Back to Journals » Journal of Asthma and Allergy » Volume 15
Chinese Central Compartment Atopic Disease: The Clinical Characteristics and Cellular Endotypes Based on Whole-Slide Imaging
Authors Kong W, Wu Q, Chen Y, Ren Y , Wang W, Zheng R, Deng H , Yuan T, Qiu H, Wang X, Luo X, Huang X, Yang Q, Zhang G , Zhang Y
Received 1 December 2021
Accepted for publication 4 March 2022
Published 15 March 2022 Volume 2022:15 Pages 341—352
DOI https://doi.org/10.2147/JAA.S350837
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Weifeng Kong,1 Qingwu Wu,1 Yubin Chen,1 Yong Ren,2 Weihao Wang,1 Rui Zheng,1 Huiyi Deng,1 Tian Yuan,1 Huijun Qiu,1 Xinyue Wang,1 Xin Luo,1 Xuekun Huang,1 Qintai Yang,1 Gehua Zhang,1 Yana Zhang1
1Department of Otolaryngology-Head and Neck Surgery, the Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Digestive Cancer Research, the Seventh Affiliated Hospital of Sun Yat-Sen University, Shenzhen, People’s Republic of China
Correspondence: Yana Zhang; Gehua Zhang, Department of Otolaryngology-Head and Neck Surgery, the Third Affiliated Hospital of Sun Yat-Sen University, No. 600 Tianhe Road, Guangzhou, 510630, People’s Republic of China, Tel +86-20-85252310, Email [email protected]; [email protected]
Purpose: Histopathologic characterizations of central compartment atopic disease (CCAD) by whole-slide imaging remains lacking. We aim to study clinical presentations and cellular endotyping diagnosis of Chinese CCAD using artificial intelligence (AI).
Methods: A total of 72 patients diagnosed with chronic rhinosinusitis with nasal polyps (CRSwNP) were enrolled. CCAD was defined by positive result of serology specific IgE, endoscopic and radiological findings. The aeroallergen sensitization status, endoscopic results, radiological findings, and symptoms were evaluated and compared between patients with CCAD (n=14), eosinophilic CRSwNP (ENP, n=32) and non-eosinophilic CRSwNP (NENP, n=26). The cellular endotypes including eosinophils, neutrophils, lymphocytes, and plasma cells were analyzed by the AI chronic rhinosinusitis evaluation platform 2.0.
Results: CCAD was most common in male (71.43%). The positive rate of aeroallergen in patients with CCAD is 100%, which is much higher than those in patients with ENP (40.63%) and NENP (23.08%). Allergic rhinitis incidence was found to be 57.14% in Chinese CCAD subjects, which is obviously higher when compared with those in patients with ENP (21.88%) or NENP (0.00%). The presence of asthma was not significantly different between groups. Chinese CCAD population demonstrated mild symptoms and lower endoscopic and radiological scores than those in patients with ENP and NENP. For cellular endotypes in CCAD subjects, the median of eosinophils, neutrophils, lymphocytes, and plasma cells was 26.55%, 0.49%, 60.85%, and 7.33%, respectively. The proportion of eosinophils in nasal tissue and peripheral blood mononuclear cells from the CCAD group is between the proportions in those patients with ENP and NENP.
Conclusion: Chinese CCAD was associated with aeroallergen sensitivity, and displayed an eosinophil-dominant inflammatory pattern. Thus, proper management with allergy control and topical steroids could be recommended for CCAD treatment.
Keywords: central compartment atopic disease, deep learning, chronic rhinosinusitis with nasal polyps, eosinophil, aeroallergen
Introduction
Chronic rhinosinusitis (CRS) is a disease of high heterogeneity characterized by persistent inflammation of sinonasal mucosa. Clinically, CRS is frequently divided into two major phenotypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). Based on the local eosinophil infiltration, CRSwNP is classified into eosinophilic CRSwNP (ENP) and non-eosinophilic CRSwNP (NENP).1
Recently, a new CRS phenotype named central compartment atopic disease (CCAD) was identified by DelGaudio and colleagues.2 CCAD is a nasal inflammatory subtype of CRSwNP that involves polypoid changes of the central compartment of the nasal cavity including middle turbinate (MT), superior turbinate (ST), and/or posterosuperior nasal septum (PSNS).2 CCAD is also defined as an inflammatory process that results from inhalant allergy, which provides a definitive link between allergy and CRSwNP.3 The involvement of the central compartment area may be due to the deposition of allergen. Previous reports have showed that the MT and NS were the most frequent sites where allergens deposited.3,4 The prevalence of allergy was higher (97.6%) while the prevalence of asthma was lower (17.1%) in CCAD patients than other CRSwNP subtypes.5
The presence of eosinophilic and non-eosinophilic endotypes among patients with CRSwNP varies with geographical region.1,6,7 Caucasian nasal polyps are mostly associated with eosinophilia, whereas less eosinophilic and more neutrophilic inflammation was identified in patients with CRSwNP in Asian countries.8 Moreover, compared with patients with CRSwNP in North America and Europe, patients with CRSwNP in China demonstrate lower comorbidity of AR, asthma, and aspirin exacerbated respiratory disease.9 Different inflammatory cell endotypes contribute to distinct treatment strategy.10 CCAD, as a new phenotype of CRS, has been studied in the North America and Australia previously,2,5,11 while the investigation of CCAD in Chinese populations has been limited. Moreover, current understanding of CCAD lacks detailed recognition of its cellular endotypes. The specific tissue inflammatory cells are excellent biomarkers for predicting disease severity and prognosis.12,13 Eosinophilia is related to higher polyp recurrence14–16 and worse olfactory function.17–19 Whereas neutrophilia is linked with corticosteroid sensitivity and more severe surgical outcomes.20–22 Thus, it is critical to give a more detailed description of CCAD histopathology.
In the current study, we aim to investigate the clinical presentations and cellular endotypes of CCAD in Chinese population based on whole-slide imaging (WSI) scanning. By clarifying the cellular endotypes of Chinese CCAD, we would have a better understanding of the histopathology in CCAD, and proper management with allergy control and topical steroids will be recommended for treatment strategies.
Materials and Methods
Subjects and Samples Collection
A retrospective analysis was performed from January to December 2019 in the Third Affiliated Hospital of Sun Yat-Sen University. The study was registered at the Chinese Clinical Trials Registry (http://www.chictr.org.cn/index.aspx) with the number ChiCTR2100048658, and was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-Sen University [(2020) 02–001-01]. This research was conducted with written informed consent from each subject. This study was conducted in accordance with the Declaration of Helsinki.
The diagnosis of CRSwNP was made according to the current European Position Paper on Rhinosinusitis and Nasal Polyps 2020.7 Eosinophilic CRSwNP was defined when the percentage of tissue eosinophils exceeded 10% of total infiltrating cells.1 CCAD was identified as previously reported,2,11 positive inhalant allergen test was accompanied with polypoid changes of MT, ST, and PSNS. These central compartment polypoid changes are evident both with nasal endoscopy and radiographic imaging. Patients with sinonasal polyps but without a polypoid change in the central compartment, or not meeting the criteria for aspirin exacerbated respiratory disease (AERD), and AFRS were listed as CRSwNP not otherwise specified.11 Patients with polypoid changes in the central compartment and concomitant sinonasal polyps were also analyzed with the CCAD cohort.11 Allergic rhinitis was diagnosed based on the concordance between a typical history of allergic symptoms and positive atopy test.23 Asthma was diagnosed based on Global Initiative for Asthma guideline.24 Patients who had an antrochoanal polyp, fungal sinusitis, cystic fibrosis, inverted papilloma, vasculitis, primary ciliary dyskinesia, aspirin exacerbated respiratory disease, or an acute respiratory tract infection were excluded from the study. Finally, 14 CCAD patients, 32 ENP subjects, and 26 patients with NENP were included. The recruitment criteria of participants is outlined by the Figure 1.
Clinical data was obtained through a review of the electronic medical record for each subject, including age, sex, disease duration, comorbidity of asthma and/or allergic rhinitis, allergy testing, endoscopic findings, computed tomography imaging data, operative reports, follow-up visits, pathology findings. Medication use with oral steroids, steroid sprays, and antibiotics were tracked for the period of continuous follow-up for all patients. Intranasal steroid sprays and oral glucocorticoids were discontinued at least 1 month and 3 months before Surgery, respectively. The demographic characteristics of enrolled subjects are listed in Table 1.
 |
Table 1 Demographic Characteristics of Enrolled Patients |
All recruited patients had received intranasal steroid spray no less than 3 months. Short-term oral medication is considered for symptomatic exacerbations of inflammation (steroid) or infection (antibiotic). These patients failed medical treatment (the symptoms existed in despite of the medical treatment mentioned). The surgery procedure was performed by one chief physician. Generally, ENP and NENP require conventional or extended functional endoscopic sinus surgery to optimize ventilation and drainage of involved sinuses via widening of sinus ostia and removal of inflamed tissue.10 For CCAD, because MT function is supposed to be critical in allergen filtering and the central compartment structures, the conservative approach focused on sculpting central compartment structures with the removal of polyps was recommended. Opening of diseased sinuses is also performed if they are involved.11 Postoperative standard maintenance therapy in any CRSwNP subtype typically includes steroid sprays, oral steroids, antibiotics, saline irrigation, and regular clinic follow-up for interval nasal endoscopy.
Symptoms and Endoscopic Assessment
Symptoms of CCAD, ENP, and NENP including nasal obstruction, rhinorrhea, hyposmia, facial fullness or pain, and Headache were assessed and total symptoms were scored based on the visual analogue scale (VAS) according to the symptom severity.7,25 All patients underwent nasal endoscopy, and the Lund-Kennedy (L-K) endoscopic score was obtained by evaluating the endoscopic appearances as previous reported.26 The endoscopic scores were evaluated by two otorhinolaryngologists who were blinded to the grouping information.
Qualitative Analysis of Computed Tomography
All subjects underwent CT scanning. As previously reported, the L-M score was assessed by evaluating the opacification of sinus.27 The results were harvested by two different otorhinolaryngologists who were blinded to the grouping information.
Allergen Test and Atopy Status
Aeroallergen sensitization was evaluated by serologic assessment as previously described.28 The specific IgE (sIgE) to airborne antigen was examined by allergy screening panel (Mediwise Analytic GmbH, Moers, Germany). Serum sIgE > 0.35 IU/mL was considered as positive. Appropriate positive and negative controls were employed. The patients were refrained from antihistamine administration for at least 7 days before serum testing. The patients were diagnosed as atopic if the serology test of any aeroallergen was positive.
Histology Assessment
The pathology samples were fixed in 10% formalin and were subjected to embedding and sectioned. Hematoxylin-eosin-stained paraffin sections (4 μm) were used to assess infiltration of eosinophils and inflammatory cells. Next, slides were scanned with an automatic digital slide scanner (Panoramic 250 FLASH, 3DHISTECH Ltd, Budapest, Hungary) to obtain the WSI. After that, the WSI was analyzed using artificial intelligence chronic rhinosinusitis evaluation platform 2.0 (AICEP 2.0), which could distinguish eosinophils, neutrophils, lymphocytes and plasma cells in nasal mucosa, as described in our previous report.29 Last, the proportions of eosinophil, neutrophil, lymphocyte and plasma cell were harvested by WSI for further analysis.
Statistical Analysis
SPSS 21 was used for statistical analysis. For continuous variables, results were presented as medians and interquartile ranges, unless stated specifically. The Kruskal–Wallis H-test was used to evaluate intergroup variability, and the Mann–Whitney U 2-tailed test was used for between-group comparison. The Chi-square test was used to compare differences in frequencies between groups. All statistical tests were two-sided, and a P value of less than 0.05 was considered statistically significant.
Results
Patients and Clinical Presentations
A total of 89 subjects were recruited for this study. Among these subjects, 10 patients who had fungal sinusitis or inverting papilloma were excluded. Seven patients whose clinical data was missing were also excluded. In total, 14 subjects with CCAD, 32 patients with ENP and 26 patients with NENP were included (Figure 1).
The demographic characteristics among groups are listed in Table 1. No significant differences in gender, age, or smoking status were found between these three groups. Interestingly, the disease duration (months) of CCAD group (24.00; 3.75–36.00) was significantly shorter than that of ENP (96.00; 27.00–120.00, P = 0.008) and NENP (48.00; 12.00–120.00, P = 0.052). The presence of inhalant allergen sensitization was obviously elevated in CCAD (100.00%) population relative to ENP (39.39%) and ENP (24.00%) populations. A greater proportion of the CCAD group (57.14%) had allergic rhinitis (AR) compared with the ENP (21.88%, P = 0.045) and NENP (0.00%, P < 0.001) groups. However, the prevalence of asthma had no significant difference among groups.
The Endoscopic Features of CCAD
On endoscopic examination, CCAD typically demonstrated mucosal inflammation and polypoid edema of the central sinonasal compartment (Figure 2A). While nasal polyps were present in the middle meatus in ENP and NENP groups (Figure 2B and C). Consistent with the endoscopic findings, the L-K scores for polyps and discharge in CCAD group were much lower than those in ENP and NENP groups (Table S1). The total L-K scores were also significantly decreased in the CCAD group (5.50; 2.00–6.50) when compared with those in ENP (7.00; 6.00–10.00, P = 0.016) and NENP (8.00; 5.00–10.00, P = 0.018) groups (Table S1).
The Central Radiological Pattern of CCAD
The radiological presentations of CCAD demonstrated lesions usually associated with central compartment, which involved the medial and floor of the sinus (Figure 3). In contrast, the lesions in ENP and NENP usually showed a diffused pattern (Figure 3). L-M scores among groups were listed in Table S2. In line with the findings of CT, the scores of frontal sinus, maxillary sinus, anterior ethmoid sinus, OMC, and total scores in CCAD population were significantly lower when compared with those in ENP and NENP (Figure 3 and Table S2). However, the scores of posterior ethmoid sinus, sphenoid sinus, and E/M ratio were only significantly reduced in CCAD group compared to those in ENP group (Figure 3 and Table S2). Consistent with previous report, we found E/M ratio is significantly increased in ENP group when compared with NENP group.
The Allergic Phenotype of Chinese CCAD
The presence of inhalant allergen sensitization was 100% in CCAD population (Figure 4 and Table 1). D. pteronyssinus was the most prevalent aeroallergen (71.43%) in Chinese CCAD patients, followed by cockroach (35.71%), mulberry (35.71%), grass pollen mix (28.57%), tree pollen mix (28.57%) and amaranth (28.57%) (Figure 4). The positive rate of D. pteronyssinus in CCAD group (71.43%) was significantly increased than that in both ENP (31.25%) and NENP (15.38%) populations (Figure 4 and Table S3). The presence of other aeroallergens including mulberry, cockroach, and grass pollen mix ambrosia in CCAD group were also dramatically elevated relative to those in both ENP and NENP populations (Figure 4 and Table S3). The positive rates of amaranth and tree pollen mix, however, were only elevated in CCAD group when compared with that in ENP subjects. We did not find any differences in positive rates of house dust, cat and dog hair and dander, or mold mix among groups (Figure 4 and Table S3).
Relatively Mild Symptoms in CCAD Patients
The total scores of VAS in CCAD group (13.50; 7.75–19.00) were less than those in both ENP (20.00; 16.25–22.75, P < 0.001) and NENP (16.50; 12.00–21.00, P = 0.046) groups (Table S4). In addition, the symptom scores for olfactory dysfunction, rhinorrhea, and facial pain/pressure were all significantly reduced in CCAD population when compared those in ENP group (Table S4). This was consistent with previous report that the olfactory function of CCAD patients was relatively better than CRSwNP not otherwise specified patients.
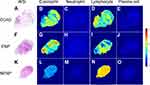
Eosinophil-Dominant Histopathological Characteristics of CCAD Based on AI Deep Learning
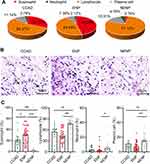
To investigate the cellular endotypes of CCAD, the infiltration status of four inflammatory cells (eosinophils, neutrophils, lymphocytes, and plasma cells) was evaluated by deep learning. The panoramic display of histopathological characteristics of biopsy tissues from patients with CCAD, ENP and NENP based on WSI were shown (Figure 5). Eosinophils and lymphocytes were the major populations in both CCAD and ENP groups (Figures 5 and 6). The proportions of eosinophils, neutrophils, lymphocytes, and plasma cells in patients with CCAD are 26.55% (20.01%, 40.03%), 0.49% (0.00%, 6.75%), 60.85% (45.31%, 66.80%), and 7.33% (4.81%, 11.32%), respectively (Figure 6A and Table S5). The percentage of eosinophils in patients with CCAD is slightly decreased when compared with ENP, but is obviously increased when compared with NENP (Figure 6B and C). In contrast, the frequencies of lymphocytes and plasma cells in CCAD patients are decreased when compared with that in patients with NENP (Figure 6C). No significant differences in the percentages of the neutrophils, lymphocytes, and plasma cells were found between patients with CCAD and patients with ENP (Figure 6C). These results demonstrated that tissues from CCAD patients share similar cellular endotypes from patients with ENP.
Altered Peripheral Blood Eosinophil Account in Patients with CCAD
To test whether CCAD is a systemic eosinophil-dominant inflammatory disease, the percentage and absolute number of eosinophils from peripheral blood was evaluated among groups. We found that the percentage, but not the absolute number of eosinophils was increased in the CCAD group when compared with NENP group. We did find that the absolute number of eosinophils in CCAD population was decreased when compared with ENP group (Table S6). The absolute numbers and percentages of white blood cells, neutrophils, and lymphocytes showed no significant differences among groups. These results displayed that CCAD is a mucosal inflammation that dominated by both peripheral blood eosinophils as well as tissue eosinophils.
Discussion
Our study, for the first time, reports the clinical characteristics and cellular endotypes based on WSI of CCAD in Chinese populations. We found that all CCAD patients have aeroallergen sensitization status, and have lower radiological and endoscopic scores when compared to patients with ENP and NENP. More importantly, the infiltration status of four major inflammatory cells (eosinophils, neutrophils, lymphocytes, and plasma cells) was evaluated by deep learning in nasal biopsies from patients with CCAD, ENP and NENP. To the best of our knowledge, for the first time, we have shown an eosinophil-dominant inflammatory pattern based on WSI.
Due to the paucity of sinus involvement, lower endoscopic and radiological scores, as well as milder symptoms were found in Chinese CCAD patients relative to subjects with ENP and NENP. These results are in line with previous reports conducted by P. Brunner.30 However, with advanced stages of CCAD, increased postobstructive changes in the sinuses may contribute to higher L-M scores and more radiologic difficulty distinguishing advanced CCAD from other forms of CRSwNP subtypes. Interestingly, results of olfactory function in CCAD patients from different groups seem to be contradictory. Research from McCormick et al31 and ours showed olfactory function in CCAD group was better than in patients with ENP and NENP. These results could be explained by the mild and limited lesions in the CCAD group. However, a study from Taiwan demonstrated that hyposmia or anosmia was the major symptom and more common in the central compartment-type CRS group.32 The distinct presentations of olfactory dysfunction may attribute to the recruited patients with different stages of CCAD.
In the current study, the proportions and distributions of distinct inflammatory cells were dissected by AICEP 2.0 in Chinese CCAD patients. CCAD in Chinese population showed a moderate tissue eosinophil infiltration [26.55% (20.01%, 40.03%)] compared to ENP [47.21% (25.23%, 62.95%)] and NENP [1.84% (0.64%, 4.50%)]. Due to the moderated eosinophilia and milder symptoms in CCAD patients compared with those in ENP patients, CCAD may be identified as the early stage of ENP. Thus, an early and aggressive intervention of CCAD might be beneficial in slowing down ENP progression. Similarly, the eosinophil percentage/absolute number in the peripheral blood from CCAD patients was greater than that in NENP subjects, but less than that in ENP subjects. These results suggest the association of blood eosinophil and tissue eosinophil infiltration.33,34 Consist with CCAD patients from Australia, America and Taiwan,35,36 Chinese CCAD also demonstrates an eosinophil-dominant type 2 inflammation. This may because type 2 signature disease in patients with CRS has evidently increased over the last 20 years, and an going “eosinophilic shift” is happening in Chinese CRS. In addition to eosinophils, infiltration of neutrophils, lymphocytes, and plasma cells was also analyzed in CCAD. Neutrophils, associated with worse prognosis and steroid insensitivity of CRS, have attracted more attentions.37,38 Chinese CCAD in southern China (Guangzhou) did not exhibit neutrophil infiltration, which further supports that CCAD is a type 2 inflammation condition and have a better progression. Therefore, CCAD could be a candidate for the biologics targeting type 2 inflammation.
Our study displayed that the prevalence of asthma in Chinese CCAD is 14.29%. Research from Marcus and colleagues demonstrated that the asthma prevalence was 17.1% in CCAD patients and 37.1% in CRSwNP not otherwise specified patients.5 One explanation for low asthma prevalence in CCAD is that the central compartment acts as a filter where aeroallergens deposit most. Only when the system overloads, does the protection shield of the lower airway decreases and asthma may manifest.5
In the current study, we found AR prevalence is 57.14% for CCAD subjects. These results were in accordance with study from America in 2020 (CCAD allergy prevalence = 97.6%).5 More importantly, we have dissected aeroallergen spectrum and demonstrated that D. pteronyssinus was the most dominant aeroallergen (71.43%) in Chinese CCAD patients. In southern China, dust mite was the most dominant allergen in patients with AR or allergic asthma.39–41 Our previous study in 2016 (n = 4085) demonstrated that the top three dominant aeroallergens for AR were house dust mites (84.4%), pet allergen (23.4%), and cockroaches (21.1%).40 Whereas research for Sichuan (Southwest of China) showed that the mite mix positive rate was 13.38% and cockroach positive rate was 2.08%.41 These results suggest patients from different areas may have distinct aeroallergen profiles. Potential function of aeroallergen, especially house dust mite, may exist and drive the eosinophil-dominant inflammatory process of CCAD.
As previously reported by our team,29,42 sampling errors were usually present based on 5 or 10 random HPFs in the diagnosis of CRSwNP. Furthermore, counting of inflammatory cells in WSI is time-consuming and laborious. Therefore, AICEP 2.0 was applied to analyze the cellular endotype of CCAD in Chinese population. In the current study, AICEP 2.0 is the first AI used to quantify distinct inflammatory cells including eosinophils, neutrophils, lymphocytes, and plasma cells based on WSI and to evaluate the density of each inflammatory cell distribution in CCAD patients. Dissection of the proportion and distribution of inflammatory cells in nasal biopsy may facilitate CCAD diagnosis and provide more tailored treatment. Since AICEP 2.0 only takes a few minutes to diagnose a patient, so it can help doctors make diagnoses and decisions more efficiently and effectively. Therefore, the use of AICEP 2.0 could save some manpower and material resources in China, especially in regions with high populations but limited doctors.
Our present study has several limitations. First, the sample size was limited and external validity of the results was missing. Some subjects failed to be included due to lacking of CT findings, endoscopic records or serology IgE test results. Thus, a larger cohort of patients and multi-center study should be conducted to further dissect characteristics of Chinese CCAD at the next opportunity. Second, sIgE and type 2 inflammation associated cytokines in nasal tissue were not detected. Identification of these biomarkers is a noteworthy research objective in the future work. It would aid in understanding of the pathophysiological mechanism and the selection of treatment strategies. Third, we did not evaluate the surgical outcome or drug prognosis of patients with CCAD. The eosinophilic nasal polyps usually exhibit a higher recurrence rate, but is sensitive to steroids and biologics such as dupilumab, mepolizumab, and omalizumab. Whether eosinophil-dominant-CCAD subjects can also benefit from steroids and biologics, or have the recurrent surgical outcomes need to be further studied in the future work.
In conclusion, Chinese CCAD is an eosinophil-dominant inflammatory pattern based on WSI, which was closely associated with aeroallergen sensitivity. CCAD patients in the present study usually display mild clinical symptoms and endoscopic and radiologic findings. CCAD may be identified as the early stage of ENP. Thus, proper management with allergy control and topical steroids could be recommended for CCAD treatment.
Data Sharing Statement
The data (age, gender, disease duration, grouping information, endoscopic surgery history, asthma status, allergic rhinitis status, smoking history, visual analogue scale of sinonasal symptoms, endoscopic Lund-Kennedy score, CT Lund- Mackay score, blood cell absolute counts and percentages, allergen test results) of this study are available on request from the corresponding author (Yana Zhang, e-mail: [email protected]). The data will also be available at Chinese Clinical Trials Registry (http://www.chictr.org.cn/index.aspx) with the number ChiCTR2100048658.
Acknowledgments
The authors would like to thank Dr. Chunkui Shao and Dr. Jianning Chen (Department of Pathology, The Third Affiliated Hospital of Sun Yat-Sen University) for scanning whole-slide imaging, as well as Dr. Shuvam Chaudhuri (Department of Pathology, Northwestern University Feinberg School of Medicine) for his careful and thorough English editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; and gave final approval of the version to be published. All authors have agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82171114, 82000958, U20A20399 and 81870704), the Key-area Research and Development Program of Guangdong Province (No. 2020B0101130015), the Guangdong Provincial Key Laboratory of Digestive Cancer Research (No. 2021B1212040006), Sun Yat-sen University Clinical Research 5010 Program (No. 2019006) and The Natural Science Foundation of Guangdong Province (No. 2021A1515011764).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124(3):
2. DelGaudio JM, Loftus PA, Hamizan AW, Harvey RJ, Wise SK. Central compartment atopic disease. Am J Rhinol Allergy. 2017;31(4):228–234. doi:10.2500/ajra.2017.31.4443
3. DelGaudio JM. Central compartment atopic disease: the missing link in the allergy and chronic rhinosinusitis with nasal polyps saga. Int Forum Allergy Rhinol. 2020;10(10):1191–1192. doi:10.1002/alr.22663
4. Zhang Y, Zhang LY, Huang F, et al. [Computational investigation of Artemisia pollen deposition in realistic nasal cavities of residents in northwest China]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;54(10):741–747. doi:10.3760/cma.j.issn.1673-0860.2019.10.007. Chinese.
5. Marcus S, Schertzer J, Roland LT, Wise SK, Levy JM, DelGaudio JM. Central compartment atopic disease: prevalence of allergy and asthma compared with other subtypes of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2020;10(2):183–189. doi:10.1002/alr.22454
6. Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138(5):1344–1353. doi:10.1016/j.jaci.2016.05.041
7. Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020;58(SupplS29):1–464. doi:10.4193/Rhin20.600
8. Zhang Y, Gevaert E, Lou H, et al. Chronic rhinosinusitis in Asia. J Allergy Clin Immunol. 2017;140(5):1230–1239. doi:10.1016/j.jaci.2017.09.009
9. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015;136(6):1431–1440. doi:10.1016/j.jaci.2015.10.010
10. Cardell LO, Stjarne P, Jonstam K, Bachert C. Endotypes of chronic rhinosinusitis: impact on management. J Allergy Clin Immunol. 2020;145(3):752–756. doi:10.1016/j.jaci.2020.01.019
11. Steehler AJ, Vuncannon JR, Wise SK, DelGaudio JM. Central compartment atopic disease: outcomes compared with other subtypes of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2021;11(11):1549–1556. doi:10.1002/alr.22819
12. Nakayama T, Yoshikawa M, Asaka D, et al. Mucosal eosinophilia and recurrence of nasal polyps - new classification of chronic rhinosinusitis. Rhinology. 2011;49(4):392–396. doi:10.4193/Rhino10.261
13. Steinke JW, Smith AR, Carpenter DJ, Patrie JT, Payne SC, Borish L. Lack of Efficacy of Symptoms and Medical History in Distinguishing the Degree of Eosinophilia in Nasal Polyps. J Allergy Clin Immunol Pract. 2017;5(6):1582–1588 e3. doi:10.1016/j.jaip.2017.04.009
14. Lou H, Meng Y, Piao Y, Wang C, Zhang L, Bachert C. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am J Rhinol Allergy. 2015;29(5):350–356. doi:10.2500/ajra.2015.29.4231
15. McHugh T, Snidvongs K, Xie M, Banglawala S, Sommer D. High tissue eosinophilia as a marker to predict recurrence for eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2018;8(12):1421–1429. doi:10.1002/alr.22194
16. Nakayama T, Sugimoto N, Okada N, et al. JESREC score and mucosal eosinophilia can predict endotypes of chronic rhinosinusitis with nasal polyps. Auris Nasus Larynx. 2019;46(3):374–383. doi:10.1016/j.anl.2018.09.004
17. Lou H, Meng Y, Piao Y, et al. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology. 2016;54(2):150–159. doi:10.4193/Rhino15.271
18. Ahn SH, Lee EJ, Ha JG, et al. Comparison of olfactory and taste functions between eosinophilic and non-eosinophilic chronic rhinosinusitis. Auris Nasus Larynx. 2020;47(5):820–827. doi:10.1016/j.anl.2020.04.006
19. Liang Z, Yan B, Liu C, Tan R, Wang C, Zhang L. Predictive significance of arachidonate 15-lipoxygenase for eosinophilic chronic rhinosinusitis with nasal polyps. Allergy Asthma Clin Immunol. 2020;16:82. doi:10.1186/s13223-020-00480-8
20. Kim DK, Kim JY, Han YE, et al. Elastase-Positive Neutrophils Are Associated With Refractoriness of Chronic Rhinosinusitis With Nasal Polyps in an Asian Population. Allergy Asthma Immunol Res. 2020;12(1):42–55. doi:10.4168/aair.2020.12.1.42
21. De Corso E, Settimi S, Tricarico L, et al. Predictors of Disease Control After Endoscopic Sinus Surgery Plus Long-Term Local Corticosteroids in CRSwNP. Am J Rhinol Allergy. 2021;35(1):77–85. doi:10.1177/1945892420936196
22. Kim DK, Lim HS, Eun KM, et al. Subepithelial neutrophil infiltration as a predictor of the surgical outcome of chronic rhinosinusitis with nasal polyps. Rhinology. 2021;59(2):173–180. doi:10.4193/Rhin20.373
23. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102(4 Pt 1):558–562. doi:10.1016/s0091-6749(98)70271-4
24. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63:8–160. doi:10.1111/j.1398-9995.2007.01620.x
25. van der Veen J, Seys SF, Timmermans M, et al. Real-life study showing uncontrolled rhinosinusitis after sinus surgery in a tertiary referral centre. Allergy. 2017;72(2):282–290. doi:10.1111/all.12983
26. Lund VJ, Kennedy DW. Quantification for staging sinusitis. The Staging and Therapy Group. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. doi:10.1177/000348949510410s02
27. Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–184.
28. Zhang YN, Song J, Wang H, et al. Nasal IL-4(+)CXCR5(+)CD4(+) T follicular helper cell counts correlate with local IgE production in eosinophilic nasal polyps. J Allergy Clin Immunol. 2016;137(2):462–473. doi:10.1016/j.jaci.2015.07.025
29. Wu Q, Chen J, Ren Y, et al. Artificial intelligence for cellular phenotyping diagnosis of nasal polyps by whole-slide imaging. EBioMedicine. 2021;66:103336. doi:10.1016/j.ebiom.2021.103336
30. Brunner JP, Jawad BA, McCoul ED, Polypoid Change of the Middle Turbinate and Paranasal Sinus Polyposis Are Distinct Entities. Otolaryngol Head Neck Surg. 2017;157:3. 519–523. doi:10.1177/0194599817711887
31. McCormick JP, Thompson HM, Cho DY, Woodworth BA, Grayson JW. Phenotypes in Chronic Rhinosinusitis. Curr Allergy Asthma Rep. 2020;20(7):20. doi:10.1007/s11882-020-00916-6
32. Lin YT, Lin CF, Liao CK, Chiang BL, Yeh TH. Clinical characteristics and cytokine profiles of central-compartment-type chronic rhinosinusitis. Int Forum Allergy Rhinol. 2021;11(7):1064–1073. doi:10.1002/alr.22759
33. Tokunaga T, Sakashita M, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70(8):995–1003. doi:10.1111/all.12644
34. Lou H, Zhang N, Bachert C, Zhang L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int Forum Allergy Rhinol. 2018;8(11):1218–1225. doi:10.1002/alr.22214
35. Grayson JW, Cavada M, Harvey RJ. Clinically relevant phenotypes in chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2019;48(1):23. doi:10.1186/s40463-019-0350-y
36. Grayson JW, Hopkins C, Mori E, Senior B, Harvey RJ. Contemporary Classification of Chronic Rhinosinusitis Beyond Polyps vs No Polyps: a Review. JAMA Otolaryngol Head Neck Surg. 2020;146(9):831–838. doi:10.1001/jamaoto.2020.1453
37. Ruan JW, Zhao JF, Li XL, et al. Characterizing the Neutrophilic Inflammation in Chronic Rhinosinusitis With Nasal Polyps. Front Cell Dev Biol. 2021;9:793073. doi:10.3389/fcell.2021.793073
38. Poposki JA, Klingler AI, Stevens WW, et al. Elevation of activated neutrophils in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2021. doi:10.1016/j.jaci.2021.11.023
39. Luo W, Hu H, Tang W, et al. Allergen sensitization pattern of allergic adults and children in southern China: a survey based on real life data. Allergy Asthma Clin Immunol. 2019;15:42. doi:10.1186/s13223-019-0357-y
40. Wang W, Huang X, Chen Z, et al. Prevalence and trends of sensitisation to aeroallergens in patients with allergic rhinitis in Guangzhou, China: a 10-year retrospective study. BMJ Open. 2016;6(5):e011085. doi:10.1136/bmjopen-2016-011085
41. Huang Z, Feng W, Wei W, Yang B, Wang L. Prevalence of food-allergen and aeroallergen sensitization among people in Sichuan, Western China: an 8-year observational study. J Clin Lab Anal. 2019;33(3):e22723. doi:10.1002/jcla.22723
42. Wu Q, Chen J, Deng H, et al. Expert-level diagnosis of nasal polyps using deep learning on whole-slide imaging. J Allergy Clin Immunol. 2020;145(2):698–701 e6. doi:10.1016/j.jaci.2019.12.002
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.