–175. | ||
Shi B, Yin C, Guo H, et al. Influence of intensive insulin therapy on the intestinal microcirculatory dysfunction in rats with sepsis. Chin Crit Care Med. 2009;21:492–494. | ||
van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114(9):1187–1195. | ||
Ince BW. Plasma clearance kinetics of unlabelled bovine insulin in rainbow trout (Salmo gairdneri). Gen Comp Endocrinol. 1982;46(4):463–472. | ||
Tong F, Tang X, Li X, Xia W, Liu D. The effect of insulin-loaded linear poly(ethylene glycol)-brush-like poly(l-lysine) block copolymer on renal ischemia/reperfusion-induced lung injury through downregulating hypoxia-inducible factor. Int J Nanomedicine. 2016;11:1717–1730. | ||
Huang H, Tian H, Li X, Zhao G. Hypoglycemic effect of chitosan-microcapsulated insulin on the blood glucose level of streptozotocin-diabetic Wistar rats. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2001;18(3):425–427. | ||
Ding Y, Vaziri ND, Coulson R, Kamanna VS, Roh DD. Effects of simulated hyperglycemia, insulin, and glucagon on endothelial nitric oxide synthase expression. Am J Physiol Endocrinol Metab. 2000;279(1):E11–E17. | ||
Tong F, Liu S, Yan B, et al. Endogenous ornithine decarboxylase/polyamine system mediated the antagonist role of insulin/PEG-CMCS preconditioning against heart ischemia/reperfusion injury in diabetes mellitus. Int J Nanomedicine. 2018;13:2507–2520. | ||
Tong F, Dong B, Chai R, et al. Simvastatin nanoparticles attenuated intestinal ischemia/reperfusion injury by downregulating BMP4/COX-2 pathway in rats. Int J Nanomedicine. 2017;12:2477–2488. | ||
Ji YY, Wang ZD, Wang SF, et al. Ischemic preconditioning ameliorates intestinal injury induced by ischemia-reperfusion in rats. World J Gastroenterol. 2015;21(26):8081–8088. | ||
Schiekofer S, Galasso G, Sato K, Kraus BJ, Walsh K. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arterioscler Thromb Vasc Biol. 2005;25(8):1603–1609. | ||
Forst T, Lübben G, Hohberg C, et al. Influence of glucose control and improvement of insulin resistance on microvascular blood flow and endothelial function in patients with diabetes mellitus type 2. Microcirculation. 2005;12(7):543–550. | ||
Jun L, Mei Y, Hongxin Z. Effect of chromium-rich yeast on mesenteric microcirculation in experimental diabetic rats. Lishizhen Medicine and Materia Medica Research. 2012;23:1406–1407. | ||
Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42(6):1075–1081. | ||
Nasiry D, Khalatbary AR, Ahmadvand H. Therapeutic potential of Juglans regia L. leaf extract against diabetic retinopathy in rat. Iran J Basic Med Sci. 2017;20(11):1275–1281. | ||
van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114(9):1187–1195. | ||
Gallo-Payet N, Hugon JS. Insulin receptors in isolated adult mouse intestinal cells: studies in vivo and in organ culture. Endocrinology. 1984;114(5):1885–1892. | ||
Hansen TK, Thiel S, Wouters PJ, Christiansen JS, van den Berghe G. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88(3):1082–1088. | ||
das UN. Current advances in sepsis and septic shock with particular emphasis on the role of insulin. Med Sci Monit. 2003;9(8):RA181–RA192. | ||
Shi B, Yin C, Guo H, Li B, Liu LP. Influence of intensive insulin therapy on the intestinal microcirculatory dysfunction in rats with sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009;21(8):492–494. |
Supplementary materials
  | Figure S1 Citosan. |
  | Figure S2 The TEM image of insulin/chitosan. |
  | Figure S3 The particle size of insulin/chitosan. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.