Back to Journals » OncoTargets and Therapy » Volume 12
Chinese perspectives on clinical efficacy and safety of alectinib in patients with ALK-positive advanced non-small cell lung cancer
Authors Yu H, Sun S, Hu X, Xia J, Wang J , Chen H
Received 20 February 2019
Accepted for publication 28 June 2019
Published 14 August 2019 Volume 2019:12 Pages 6481—6495
DOI https://doi.org/10.2147/OTT.S185115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Hui Yu,1 Si Sun,1 Xingjiang Hu,2 Jinjing Xia,3 Jialei Wang,1 Haiquan Chen4
1Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China; 2Zhejiang Provincial Key Laboratory for Drug Evaluation and Clinical Research, First Affiliated Hospital, Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China; 3Department of Medical Science Oncology, Shanghai Roche Pharmaceuticals Ltd., Shanghai, People’s Republic of China; 4Department of Thoracic Surgery, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China
Abstract: The incidence of lung cancer is increasing in China, in contrast to trends in Western countries, due to the increasing numbers of smokers and high levels of air pollution. Non-small-cell lung cancer (NSCLC) is the most common form of lung cancer, accounting for approximately 85% of lung cancers. Better understanding of the pathogenesis of NSCLC has led to the identification of multiple genetic mutations and chromosomal translocations such as those in the anaplastic lymphoma kinase (ALK) gene. To facilitate the identification of treatment targets, multiple guidelines (European Society for Medical Oncology, National Comprehensive Cancer Network, and American Society of Clinical Oncology) now recommend screening for genetic factors to help guide treatment decisions. In recent years, multiple ALK inhibitors have been developed to treat NSCLC, including the first-generation tyrosine kinase inhibitor (TKI) crizotinib; second-generation TKIs such as ceritinib, ensartinib, brigatinib, and alectinib; the third-generation TKI lorlatinib; and the fourth-generation TKI repotrectinib. These agents differ in structure, potency, and activity, both systemically and their effects on central nervous system (CNS) metastases. Recently, alectinib was approved in China to treat patients with locally advanced or metastatic NSCLC that were ALK+. Alectinib has demonstrated activity against NSCLC, including metastases within the CNS, with better tolerability than crizotinib. These ALK inhibitors represent significant advances in the treatment of NSCLC and yet patients will likely still exhibit disease progression. Alectinib offers greater potency with greater specificity as well as a better toxicity profile than many other TKIs that are currently available. Here, we review the role of ALK as a therapeutic target in NSCLC, the testing methods for identifying ALK-rearranged NSCLC, and the various TKIs currently being used or explored for treatment in this setting, with a focus on alectinib from a Chinese perspective.
Keywords: NSCLC, anaplastic lymphoma kinase, alectinib, tyrosine kinase inhibitor
Introduction
In China, the incidence of cancer is on the rise with lung cancer being the most common cancer type diagnosed and the most common cause of cancer-related deaths.1–5 It is known that lung cancer morbidity and mortality trends reflect the prevalence of tobacco exposure 20–30 years earlier.6,7 Therefore, due to the ongoing high levels of tobacco exposure and air pollution in China, both of which are significant risk factors in the development of lung cancer, the health burden of lung cancer is expected to increase in the future.8–10
Non-small-cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for 85–90% of all lung cancer cases globally, and is associated with high rates of mortality.1–5,11,12 According to the most recently released SEER Cancer Statistics Review, the 5-year survival rate (2009–2015) for NSCLC is 25.1%.13 Improved understanding of the pathogenesis of NSCLC has resulted in the development of treatments that target various genetic mutations that drive the development and progression of NSCLC. This includes translocations of the anaplastic lymphoma kinase (ALK) gene14 and the ROS1 proto-oncogene 1 receptor tyrosine kinase (ROS1) gene;14 rearrangements in kinase-encoding genes such as epidermal growth factor receptor (EGFR),14 Kirsten ras (KRAS),14 v-raf murine sarcoma viral oncogene homolog B (BRAF),14 neurotrophic tyrosine receptor kinase (NTRK),14 and rearranged during transfection (RET);14 fusions of neuregulin 1 (NRG1)15 and fibroblast growth factor receptor 1/3 (FGFR1/3);16 human epidermal growth factor receptor 2 (HER2) insertion;16 and AKT serine/threonine kinase 1 (AKT1) mutation.17 Some reports show that these mutations are mutually exclusive, while other reports show that some tumors can have concomitant mutations.18–21 These mutations are capable of influencing responses to targeted therapy.14 It has, therefore, become standard clinical practice to test for gene mutations and fusions in patients with NSCLC and to tailor treatment strategies accordingly.22
In the present review, we briefly discuss the role of ALK mutations and translocations in NSCLC, the testing methods for identifying ALK-positive (ALK+) NSCLC, and present the evidence for approved ALK-targeted therapies. This review focuses on alectinib in Chinese patients due to its recent and rapid approval (August 2018) as a monotherapy for locally advanced or metastatic ALK+ NSCLC in China.
Mechanism of action of ALK
The ALK gene is a member of the insulin receptor superfamily. It is located on the short arm of chromosome 2 (2p23) and encodes a receptor tyrosine kinase.23,24 Similar to other receptor tyrosine kinases, ALK contains an extracellular domain, a transmembrane segment, and a cytoplasmic receptor kinase segment.24 ALK mutations have been implicated in tumorigenesis, with involvement in the initiation and progression of several cancer types, including lymphomas, neuroblastoma, and NSCLC.25,26 ALK translocations typically cause an increase in tyrosine kinase activity, resulting in increased cell proliferation and survival via their effects on signaling pathways that include phospholipase Cγ, phosphatidylinositol 3-kinase (PI3K)–protein kinase B (AKT), mammalian target of rapamycin (mTOR), and mitogen-activated protein kinase (MAPK) signaling cascades, among others.26 Translocation events at the ALK locus generate a variety of ALK fusion proteins, such as NPM1–ALK, that are found in multiple types of cancers.26 ALK activation in cancer may also arise through overexpression and point mutation of full-length ALK.27–29
Anaplastic lymphoma kinase was first identified as the receptor tyrosine kinase in a novel fusion gene arising from chromosomal translocation t(2;5) in anaplastic large-cell lymphoma.30 This rearrangement results in a nucleophosmin (NPM1)–ALK fusion protein30,31 and subsequent constitutive activation of the ALK kinase, which is normally regulated by the extracellular ligand-binding domain of the full-length receptor.25,30 Although ALK is highly expressed during embryogenesis and appears to be involved in brain and neural development,32 its precise physiological role in mammals remains unclear. ALK, however, is not critical for viability as Alk−/− mice are viable.33
NSCLC and ALK
In 2007, the fusion oncogene echinoderm microtubule-associated protein-like 4 (EML4)–ALK was shown to be present in 3% to 6% of patients with NSCLC.34 This fusion oncogene is the result of a chromosome inversion (inv[2][p21;p23]) in which EML4 fuses with the juxta membranous portion of ALK and replaces the extracellular and intramembranous portions.34 EML4-ALK fusions form ligand-independent dimers via the coiled-coil of EML4;35 this ligand-independent dimerization results in constitutive downstream signaling of canonical ALK pathways.34 The EML4–ALK oncogene was shown to induce tumor formation in nude mice.26,34 Figure 1 shows an overview of the EML4–ALK pathways involved in tumorigenesis.
ALK gene rearrangements appear to arise more frequently in specific NSCLC patient populations. Demographic characteristics associated with more frequent rearrangements include young, female, never- or light-smoker status, or the presence of tumors with an adenocarcinoma histology.23,36–39 The reported prevalence of ALK rearrangements in Chinese patients ranges between 3.3% and 11.6%, compared with 30% of patients with EGFR mutations;40–42 however, the prevalence of ALK rearrangements in the Chinese population is similar to that reported in other Asian populations (for example, 7% in Japanese patients).23 In general, the prevalence of ALK rearrangements in Asian populations is slightly greater than that in non-Asian populations.43–46 It has been reported that the clinicopathologic features of ALK-rearranged lung adenocarcinoma in Western patients may differ from those in other populations.47 However, there are suggestions that this discrepancy may result from differing pathologic interpretations rather than actual ethnicity-related genetic differences in patients with ALK fusion oncogene-positive lung cancer.26
Testing methods for ALK-rearranged NSCLC
The general consensus of the International Association for the Study of Lung Cancer Atlas48 is that all patients presenting with advanced NSCLC should be screened for the presence of ALK gene rearrangements.14 ALK–EML4 gene fusions and ALK rearrangements in NSCLC can be identified in clinical specimens using several techniques: immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), reverse transcriptase polymerase chain reaction (RT-PCR), and next generation sequencing (NGS).49 Figure 2 shows the currently recommended testing methods for ALK-rearranged NSCLC.
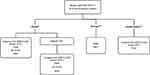 |
Figure 2 Testing methods for ALK-rearranged NSCLC as used in China,57 Europe140 and the United States.141 Notes: aPreferred method.57 bConducted in a laboratory meeting the qualifications of the National Center for Clinical Laboratories.57 cOnly performed when the Ventana ALK (D5F3) CDx assay is not reasonably available, primary screening only.57 dAny IHC assay that has been validated against FISH.140 Abbreviations: NSCLC, non-small cell lung cancer; ALK, anaplastic lymphoma kinase; FISH, fluorescent in situ hybridization; RT-PCR, reverse transcription polymerase chain reaction; IHC, immunohistochemistry. |
FISH was clinically validated in studies with the first-generation tyrosine kinase inhibitor (TKI), crizotinib, prior to its approval by the US Food and Drug Administration (FDA) for ALK-rearranged NSCLC.50 The ALK FISH approach is the gold standard and uses ALK break-apart probes that label the 5′ and 3′ ends of the ALK gene with fluorescent probes. Rearrangements in ALK result in a split appearance of the signal or loss of the 5′ signal in at least 15% of the cells counted.51 FISH can be performed on formalin-fixed paraffin-embedded tissue or snap-frozen samples, but its use is limited by high costs, suboptimal reliability, and technical complexity.49
IHC is a widely available, cost-effective, and rapid technique for ALK screening that can be performed before FISH analysis.52,53 In 2013, China was the first country to approve the VENTANA ALK (D5F3) CDx Assay, which uses IHC to identify the presence of ALK+ gene rearrangements. This assay is a key step in qualitative identification, which is used to assess the presence of ALK protein in samples from patients with ALK+ NSCLC, and is often subsequently confirmed by FISH analysis. At present, Ventana IHC (D5F3) remains the most widely used testing method for ALK+ NSCLC.54,55 Furthermore, it was recommended by Chinese guidelines as the first choice for ALK+ NSCLC diagnosis in 2018.56–58
Quantitative RT-PCR represents a highly sensitive method capable of detecting ALK mRNA that has both fusions and mutations. However, it has some limitations including (1) the requirement for frozen or fresh tissues or cells for RNA extraction,49 and (2) the existence of many variants of EML4-ALK, which raises the possibility of additional variant fusions, making multiplexed RT-PCR assays difficult to optimize for clinical use.59
Emerging technologies for testing include NGS and liquid biopsy.60,61 NGS is based on highly multiplexed PCR amplicon-based targeted sequencing for oncogenic fusions and represents a viable alternative to FISH because it can be performed on formalin-fixed paraffin-embedded samples, with minimal RNA input needed.61 Although NGS requires a supporting e-infrastructure to be performed,62 it offers the advantage of being able to test for a broader range of mutations simultaneously as well as for both somatic and germline mutations.60,61 Liquid biopsy involves examining cell-free DNA or circulating tumor DNA, a process that is advantageous when testing for metastatic cancers, but this method offers less sensitivity than tissue-testing for early stage cancers.60
The aforementioned techniques differ in their relative sensitivities and specificities, their usefulness for detecting a given type of mutation (eg, whether it involves a fusion or not), and their ease of conduct and affordability. One study assessed patients whose samples were tested by both Ventana IHC and RT-PCR; in addition, some of the samples were also tested by NGS.56 In this study, it was determined that Ventana IHC was both a reliable and rapid method to identify suitable candidates for ALK gene-targeted therapy. An economic analysis from a Chinese healthcare system perspective investigated the cost-effectiveness of three ALK rearrangement testing methods: Ventana IHC, qRT-PCR, and IHC following FISH confirmation.63 Here, it was shown that gene-guided therapy was a cost-effective option with or without Chinese patient-assisted programs. The results of this economic analysis were supported in a more recent follow-up study, which compared the cost-effectiveness and quality-adjusted life-years when using NGS or multiplex PCR testing to guide therapy.64 Furthermore, ALK testing under real-world settings across 31 hospitals around China is currently under investigation.
Chinese guidelines on the diagnosis and treatment of ALK+ NSCLC, set by the Chinese Society of Clinical Oncology Cancer Markers Expert Committee and the Chinese Expert Consensus Opinion of the diagnosis of ALK-positive NSCLC, provide guidance on choosing the most suitable technique for identifying ALK gene fusions and rearrangements.57,65 As per their assessment, the highest level of evidence supports the use of Ventana IHC to screen for ALK+ NSCLC, which became widely accepted based on findings from later clinical studies, including the ASCEND-4 and ALEX studies of alectinib.57,65–67 In contrast, the RT-PCR technique was recognized as being able to detect known fusion genes, but its performance requires an appropriately qualified laboratory.57,65 Lastly, the committee determined that the blood sample testing was still considered experimental and should only be performed in certain circumstances.57,65
In China, a multicenter survey of 932 patients with advanced NSCLC showed that 71.4% of patients were tested for EGFR gene mutations, 44.7% were tested for ALK gene fusions, and 13.7% were tested for ROS1 gene fusions.68 The clinical assays most used in China to determine ALK involvement in NSCLC are IHC and RT-PCR, with IHC being the most widely used testing method for ALK.69 The use of FISH is still rare due to the high cost and extensive operational requirements.69 To date, the China National Medical Products Administration (NMPA) has approved three FISH kits, two IHC kits, six RT-PCR kits, and four NGS solutions, and current Chinese guidelines recommend NMPA-approved kits and methods in clinical ALK+ NSCLC diagnosis.51 Although various options to test for ALK rearrangements are available, the 2018 Chinese guidelines state that their highest recommendation is for the Ventana IHC.57,65 However, in acknowledging that this test may not be available at all sites, the guidelines committee recommends either forwarding samples to laboratories that could perform the Ventana IHC, or performing a primary screening locally using routine IHC and subsequently sending positive samples to other laboratories for confirmation by recommended methods, including FISH, Ventana IHC, or RT-PCR.57,65
ALK-targeted therapy in NSCLC
Overview of NSCLC treatment and the role of ALK inhibitors
During recent years, several ALK inhibitors that demonstrate significant benefits in the treatment of ALK+ NSCLC compared with conventional chemotherapy have become available in clinical practice around the world or are under clinical investigation.37,39,70–72 Although the first generation TKI crizotinib is effective in patients with NSCLC compared with standard chemotherapy,39 it is limited by the relatively shorter progression-free survival (PFS), its associated toxicities, and the disease progression of central nervous system (CNS) metastases due to its poor penetration of the blood–brain barrier.49,73,74 Subsequent generations of TKIs were developed to achieve improved outcomes and fewer toxicities including second generation TKIs such as ceritinib, ensartinib, brigatinib, and alectinib.23,75 The pharmacokinetic properties of the ALK inhibitors crizotinib, ceritinib, ensartinib, brigatinib, and alectinib are shown in Table 1, which outlines the absorption, distribution, metabolism, and excretion rates of each agent. Efficacy results for ALK inhibitors are presented in Table 2.
 |
Table 1 Key pharmacokinetics of first- and second-line ALK inhibitors |
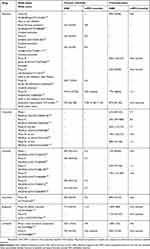 |
Table 2 Clinical outcomes of phase I–III studies of ALK inhibitors in crizotinib-naïve or crizotinib resistant patients, with or without chemotherapy |
Limitations of ALK inhibitors
A limitation of the use of some members of this class of TKIs is the emergence of treatment resistance.76,77 Several mechanisms of resistance to each targeted therapy have been identified, but these can be broadly categorized into two main classes: (1) alteration of the driver oncogene, or (2) activation of a critical parallel or downstream signaling pathway(s) that promotes pro-survival signaling.14 Resistance to crizotinib involves mechanisms such as secondary mutations within the ALK tyrosine kinase domain and activation of alternative signaling pathways.78 Ceritinib, more potent than crizotinib, is active against multiple ALK mutations that appear to result in resistance to crizotinib.79,80 In the case of ceritinib, the increased potency appears to be associated with a higher rate of adverse events.81 Alectinib is a second-generation ALK inhibitor that has both high selectivity82 and efficient blood–brain barrier penetrance;83,84 these characteristics are believed to contribute to the prevention of treatment resistance in naïve patients. In addition, alectinib can overcome crizotinib treatment resistance in patients who have relapsed.85
Each TKI can target different oncogenic drivers, which can affect the likelihood of treatment resistance occurring through secondary mutations or bypass mechanisms. Targets for each TKI are as follows: alectinib (inhibitor of ALK tyrosine kinase and RET kinase, including the ALK L1196M mutant); ensartinib (ROS proto-oncogene 1 receptor tyrosine kinase, ROS1; MET protocol-oncogene receptor tyrosine kinase, MET; STE20 like kinase, SLK; AXL receptor tyrosine kinase, AXL; leukocyte receptor tyrosine kinase, LTK; ABL proto-oncogene 1 non-receptor tyrosine kinase, ABL1; and EPH receptor A2, EPHA2); brigatinib (inhibitor of ALK and EGFR, including ALK L1196M and EGFR T790M mutants); lorlatinib (ALK and ROS1); and repotrectinib (ALK, ROS1, and TRK).14,23,75–87
The treatment landscape in China
The first Chinese guidelines for the diagnosis and treatment of primary lung cancer were published in 2003, and then updated in 2011, 2015, and 2018.57,88 First-line drug regimens in cases of advanced NSCLC include platinum-doublet chemotherapy and targeted molecular therapy drugs, such as gefitinib, erlotinib, or icotinib if EGFR mutations are detected; or crizotinib if ALK fusion genes are detected. Treatment of NSCLC in China is characterized by many factors, which include the accessibility and availability of diagnostic assays and treatments, insurance reimbursement rates,89 and the accuracy of decision making in the Chinese healthcare system.90
More recently, Chinese guidelines for the treatment and diagnosis of ALK+ and ROS1+ NSCLC were published.57 The first TKI to be approved by the Chinese FDA for advanced ALK+ NSCLC was crizotinib in 2013.91 Global studies have shown that treatment resistance to crizotinib is inevitable;70,73 however, a retrospective study observed that continuation of crizotinib therapy in Chinese patients beyond the initial disease progression may provide further benefits.92 The first second-generation TKI to be developed was ceritinib, which initially showed efficacy in the ASCEND-193 trial, and was approved by the US FDA shortly thereafter, in 2015.81 However, a subsequent phase III study94 showed inferior efficacy of ceritinib compared with the results of a phase II study.95 Furthermore, with the publication of the ALEX66 and J-ALEX96 trials, alectinib achieved a median PFS of 34.8 months (95% CI: 17.7–not estimable) compared with 10.9 months (95% CI: 9.1–12.9) with crizotinib, making it the drug of choice for advanced ALK+ NSCLC (HR 0.43 [95% CI: 0.32–0.58]).97
Following priority review, alectinib was approved for use in China in August 2018 as a monotherapy to treat patients with locally advanced or metastatic ALK+ NSCLC.98 This approval allows the use of alectinib for both ALK-inhibitor naïve or treated patients. Other second-generation TKIs, ensartinib and brigatinib; the third-generation TKI lorlatinib; and the fourth-generation TKI repotrectinib are not currently available in China.
Alectinib
Alectinib (CH5424802/RO5424802) is a potent and highly selective second-generation inhibitor of ALK tyrosine kinase that acts only on ALK+ NSCLC.99,100 It also inhibits RET kinase activity and, thus, may prove efficacious against RET fusion+ tumors.101 Furthermore, alectinib exhibits activity against multiple gate-keeper mutations that impart resistance to crizotinib, and can cross the blood–brain barrier.83,102–106 Notably, alectinib shows activity both in patients who are crizotinib treatment-naïve and in patients who have demonstrated resistance to crizotinib (Table 2).99,107 In contrast to ceritinib, alectinib has a lower incidence of adverse events.81 The clinical outcomes of phase I–III studies of alectinib are presented in Table 2.
Prior to its indication in China last year, alectinib was approved in the EU for ALK+ advanced NSCLC patients either as a first-line treatment108 or for those previously treated with crizotinib,109 and in the US for ALK+ metastatic NSCLC as detected by an US FDA-approved test.110
Mode of action
The unique chemical structure of alectinib means it 1) targets both ALK rearrangements and RET fusion+ tumors, but not MET or ROS1 kinase activity, and 2) overcomes acquired resistance to crizotinib through its ability to target the mutations that develop with crizotinib treatment.83,102–106 Its features include a scaffold-like structure with lipophilic properties and an ability to cross the blood–brain barrier, possibly because it is not a substrate of the key efflux transporter that delays blood–brain barrier penetration. Additionally, an increased potency over crizotinib is evident in the three-fold increase in in vitro ALK inhibition (53 nM alectinib versus 150.8 nM crizotinib).99 Together, these characteristics may contribute to its ability to overcome resistance to other ALK inhibitors caused by mutations83,84,100,102,111 and its increased potency over crizotinib for treating CNS metastases.83,84 Successful treatment of CNS metastases is demonstrated based on a pooled analysis of data from two phase II studies (NCT01871805 and NCT01801111) that reported a 64.0% CNS objective response rate (95% confidence interval [CI]: 49.2–77.1) and a median CNS duration of response of 10.8 months (95% CI: 7.6–14.1) in crizotinib-refractory ALK+ NSCLC patients with measurable CNS disease (n=50) at baseline.112
Metabolism and pharmacokinetics
Alectinib is primarily metabolized by the cytochrome P450 (CYP) 3A4 enzyme, producing its major active metabolite M4 (Table 1).113 Most of the drug is excreted in the feces.113 The pharmacokinetics of alectinib relative to other ALK inhibitors are presented in Table 1. Alectinib exhibits a time to maximum concentration of 3–5 hrs, and a half-life of 33 hrs.114 The maximum steady-state concentration for alectinib is higher than that of crizotinib, by approximately 4-fold, while alectinib achieves this concentration earlier than crizotinib (Table 1).114,115 It is important to note that the pharmacokinetics of alectinib does not appear to differ by race. Analyses of pharmacokinetic data from the phase III ALEX trial of alectinib, which was prospectively stratified by Asian and non-Asian patients, did not exhibit any notable differences.116
Clinical efficacy of alectinib
The global phase III ALEX study demonstrated prolonged PFS in newly diagnosed patients receiving alectinib versus those receiving crizotinib.97 The study reported a median PFS of 34.8 months (95% CI: 17.7–not estimable), with only 12% of patients developing brain metastases, as compared with 10.9 months (95% CI: 9.1–12.9) and 45%, respectively, in patients treated with crizotinib. In another phase III trial (ALUR) across 13 countries in Europe and Asia, alectinib versus platinum-based chemotherapy in crizotinib-pretreated patients exhibited prolonged median PFS, both investigator assessed and independent review committee assessed: 9.6 months (95% CI: 6.9–12.2) with alectinib and 1.4 months (95% CI: 1.3–1.6) with chemotherapy (investigator assessed) with a hazard ratio (HR) of 0.15 (95% CI: 0.08–0.29; p<0.001).85 Therefore, the long-term benefits of alectinib in ALK inhibitor-naïve patients have been demonstrated in a number of different populations.117
A systematic meta-analysis of eight alectinib trials was published recently and presented the overall pooled efficacy and safety results, and results stratified by prior treatment status.118 The pooled overall response rate (ORR) was 70% (95% CI: 57–82), with ALK-inhibitor-naïve patients having an ORR of 87% (95% CI: 81–92) versus crizotinib-resistant patients, who had an ORR of 52% (95% CI: 46–58).118 Table 2 summarizes clinical efficacy results from individual trials of alectinib.
A recent report of the pooled efficacy data after 15- and 18-month follow-up of two key phase II studies (NCT01871805 and NCT01801111; n=255) showed that alectinib has a durable response rate (median =14.9 months; 95% CI: 11.1–20.4) with a 78.8% disease control rate (95% CI: 72.3–84.4) and median PFS of 8.3 months (95% CI: 7.0–11.3).119 There were also no specific ethnic differences in the efficacy of alectinib observed.
Clinical evidence in Chinese patients
The superior efficacy of alectinib compared with crizotinib has been reported in an Asian versus non-Asian patient subgroup analysis in the global phase III ALEX study66,116 and in the ALESIA phase III randomized clinical trial, which comprised patients from China, South Korea, and Thailand.120 The ALESIA study reported a significant improvement in both investigator-assessed (HR: 0.22; 95% CI: 0.13–0.38; p<0.0001; median PFS not estimable [alectinib] vs 11.1 months [crizotinib]) and independent review committee-assessed (HR: 0.37; 95% CI: 0.22–0.61; p<0.0001) PFS in the alectinib vs crizotinib groups.120 The percent of patients experiencing disease progression or death was higher with crizotinib versus alectinib treatment (60% vs 21%, respectively).120 Although patients receiving alectinib were treated for a longer period than those receiving crizotinib (14.7 vs 12.6 months, respectively), fewer grade 3–5 adverse events (alectinib, 36 [29%] of 125; crizotinib, 30 [48%] of 62) and serious adverse events (alectinib, 19 [15%] of 125; crizotinib, 16 [26%] of 62) were reported in the alectinib vs crizotinib groups.120 Pharmacokinetic results from a subset of 20 frequently sampled Chinese patients (600 mg alectinib, twice daily) demonstrated nearly identical pharmacokinetic profiles to white patients (historical data from a phase II global study).120,121 These clinical data are consistent with data reported for the ALEX study,66 suggesting that alectinib could be suitable to treat both crizotinib-naïve and crizotinib-refractory patients with ALK+ NSCLC, irrespective of ethnicities.
The ALEX trial also included 43 Chinese patients, of which 25 received alectinib and 18 received crizotinib, and results were reported at ESMO-Asia 2017.116 Median PFS (independent review committee-assessed) was longer in alectinib-treated patients (25.7 vs 14.8 months; HR: 0.57, 95% CI: 0.24–1.38; p=0.16), suggesting the efficacy of alectinib in Chinese patients is consistent with that in the global population.
Activity in the CNS
Brain metastases are a common complication during the advanced stages of NSCLC, particularly in patients who have ALK gene rearrangements.122 Therefore, systemic therapies with intracranial efficacy are the preferred long-term treatment option for patients with ALK+ NSCLC. Alectinib has a unique physical structure that contributes to its potency, including an improved ability to penetrate the blood–brain barrier compared to other TKIs.83,84 Alectinib has shown potential efficacy against brain metastases as reported in the pooled analysis of data from two phase II studies (NCT01871805 and NCT01801111) involving crizotinib-refractory ALK+ NSCLC patients with measurable CNS disease (n=50) at baseline, which reported a 64.0% CNS ORR (95% CI: 49.2–77.1) and a median CNS duration of response of 10.8 months (95% CI: 7.6–14.1).110 In a retrospective study of patients with advanced ALK+ NSCLC treated with alectinib, the ORR was 73.3% and the disease control rate was 100.0%, with a median CNS duration of response of 19.3 months.123
Patients receiving alectinib in the phase III study, ALEX, exhibited longer times to CNS progression than those patients receiving crizotinib, and the two groups were balanced in CNS disease levels at baseline.111 A recently published update of the phase III ALEX study supports the superior CNS efficacy of alectinib over crizotinib.124 Here, it was reported that alectinib had a significantly longer time to CNS progression, regardless of the presence of CNS metastases at baseline. Notably, when evaluating the cumulative incidence rate (CIR) of CNS progression in patients without baseline CNS metastases, it was shown that the 12-month CIR was only 4.6% in the alectinib group compared with 31.5% in the crizotinib group.125 In addition, the CIR of CNS progression in patients with brain metastases at baseline was 16.0% and 58.3%, respectively. These data demonstrate a superior efficacy and capability of delaying CNS progression for patients treated with alectinib, even in patients without brain metastases at baseline.
A systematic meta-analysis has also described the clinical outcomes among patients with ALK+ NSCLC and brain metastases.89 Patients who received alectinib exhibited a pooled ORR of 52.0% (95% CI: 45.0–59.0), with an ORR of 59.0% (95% CI: 47.0–71.0) among ALK-inhibitor-naïve patients versus 48.0% (95% CI: 38.0–57.0) among crizotinib-resistant patients.89 In the phase III ALUR study of European and Asian patients previously treated with crizotinib, it was shown that there was a greater CNS ORR in patients receiving alectinib versus those receiving chemotherapy (54.2% vs 0%, p<0.001).85 Similarly, Asian patients receiving alectinib in the ALESIA study exhibited larger CNS ORR compared with patients receiving crizotinib: 73% vs 22%, respectively, in patients with and without measurable CNS lesions at baseline.120
The observed efficacy of alectinib in ALK+ NSCLC patients without brain metastases suggests that alectinib may also prevent NSCLC progression to the brain. Therefore, alectinib is a CNS active ALK TKI that is not only active against baseline brain metastases but may also provide long-term benefits to the patient through its ability to potentially prevent the development of further brain metastases.
Safety profile of alectinib
Alectinib is generally well tolerated and has been reported to have a good toxicity profile.119,126,127 The incidence of adverse events in clinical trials is relatively low and this has been reported in both patients with and without baseline brain metastases.85,112 A systematic meta-analysis of eight clinical trials reported that the most common adverse events were constipation (29%), anemia (25%), myalgia (18%), peripheral edema (18%), dysgeusia (18%), and blood creatine phosphokinase increased (18%).118 This meta-analysis also reported a pooled discontinuation rate of 7% (95% CI: 4–10) and a pooled dose reduction or interruption rate of 33% (95% CI: 24–42) among alectinib-treated patients.118
The Japanese J-ALEX clinical trial compared alectinib (300 mg b.i.d) to crizotinib (250 mg b.i.d) and showed that alectinib had a better safety profile than crizotinib.96 In comparison to crizotinib, the incidence of grade 3/4 adverse events (26% versus 52%) and proportion of patients who discontinued treatment (9% versus 20%) were lower in alectinib-treated patients. Furthermore, the phase III ALEX trial also reported fewer grade 3–5 adverse events in treatment-naïve patients receiving alectinib relative to those receiving crizotinib.66 The phase III ALEX trial was a global study and showed that at a dose of 600 mg (b.i.d), grade 3–5 adverse events were reported in 41% of patients, with 11% of patients discontinuing treatment. In both the J-ALEX and ALEX trials, the incidence of adverse events was consistently lower compared to crizotinib except for myalgia and anemia, which were more frequent after alectinib treatment. Similar results were also observed in the phase III ALUR trial of crizotinib-pretreated patients and in the phase III ALESIA trial in Asian patients: fewer grade ≥3 adverse events and fewer adverse events leading to discontinuation occurred in patients receiving alectinib versus those receiving the other treatment.85,120
Conclusions
NSCLC has a very poor prognosis;13 however, the development of treatments that target pathways involved in tumorigenesis can improve patient survival. Targeting the NSCLC oncogenic-driver gene ALK has resulted in the development of various ALK inhibitors with notable effects in prolonging patient survival. Currently recommended testing methods for identifying ALK gene rearrangements in NSCLC patients include the use of Ventana IHC, which is recommended by the Chinese guidelines.57,65 Once the ALK gene mutation status has been assessed, current TKI therapies confer efficacy, albeit with the risk of toxicities or eventual regression in some cases. Several of these agents, however, have multiple targets that may contribute to the development of resistance (eg, crizotinib) or that may result in greater toxicity (eg, ceritinib). Alectinib, a TKI that potently and selectively targets the ALK and MET pathways, results in prolonged PFS, even in patients with CNS involvement or patients with or without previous exposure to crizotinib, with lower rates of adverse events and dose reductions or interruptions due to adverse events. Recently approved in China, alectinib may prove to be the best first-line agent to help improve the outcomes of ALK+ locally advanced or metastatic NSCLC patients.
Acknowledgments
We thank James Graham, PhD, and Sarah Bubeck, PhD, of Edanz Medical Writing, for providing medical writing assistance, which was funded by Shanghai Roche Pharmaceuticals Ltd. This work was funded by Shanghai Roche Pharmaceuticals Ltd. Hui Yu and Si Sun contributed equally as co-first authors in this study.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Jinjing Xia is an employee of Shanghai Roche Pharmaceuticals Ltd. All other authors report no conflicts of interest in this work.
References
1. Zhou C. Lung cancer molecular epidemiology in China: recent trends. Transl Lung Cancer Res. 2014;3:270–279.doi:10.3978/j.issn.2218-6751.2014.09.01
2. Gridelli C, Peters S, Sgambato A, et al. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev. 2014;40:300–306. doi:10.1016/j.ctrv.2013.07.002
3. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21294
4. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338
5. Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26:48–58.
6. Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the future. J Clin Oncol. 2005;23:3175–3185. doi:10.1200/JCO.2005.10.462
7. Peace LR. A time correlation between cigarette smoking and lung cancer. Statistician. 1985;34:371–381. doi:10.2307/2987825
8. Liu BQ, Peto R, Chen ZM, et al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. BMJ. 1998;317:1411–1422. doi:10.1136/bmj.317.7159.682
9. Yang L, Parkin DM, Li L, et al. Time trends in cancer mortality in China: 1987–1999. Int J Cancer. 2003;106:771–783. doi:10.1002/ijc.11300
10. Chen W, Zheng R, Zeng H, Zhang S. Epidemiology of lung cancer in China. Thorac Cancer. 2015;6:209–215. doi:10.1111/tca.2015.6.issue-2
11. Barzi A, Pennell NA. Targeting angiogenesis in non-small cell lung cancer. Eur J Clin Med Oncol. 2010;2:31–42.
12. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi:10.3322/caac.21208
13. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2016. National Cancer Institute; Posted April 2019. Available from: https://seer.cancer.gov/csr/1975_2016/.
14. Addeo A, Tabbò F, Robinson T, et al. Precision medicine in ALK rearranged NSCLC: A rapidly evolving scenario. Crit Rev Oncol/Hematol. 2018;122:150–156. doi:10.1016/j.critrevonc.2017.12.015
15. Fernandez-Cuesta L, Plenker D, Osada H, et al. CD74–NRG1 fusions in lung adenocarcinoma. Cancer Discov. 2014;4:415–422. doi:10.1158/2159-8290.CD-13-0646
16. Zheng D, Wang R, Ye T, et al. MET exon 14 skipping defines a unique molecular class of non-small cell lung cancer. Oncotarget. 2016;5:41691–41702.
17. Do H, Solomon B, Mitchell PL, Fox SB, Dobrovic A. Detection of the transforming AKT1 mutation E17K in non-small cell lung cancer by high resolution melting. BMC Res Notes. 2008;1:14. doi:10.1186/1756-0500-1-23
18. Gainor JF, Varghese AM, Ou S-HI, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19:4273–4281. doi:10.1158/1078-0432.CCR-13-0318
19. Lee T, Lee B, Choi YL, et al. Non-small cell lung cancer with concomitant EGFR, KRAS, and ALK mutation: clinicopathologic features of 12 cases. J Pathol Transl Med. 2016;50:197. doi:10.4132/jptm.2016.03.09
20. Sweiss RF, Thomas S, Bank B, et al. Concurrent EGFR mutation and ALK translocation in non-small cell lung cancer. Cureus. 2016;8:e513.
21. Lo Russo G, Imbimbo M, Corrao G, et al. Concomitant EML4-ALK rearrangement and EGFR mutation in non-small cell lung cancer patients: a literature review of 100 cases. Oncotarget. 2017;8:59889–59900. doi:10.18632/oncotarget.17431
22. Stella GM, Scabini R, Inghilleri S, et al. EGFR and KRAS mutational profiling in fresh non-small cell lung cancer (NSCLC) cells. J Cancer Res Clin Oncol. 2013;139:1327–1335. doi:10.1007/s00432-013-1444-y
23. Wu J, Savooji J, Liu D, et al. Second- and third-generation ALK inhibitors for non-small cell lung cancer. J Hematol Oncol. 2016;9:19. doi:10.1186/s13045-016-0251-8
24. Duyster J, Bai R-Y, Morris SW. Translocations involving anaplastic lymphoma kinase (ALK). Oncogene. 2001;20:5623. doi:10.1038/sj.onc.1204594
25. Iwahara T, Fujimoto J, Wen D, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439. doi:10.1038/sj.onc.1200849
26. Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13:685.
27. Kruczynski A, Delsol G, Laurent C, et al. Anaplastic lymphoma kinase as a therapeutic target. Expert Opin Ther Targets. 2012;16:1127–1138. doi:10.1517/14728222.2012.719498
28. Mossé YP, Wood A, Maris JM. Inhibition of ALK signaling for cancer therapy. Clin Cancer Res. 2009;15:5609–5614. doi:10.1158/1078-0432.CCR-09-0547
29. Palmer RH, Vernersson E, Grabbe C, et al. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009;420:345–361. doi:10.1042/BJ20082397
30. Morris S, Kirstein M, Valentine M, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. doi:10.1126/science.8122112
31. Shiota M, Nakamura S, Ichinohasama R, et al. Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood. 1995;86:1954–1960.
32. Webb TR, Slavish J, George RE, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther. 2009;9:331–356. doi:10.1586/14737140.9.3.331
33. Weiss JB, Xue C, Benice T, Xue L, Morris SW, Raber J. Anaplastic lymphoma kinase and leukocyte tyrosine kinase: functions and genetic interactions in learning, memory and adult neurogenesis. Pharmacol Biochem Behav. 2012;100(3):566–574. doi:10.1016/j.pbb.2011.10.024
34. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561. doi:10.1038/nature05984
35. Soda M, Takada S, Takeuchi K, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A. 2008;105:19893–19897. doi:10.1073/pnas.0805381105
36. Iragavarapu C, Mustafa M, Akinleye A, et al. Novel ALK inhibitors in clinical use and development. J Hematol Oncol. 2015;8:17. doi:10.1186/s13045-015-0122-8
37. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi:10.1056/NEJMoa1011205
38. Shaw AT, Costa DB, Iafrate AJ, et al. Clinical activity of the oral ALK and MET inhibitor PF-02341066 in non-small lung cancer (NSCLC) with EML4-ALK translocations. J Thorac Oncol. 2009;4:S305–S306. doi:10.1097/JTO.0b013e3181b6be12
39. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi:10.1056/NEJMoa1214886
40. An SJ, Chen ZH, Su J, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS One. 2012;7:e40109. doi:10.1371/journal.pone.0040109
41. Li Y, Li Y, Yang T, et al. Clinical significance of EML4-ALK fusion gene and association with EGFR and KRAS gene mutations in 208 Chinese patients with non-small cell lung cancer. PLoS One. 2013;8:e52093. doi:10.1371/journal.pone.0052093
42. Zhang X, Zhang S, Yang X, et al. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer. 2010;9:188. doi:10.1186/1476-4598-9-254
43. Barlesi F, Blons H, Beau-Faller M, et al. Biomarkers (BM) France: results of routine EGFR, HER2, KRAS, BRAF, PI3KCA mutations detection and EML4-ALK gene fusion assessment on the first 10,000 non-small cell lung cancer (NSCLC) patients (pts). J Clin Oncol. 2013;31:8000. doi:10.1200/JCO.2013.49.0219
44. Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–4283. doi:10.1158/1078-0432.CCR-08-0168
45. Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14:6618–6624. doi:10.1158/1078-0432.CCR-08-1018
46. Wu YC, Chang IC, Wang CL, et al. Comparison of IHC, FISH and RT-PCR methods for detection of ALK rearrangements in 312 non-small cell lung cancer patients in Taiwan. PLoS One. 2013;8:e70839. doi:10.1371/journal.pone.0070839
47. Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15:5216–5223. doi:10.1158/1078-0432.CCR-09-0547
48. International Association for the Study of Lung Cancer. IASLC Atlas of ALK and ROS1 Testing in Lung Cancer.
49. Li G, Dai WR, Shao FC. Effect of ALK-inhibitors in the treatment of non-small cell lung cancer: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2017;21:3496–3503.
50. US Food and Drug Administration. FDA approves xalkori with companion diagnostic for a type of late-stage lung cancer [press release]. 2011. Available from: http://web.archive.org/web/20110901151213/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm269856.htm .
51. Shaw AT, Solomon B, Kenudson MM. Crizotinib and testing for ALK. J Natl Compr Canc Netw. 2011;9:1335–1341. doi:10.6004/jnccn.2011.0115
52. Cutz JC, Craddock KJ, Torlakovic E, et al. Canadian anaplastic lymphoma kinase study: a model for multicenter standardization and optimization of ALK testing in lung cancer. J Thorac Oncol. 2014;9:1255–1263. doi:10.1097/JTO.0000000000000011
53. Zhou J, Zhao J, Sun K, et al. Accurate and economical detection of ALK positive lung adenocarcinoma with semiquantitative immunohistochemical screening. PLoS One. 2014;9:e92828. doi:10.1371/journal.pone.0092828
54. Ying J, Guo L, Qiu T, et al. Diagnostic value of a novel fully automated immunochemistry assay for detection of ALK rearrangement in primary lung adenocarcinoma. Ann Oncol. 2013;24:2589–2593. doi:10.1093/annonc/mdt295
55. Liu L, Zhan P, Zhou X, et al. Detection of EML4-ALK in lung adenocarcinoma using pleural effusion with FISH, IHC, and RT-PCR methods. PLoS One. 2015;10:e0117032. doi:10.1371/journal.pone.0117032
56. Xu CW, Wang WX, Chen YP, et al. Simultaneous VENTANA IHC and RT-PCR testing of ALK status in Chinese non-small cell lung cancer patients and response to crizotinib. J Transl Med. 2018;16:93. doi:10.1186/s12967-018-1468-9
57. Zhang XC, Lu S, Zhang L. Chinese guidelines for the treatment and diagnosis of anaplastic lymphoma kinase-positive and ROS1-positive non-small-cell lung cancer [Chinese]. Chin J Pathol. 2018;47:241–247.
58. Zhang YG, Jin ML, Li L, et al. Evaluation of ALK rearrangement in chinese non-small cell lung cancer using FISH, immunohistochemistry, and real-time quantitative RT- PCR on paraffin-embedded tissues. PLoS One. 2013;8:e64821. doi:10.1371/journal.pone.0064821
59. Hout DR, Schweitzer BL, Lawrence K, et al. Performance of a RT-PCR assay in comparison to FISH and immunohistochemistry for the detection of ALK in non-small cell lung cancer. Cancers. 2017;9:99. doi:10.3390/cancers9080099
60. Yohe S, Thyagarajan B. Review of clinical next-generation sequencing. Arch Pathol Lab Med. 2017;141:1544–1557. doi:10.5858/arpa.2016-0501-RA
61. Vendrell JA, Taviaux S, Beganton B, et al. Detection of known and novel ALK fusion transcripts in lung cancer patients using next-generation sequencing approaches. Sci Rep. 2017;7:12510. doi:10.1038/s41598-017-12679-8
62. Spjuth O, Bongcam-Rudloff E, Dahlberg J, et al. Recommendations on e-infrastructures for next-generation sequencing. Gigascience. 2016;5:26. doi:10.1186/s13742-016-0132-7
63. Lu S, Zhang J, Ye M, Wang B, Wu B. Economic analysis of ALK testing and crizotinib therapy for advanced non-small-cell lung cancer. Pharmacogenomics. 2016;17:985–994. doi:10.2217/pgs-2016-0017
64. Lu S, Yu Y, Fu S, Ren H, Gangopadhyay N. Cost-effectiveness of ALK testing and first-line crizotinib therapy for non-small-cell lung cancer in China. PLoS One. 2018;13:e0205827. doi:10.1371/journal.pone.0205827
65. Zhang X, Lu S, Zhang L, et al. The Chinese expert consensus opinion of the diagnosis of ALK-positive NSCLC (2013 version) [Chinese]. Chin J Pathol. 2013;6:402–406.
66. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi:10.1056/NEJMoa1704795
67. Hida T, Seto T, Horinouchi H, et al. Phase II study of ceritinib in alectinib-pretreated patients with anaplastic lymphoma kinase-rearranged metastatic non-small-cell lung cancer in Japan: ASCEND-9. Cancer Sci. 2018;109:2863–2872. doi:10.1111/cas.13721
68. Zhou Q, Song Y, Zhang X, et al. A multicenter survey of first-line treatment patterns and gene aberration test status of patients with unresectable Stage IIIB/IV nonsquamous non-small cell lung cancer in China (CTONG 1506). BMC Cancer. 2017;17:462. doi:10.1186/s12885-017-3451-x
69. Niu FY, Wu Y-L. Personalized treatment strategies for non-small-cell lung cancer in Chinese patients: the role of crizotinib. Onco Targets Ther. 2015;8:999–1007.
70. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi:10.1056/NEJMoa1410490
71. Su S, Wu YL. Clinical trials of tyrosin kinase inhibitor for lung cancer in China: a review. J Hematol Oncol. 2017;10:147. doi:10.1186/s13045-017-0514-z
72. Khan M, Lin J, Liao G, et al. ALK inhibitors in the treatment of ALK positive NSCLC. Front Oncol. 2019;8:557. doi:10.3389/fonc.2018.00557
73. Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–1888. doi:10.1200/JCO.2014.59.0539
74. Betts K, Song J, Guo J, et al. Real-world treatment patterns and brain metastases development in ALK-positive non-small cell lung cancer.
75. Horn L, Infante JR, Reckamp KL, et al. Ensartinib (X-396) in ALK-positive non-small cell lung cancer: results from a first-in-human phase I/II, multicenter study. Clin Cancer Res. 2018;24:2771–2779. doi:10.1158/1078-0432.CCR-17-2398
76. Isozaki H, Takigawa N, Kiura K. Mechanisms of acquired resistance to ALK inhibitors and the rationale for treating ALK-positive lung cancer. Cancers. 2015;7:763–783. doi:10.3390/cancers7020763
77. Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18:1472–1482. doi:10.1158/1078-0432.CCR-11-2906
78. Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol. 2016;27(Suppl3):iii42–iii50. doi:10.1093/annonc/mdw141
79. Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662–673. doi:10.1158/2159-8290.CD-13-0646
80. Marsilje TH, Pei W, Chen B, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulf onyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem. 2013;56:5675–5690.
81. Khozin S, Blumenthal GM, Zhang L, et al. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res. 2015;21:2436–2439. doi:10.1158/1078-0432.CCR-14-3157
82. Santarpia M, Altavilla G, Rosell R. Alectinib: a selective, next-generation ALK inhibitor for treatment of ALK-rearranged non-small-cell lung cancer. Expert Rev Respir Med. 2015;9:255–268. doi:10.1586/17476348.2015.1009040
83. Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74:1023–1028. doi:10.1007/s00280-014-2578-6
84. Masters GA, Temin S, Azzoli CG, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015;33:3488–3515. doi:10.1200/JCO.2015.62.1342
85. Novello S, Mazieres J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018;29:1409–1416. doi:10.1093/annonc/mdx807
86. Huang WS, Liu S, Zou D, et al. Discovery of Brigatinib (AP26113), a phosphine oxide-containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J Med Chem. 2016;59:4948–4964. doi:10.1021/acs.jmedchem.6b00306
87. Drilon AE, Ou S-HI, Cho BC, et al. A phase 1 study of the next-generation ALK/ROS1/TRK inhibitor ropotrectinib (TPX-0005) in patients with advanced ALK/ROS1/NTRK+ cancers (TRIDENT-1). J Clin Oncol. 2018;36:2513. doi:10.1200/JCO.2018.36.15_suppl.2513
88. Zhi XY, Yu JM, Shi YK. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version). Cancer. 2015;122:162.
89. Li X, Zhou Q, Wang X, et al. The effect of low insurance reimbursement on quality of care for non-small cell lung cancer in China: a comprehensive study covering diagnosis, treatment, and outcomes. BMC Cancer. 2018;18:683. doi:10.1186/s12885-018-4242-8
90. Yang LL, Zhang XC, Yang XN, et al. Lung cancer treatment disparities in China: A question in need of an answer. Oncologist. 2014;19:1084–1090. doi:10.1634/theoncologist.2014-0007
91. Daverman R Prizer Inc. gains China approval of drug for kinase-specific lung cancer. Available from: https://www.biospace.com/article/releases/pfizer-inc-gains-china-approval-of-drug-for-kinase-specific-lung-cancer-/.
92. Hong X, Chen Q, Ding L, et al. Clinical benefit of continuing crizotinib therapy after initial disease progression in Chinese patients with advanced ALK-rearranged non-small-cell lung cancer. Oncotarget. 2017;8:41631–41640. doi:10.18632/oncotarget.15892
93. Kim DW, Mehra R, Tan DSW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–463. doi:10.1016/S1470-2045(15)00614-2
94. Shaw AT, Kim TM, Crino L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18:874–886. doi:10.1016/S1470-2045(17)30072-4
95. Crino L, Ahn MJ, De Marinis F, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol. 2016;34:2866–2873. doi:10.1200/JCO.2015.65.5936
96. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi:10.1016/S0140-6736(17)31928-1
97. Camidge DR, Mok T, Gadgeel SM, et al. Updated efficacy and safety data from the global phase III ALEX study of alectinib (ALC) vs crizotinib (CZ) in untreated advanced ALK+ NSCLC.
98. China National Drug Administration grants rapid approval of Roche’s Alecensa (alectinib) as a treatment for ALK-positive lung cancer [press release]. August 20, 2018. Available from: https://www.roche.com/media/releases/med-cor-2018-08-20.htm.
99. Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19:679–690. doi:10.1016/j.ccr.2011.04.004
100. Vavala T, Novello S. Alectinib in the treatment of ALK-positive non-small cell lung cancer: an update on its properties, efficacy, safety and place in therapy. Ther Adv Med Oncol. 2018;10:1758835918789364. doi:10.1177/1758835918789364
101. Kodama T, Tsukaguchi T, Satoh Y, et al. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol Cancer Ther. 2014;13:2910–2918. doi:10.1158/1535-7163.MCT-14-0274
102. Kodama T, Tsukaguchi T, Yoshida M, Kondoh O, Sakamoto H. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett. 2014;351:215–221. doi:10.1016/j.canlet.2014.05.020
103. Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi:10.1158/2159-8290.CD-16-0596
104. Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res. 2015;21:2227–2235. doi:10.1158/1078-0432.CCR-14-2791
105. Awad MM, Shaw AT. ALK inhibitors in non-small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol. 2014;12:429–439.
106. Mologni L, Ceccon M, Pirola A, et al. NPM/ALK mutants resistant to ASP3026 display variable sensitivity to alternative ALK inhibitors but succumb to the novel compound PF-06463922. Oncotarget. 2015;6:5720–5734. doi:10.18632/oncotarget.3122
107. Kinoshita K, Oikawa N, Tsukuda T. Chapter Nineteen - anaplastic lymphoma kinase inhibitors for the treatment of ALK-positive cancers. In: Desai MC, editor. Annual Reports in Medicinal Chemistry. Vol. 47. Cambridge (MA): Academic Press; 2012:281–293.
108. European Commission approves Roche’s Alecensa (alectinib) as first-line treatment in ALK-positive lung cancer [press release]. December 21, 2017. Available from: https://www.roche.com/investors/updates/inv-update-2017-12-21.htm.
109. Roche receives EU approval of Alecensa (alectinib) for people with previously treated ALK-positive non-small cell lung cancer [press release]. February 21, 2017. Available from: https://www.roche.com/media/releases/med-cor-2017-02-21.htm.
110. FDA approves Genentech’s Alecensa (alectinib) as first-line treatment for people with specific type of lung cancer [press release]. November 6, 2017. Available from: https://www.gene.com/media/press-releases/14689/2017-11-06/fda-approves-genentechs-alecensa-alectin.
111. Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–1128. doi:10.1016/S1470-2045(13)70510-2
112. Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of CNS response to Alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34:4079–4085. doi:10.1200/JCO.2016.68.4639
113. Chugai Pharmaceutical Co Ltd. Alecensa (alectinib) [Japanese prescribing information]. 2014. Available from: https://chugai-pharm.jp/hc/ss/pr/drug/alc_cap0150/guide/PDF/lg/alc_150_guide_lg.pdf.
114. Genentech USA Inc. Alecensa (Alectinib) [prescribing information]. Available from: https://www.gene.com/download/pdf/alecensa_prescribing.pdf.
115. Muller IB, De Langen AJ, Honeywell RJ, Giovannetti E, Peters GJ. Overcoming crizotinib resistance in ALK-rearranged NSCLC with the second-generation ALK-inhibitor ceritinib. Expert Rev Anticancer Ther. 2016;16:147–157. doi:10.1586/14737140.2016.1131612
116. Mok TSK, Peters S, Camidge DR, et al. Alectinib (ALC) vs crizotinib (CRZ) in treatment-naïve ALK+ non-small-cell lung cancer (NSCLC): Asian vs non-Asian subgroup analysis of the ALEX study. Ann Oncol. 2017;28:X186–X195. doi:10.1093/annonc/mdx075
117. Tamura T, Kiura K, Seto T, et al. Three-year follow-up of an Alectinib phase I/II study in ALK-positive non-small-cell lung cancer: AF-001JP. J Clin Oncol. 2017;35:1515–1521. doi:10.1200/JCO.2016.70.5749
118. Fan J, Xia Z, Zhang X, et al. The efficacy and safety of alectinib in the treatment of ALK+ NSCLC: a systematic review and meta-analysis. Onco Targets Ther. 2018;11:1105–1115. doi:10.2147/OTT.S156170
119. Yang JC, Ou SI, De Petris L, et al. Pooled systemic efficacy and safety data from the pivotal phase ii studies (NP28673 and NP28761) of Alectinib in ALK-positive non-small cell lung cancer. J Thorac Oncol. 2017;12:1552–1560. doi:10.1016/j.jtho.2016.09.002
120. Zhou C, Kim SW, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7:437–446. doi:10.1016/S2213-2600(19)30053-0
121. Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34:661–668. doi:10.1200/JCO.2015.63.9443
122. Toyokawa G, Seto T, Takenoyama M, Ichinose Y. Insights into brain metastasis in patients with ALK+ lung cancer: is the brain truly a sanctuary? Cancer Metastasis Rev. 2015;34(4):797–805. doi:10.1007/s10555-015-9592-y
123. Lin JJ, Jiang GY, Joshipura N, et al. Efficacy of alectinib in patients with ALK-positive NSCLC and symptomatic or large CNS metastasis. J Thorac Oncol. 2019;14:683–690. doi:10.1016/j.jtho.2018.12.002
124. Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018;29:2214–2222. doi:10.1093/annonc/mdx807
125. Gadgeel S, Shaw AT, Barlesi F, et al. Cumulative incidence rates for CNS and non-CNS progression in two phase II studies of alectinib in ALK-positive NSCLC. Br J Cancer. 2018;118:28–42. doi:10.1038/bjc.2017.395
126. Larkins E, Blumenthal GM, Chen H, et al. FDA approval: alectinib for the treatment of metastatic, ALK-positive non-small cell lung cancer following crizotinib. Clin Cancer Res. 2016;22:5171–5176. doi:10.1158/1078-0432.CCR-16-0190
127. Camidge DR, Gadgeel S, Oi SH, et al. MA07.02 Updated efficacy and safety data from the phase 2 NP28761 study of alectinib in ALK-positive non-small-cell lung cancer. J Thorac Oncol. 2017;12:S378. doi:10.1016/j.jtho.2016.09.002
128. ARIAD Pharmaceuticals Inc. ALUNBRIG™ (brigatinib) [US prescribing information]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208772lbl.pdf.
129. Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol. 2013;14:590–598. doi:10.1016/S1470-2045(13)70142-6
130. Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–1019. doi:10.1016/S1470-2045(12)70140-7
131. Blackhall F, Ross Camidge D, Shaw AT, et al. Final results of the large-scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK-positive non-small-cell lung cancer. ESMO Open. 2017;2:e000219. doi:10.1136/esmoopen-2017-000219
132. Wu YL, Zhou J, Shi YK, et al. Results of PROFILE1029, a phase III comparison of first-line crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:1539–1548. doi:10.1016/j.jtho.2018.06.012
133. Felip E, Orlov S, Park K, et al. ASCEND-3: A single-arm, open-label, multicenter phase II study of ceritinib in ALKi-naïve adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33:8060. doi:10.1200/jco.2015.33.15_suppl.8060
134. Soria JC, Chiari R, Paz-Ares L, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi:10.1016/S0140-6736(17)30123-X
135. Zhang L, Shi Y, Tan DSW, et al. ASCEND 6: single-arm, open label, multicenter phase 1/2 study of ceritinib in Chinese pts with advanced ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with crizotinib. Ann Oncol. 2016;27(Supplement_9):ix139–ix156. doi:10.1093/annonc/mdw141
136. Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:1683–1696. doi:10.1016/S1470-2045(16)30392-8
137. Camidge DR, Ahn MJ, Yang JC, et al. Brigatinib versus crizotinib in ALK-positive non–small-cell lung cancer. N Engl J Med. 2018;379:2027–2039. doi:10.1056/NEJMoa1810171
138. Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi:10.1016/S1470-2045(17)30072-4
139. Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi:10.1016/S1470-2045(18)30144-X
140. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: EMSO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):iv192–iv237. doi:10.1093/annonc/mdx807
141. Ettinger DS, Alsner DL, Wood DE, et al. NCCN guidelines insights: non-small cell lung cancer, version 5.2018. J Natl Compr Canc Netw. 2018;16:807–821. doi:10.6004/jnccn.2018.0062
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.