Back to Journals » International Journal of Nanomedicine » Volume 14
Chimeric hepatitis B virus core particles displaying Neisserial surface protein A confer protection against virulent Neisseria meningitidis serogroup B in BALB/c mice
Authors Hou Y, Yan T, Cao H, Liu P, Zheng K, Li Z, Deng Q, Hu S
Received 21 February 2019
Accepted for publication 1 August 2019
Published 16 August 2019 Volume 2019:14 Pages 6601—6613
DOI https://doi.org/10.2147/IJN.S206210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
YongLi Hou,1 Ting Yan,2 Hui Cao,1 Peng Liu,1 Kang Zheng,1 Zhenyu Li,1 Qing Deng,1 SiHai Hu1
1Hunan Provincial Key Laboratory for Special Pathogens Prevention and Control, Institution of Pathogenic Biology, Hengyang Medical College, University of South China, Hengyang 421001, People’s Republic of China; 2Department of Health Services, Air Force Medical University, Xi’an, Shaanxi 710032, People’s Republic of China
Correspondence: SiHai Hu
Institution of Pathogenic Biology, Hengyang Medical College, University of South China, 28 West Changsheng Road, Hengyang 421001, Hunan, People’s Republic of China
Tel +86 1 397 340 7567
Fax +86 734 828 2907
Email [email protected]
Purpose: The primary goal of the present study was to explore and evaluate the highly conserved Neisserial surface protein A (NspA) molecule, fused with truncated HBV virus-like particles (VLPs), as a candidate vaccine against the virulent Neisseria meningitidis serogroup B (NMB).
Methods: NspA was inserted into the major immunodominant region of the truncated hepatitis B virus core protein (HBc; amino acids 1–144). The chimeric protein, HBc-N144-NspA, was expressed from a prokaryotic vector and generated HBc-like particles, as determined by transmission electron microscopy. Further, the chimeric protein and control proteins were used to immunize mice and the resulting immune responses evaluated by flow cytometry, enzyme-linked immunosorbent assay, and analysis of serum bactericidal activity (SBA) titer.
Results: Evaluation of the immunogenicity of the recombinant HBc-N144-NspA protein showed that it elicited the production of high levels of NspA-specific total IgG. The SBA titer of HBc-N144-NspA/F reached 1:16 2 weeks after the last immunization in BALB/c mice, when human serum complement was included in the vaccine. Immunization of HBc-N144-NspA, even without adjuvant, induced high levels of IL-4 and a high IgG1 to IgG2a ratio, confirming induction of an intense Th2 immune response. Levels of IL-17A increased rapidly in mice after the first immunization with HBc-N144-NspA, indicating the potential for this vaccine to induce a mucosal immune response. Meanwhile, the immunization of HBc-N144-NspA without adjuvant induced only mild inflammatory infiltration into the mouse muscle tissue.
Conclusion: This study demonstrates that modification using HBc renders NspA a candidate vaccine, which can trigger protective immunity against NMB.
Keywords: hepatitis B core protein, Neisserial surface protein A, virus-like particles, recombinant protein vaccine, Neisseria meningitidis serogroup B
Introduction
Neisseria meningitidis is an aerobic gram-negative bacterium and an obligate human parasite that can cause pyogenic infection.1,2 Septicemia can lead to invasion through the blood–brain barrier (BBB), resulting in brain and spinal cord injuries and even causing permanent brain damage. Monitoring data show that China experienced a five-fold increase in the prevalence of serogroup A meningococcal disease (MenA) from 1939 to 1978; however, MenA has been associated with decreased morbidity since the application of the MenA polysaccharide vaccine in China in the 1980s.3 Currently, due to the low immunogenicity of the capsular polysaccharide of MenB, this is the most common strain responsible for epidemics, and outbreaks of serogroup B meningococcal disease have become a global health problem.4 Therefore, it is necessary to develop an effective vaccine to prevent MenB. MenB vaccines based on outer membrane vesicles have become the focus of considerable research efforts. With the introduction of reverse vaccinology, some minor highly conserved proteins have been identified based on the analysis of the N. meningitidis serogroup B (NMB) genome, using data from the Molecular Biology Software and Genome Database (GDB). Published data show that a MenB vaccine based on recombinant proteins can elicit a robust bactericidal immune response against a broad range of serogroup B isolates in adults, adolescents, and infants.5 However, the first new vaccine, termed 4CmenB, could not confer protection against all invasive MenB strains. In addition, it is not yet possible to accurately determine the most effective components of this vaccine against meningitis. Furthermore, the genetic diversity of group B meningococcus means that not all MenB strains contain genes encoding each antigen, and the expression of antigens can vary with time or location.6 Thus, a highly conserved antigen is crucial for the development of a new, efficient vaccine.
Neisserial surface protein (NspA), which was previously identified by Martin et al in 1997, is expressed in approximately 90% NMB strains examined to date.7 An NspA-specific monoclonal antibody (mAb), referred to as Me-1, reacts with 99% of the meningococcal strains tested, indicating that the epitope recognized by this particular mAb is widely distributed and highly conserved. NspA is a highly immunogenic antigen against all pathogenic Neisserial serogroups in mice, and there is evidence that it belongs to the OPa protein family, which mediates cell adhesion. In a mouse model, NspA induced a protective immune response against serogroups A, B, and C.8 Published data showed that NspA can readily access the surface of the cell to evoke complement-mediated bactericidal activity via anti-NspA mAbs. These characteristics indicate that NspA is an attractive candidate for a broad-range effective meningococcal vaccine and is efficient in eliciting serum bactericidal activity (SBA). Moreover, a Phase I clinical trial of a recombinant Neisseria surface protein A (rNspA) vaccine showed that unfolded rNspA meningococcal vaccine was well tolerated and immunogenic in healthy adult volunteers; however, it did not elicit bactericidal antibodies. This failure to elicit bactericidal antibodies is considered to have occurred because NspA binds complement regulator factor H (FH), thereby inhibiting complement system activation on the cell surface.9 This result was not anticipated but has inspired additional studies. The structure of an outer surface protein of Borrelia burgdorferi, CspZ, which also acts as a complement inhibitor, was modified by Ashley et al, via fusion to virus-like particles (VLPs), generating modified VLP-CspZ to eliminate binding to FH. Stronger immunogenic response and greater bactericidal antibody titers were detected in mice vaccinated with modified VLP-CspZ.10 Thus, we investigated whether the modification using VLPs could similarly affect the immunogenicity of NspA in this study.
VLPs have generated tremendous changes in vaccinology since the 1980s, as they can serve as carriers by displaying the products of foreign genes on their surfaces.,11 VLPs play a crucial role in current vaccination and antiviral therapy approaches because they of their numerous important features, including their size (<200 nm in diameter), which allows them to access lymphatic drainage vessels via lymphatic stomata, which are also approximately 200 nm in size, and their highly symmetrical and repetitive surface geometric structures, which allow them to display multiple copies of foreign inserted sequences.12 In addition, VLPs can elicit both innate and adaptive immune response and represent a commercially available and safe template for vaccine development. For example, the current human papillomavirus (HPV) vaccines, Gardasil® and Cervarix®, are based on VLPs derived from the HPV principal shell proteins, L1 and L2.13 In addition, the first malaria vaccine based on a VLP, Mosquirix, has been approved for application in the regular immunization program in African countries.14 The hepatitis B core monomer (HBc) has also been verified as a potential VLP modifier for use in vaccine development; it contains 183 amino acids comprising three vital regions: the N- and C-terminal domains and the immunodominant e1 loop, including a particle assembly region (amino acid (aa) 1–144). Experimental data demonstrate that the e1 loop in the major immunodominant region (MIR) of HBc is a promising site for insertion of foreign sequences. Further, successful assembly is not impaired by the insertion of foreign genes.15,16
The present study shows that truncated HBc can readily form VLPs with abundant spikes covering their surfaces. We describe the construction of a chimeric protein, by insertion of the sequence encoding NspA into a truncated form of HBc, HBc-N144. The resulting complex was termed HBc-N144-NspA and generated a modified NspA structure, which limited the interaction of NspA and FH and could elicit a potent innate or adaptive immune responses following MenB challenge.
Materials and methods
Plasmids, bacterial strains, and cells
The plasmid pET28a HBc-N144 was constructed by truncating the whole hepatitis B virus (HBV) genome at the site of the N-terminal 144 aa and inserting the NspA gene after between aa 78 and 79 of the truncated pET28a-HBc-N144. Overlapping PCR technology was used to generate the fuzed plasmid, pET28a-HBc-N144-NspA, and both steps were performed by Sangon Biotech (Shanghai, China).17 All recombinant plasmids were modified by codon optimization. NMB strain MC58 was purchased from American Type Culture Collection (ATCC; Manassas, VA, USA),which was maintained at was stored at –80°C in the laboratory.
Expression and purification of recombinant proteins
Plasmids encoding modified His-tagged recombinant proteins were transformed into Escherichia coli BL21 (DE3) competent cells. Bacteria were grown overnight at 37°C in Luria–Bertani18 medium containing 50 μg/mL kanamycin. Then, the cultivated cells were inoculated into fresh LB medium at a dilution of 1:100 and incubated in a shaker at 37°C until the optical density at 600 nm (OD600) reached 0.5. Protein expression was induced using different concentrations of isopropyl-β-D-thiogalactopyranoside (IPTG) (0.1 mM, 0.5 mM, and 1 mM). We used the SDS-PAGE to analyze the cellular localization of the recombinant protein. Then, after shaking for 4 hrs, bacteria were centrifuged at 4000 g (4°C, 20 mins), and the resulting pellets resuspended in lysis buffer (pH 8.0, 50 mM NaH2PO4, 10 mM Tris–HCl, 250 mM NaCl, 100 µg/mL PMSF, 1 µg/mL aprotinin) by shaking overnight at 4°C. Bacterial cells were lysed by ultrasonication and then centrifuged at 24,000 g (4°C and 20 mins). Clear supernatants were added to Ni2+ NTA columns (Sangon Biotech), as recommended by the manufacturer.Unbound proteins were removed using wash buffer (50 mM Tris, 100 mM NaCl, and 50 mM imidazole buffer, pH 8.0), and bound proteins were eluted with elution buffer (50 mM Tris, 100 mM NaCl, and 250 mM imidazole buffer, pH 8.0).
Purification and assembly of VLPs
E. coli cells were harvested by centrifugation, resuspended, and sonicated in binding buffer (0.5 M NaCl, 10 mM Tris, and 100 mM NaH2PO4, pH 8.0). VLP inclusion bodies were collected by centrifugation at 4000 g (15 mins, 4°C), and the pellet was resuspended in binding buffer containing 8 M urea and dissolved for 2 hrs at 4°C. After centrifugation, the His-tagged proteins were purified using Ni-NTA His-Bind resin. Then, purified proteins were dialyzed against renaturation buffer (0.15 M NaCl, 20 mM Tris, 0.2 mM glutathione (GSH), and 1 mM oxidized glutathione (GSSG) pH 8.0) for protein renaturation and VLP self-assembly. A gradient of decreasing concentrations of urea (6 M to 0 M in 0.5 M decrements) was applied at 4°C, and the samples were finally dialyzed against PBS (pH 8.0).
Evaluation of purified proteins
All eluted proteins were analyzed by SDS-PAGE, and a Multiscan Spectrum system was used to determine protein characteristics. Purified chimeric proteins were diluted to different concentrations with PBS in preparation for morphologic observation and immunological investigations.
Recombinant NspA
The plasmid encoding recombinant NspA, from the NMB MC58 standard strain genomic DNA (NCBI accession no. NC_003112.2), was transformed into E. coli BL21 (DE3) cells, and different concentrations of IPTG were used to induce rNspA expression. Subsequently, recombinant protein was purified by adding separated bacterial supernatant to Ni2+ NTA columns (Sangon Biotech) and then quantified using a bicinchoninic acid (BCA) assay on a Multiscan Spectrum system (Gatan, Inc, Abingdon, UK).
SDS-PAGE and Western blotting
Electrophoresis was performed using 12–15% SDS-PAGE to characterize the molecular weights of the different recombinant proteins. To confirm the immunological characteristics of the purified proteins, serum samples were diluted with PBS containing Tween 20 (PBST), and samples were collected from immunized mice in the rNspA and HBc-N144-NspA groups, respectively, bound to the anti-His-tag antibody. Briefly, purified proteins were transferred to a Polyvinylidene Fluoride membrane (PVDF). The PVDF membrane was blocked with blocking buffer and incubated with the anti-His antibody.19 Protein bands were visualized using horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (Sigma, St. Louis, MO, USA) and using chemiluminescence kit for detection (GE Healthcare, Chicago, IL, USA)
Electron microscopy
Purified fusion proteins were diluted to different concentrations to determine the morphologies of HBc-N144 and HBc-N144-NspA in VLPs. Diluted samples were placed onto a carbon-coated e(EM) grids and incubated for 10 mins at room temperature. Solutions on EM grids were then removed and samples stained with sodium phosphotungstate for 5 mins, and then observed by transmission electron microscopy (TEM) (Tecnai G2 F30).
Mouse immunization
Specific pathogen-free (SPF) female BALB/c mice (The Center for Animal Experiments of the University of South China), aged 6–8 weeks, were immunized by intramuscular injection (i.m.) of 50 μg of recombinant proteins, HBc-N144, HBc-N144-NspA, and rNspA, three times at 2-week intervals. VLPs (). Negative control was injected with 100 μL of PBS. Freund’s complete adjuvant was used for the first immunization and Freund’s incomplete adjuvant for the two subsequent immunizations. In addition, to examine adjuvant function, we immunized mice with HBc-N144-NspA without any adjuvant. Serum samples and liquid samples from vaginal flushing were collected 0, 2, 4, and 6 weeks after the first immunization for analysis. Two weeks after the final immunization, splenocytes were collected for flow cytometry (FCM) and ELISA. In addition, muscular tissue was collected from injection sites of mice in different groups for pathological analysis. All animal experiments were approved by the Animal Welfare Committee of the University of South China and were conducted in accordance with institutional regulations.
Serological tests
ELISA
Serum and mucosal secreted antibodies specific for rNspA, including IgG, IgG1, and IgG2a, were detected by ELISA. Briefly, rNspA (10 μg/mL) served as the coating antigen, and specific antibodies were collected from immunized mice, serial dilutions made in PBST, and samples then added to ELISA plates coated with rNspA and incubated for 1 hr at 37°C, followed by incubation with HRP-conjugated goat anti-mouse IgG, IgG1, or IgG2a secondary antibodies in each well.Plates were washed five times and developed with tetramethylbenzidine (TMB) solution in the dark for 15 mins. Enzyme reactions were stopped by addition of 2 M H2SO4, and the plates were measured using an OD450 microplate reader (Bio-Rad, Hercules, CA, USA).
Cytokine evaluation
Splenocytes were harvested from each group to evaluate levels of cytokines following stimulation with specific antigens for 48 hrs at 37°C in 5% CO2. Briefly, splenocyte suspensions were prepared from the spleens of individual experimental mice for quantitative measurement of anti-mouse IFN-γ, IL-4, and IL-17A using ELISA kits (eBioscience, Thermo Fisher Scientific Waltham, MA, USA) according to the manufacturer’s instructions.
In vitro SBA
Serum samples from immunized mice in each group were used for the in vitro SBA. MenB (MC58) was cultured on chocolate agar plates at 37°C in 5% CO2 for 24 hrs. To obtain the appropriate bacterial concentration for investigation, the bacterial colonies were collected and blended with prewarmed fastidious broth (FB). The concentration of the bacterial mixture was approximately 3×103 CFU/mL. After heating the mouse serum samples at 56°C for 30 mins, 20 μL of normal human serum was added to the mixed bacteria and serum samples in a serial dilution. Human complement was mixed with the examined serum at a ratio of 1:1. Serum samples were then diluted at ratios of 1:2, 1:4, 1:8, 1:16, 1:32, and 1:64, then cultivated for 24 hrs. Mixtures resulting in a 50% bacterial killing rate were considered positive.
Live NMB challenge
Challenge with NMB (MC58) was conducted during the second week after the final immunization. Briefly, five immunized mice were randomly selected from each group, then injected intraperitoneally with a lethal dose of MC58 and monitored daily for survival for 14 days.
FCM
Splenocytes were resuspended in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin–L-glutamine and seeded at a density of 1×106 cells/well. Then, cells were stimulated for 5 hrs with phorbol 12-myristate 13-acetate (PMA, BD Biosciences) and washed in staining buffer (PBS containing 2% FBS), followed by surface staining with anti-CD3 and anti-CD4 fluorescent antibodies. Next, cells were washed, fixed, and permeabilized using a commercially available Cytofix/Cytoperm kit (BD Biosciences) and stained for intracellular cytokine expression using phycoerythrin-conjugated anti-mouse interferon-γ (IFN-γ) (BD Biosciences) and IL-4 antibodies. After washing, cells were resuspended in staining buffer for analysis using a BD FACS II flow cytometer (BD Biosciences) and FlowJo 7.6.5 software.
Statistical analysis
The significance of the differences was determined by unpaired parametric test (Student’s t-test for two groups or one-way ANOVA for more than three groups). Bacterial burden was analyzed by the nonparametric Mann–Whitney test. Differences were considered significant at the p<0.05 level. GraphPad Prism 5.0 software (San Diego, CA, USA) was used for statistical analysis of the data.
Results
Expression and characterization of HBc-N144-NspA and HBc-N144 VLPs in E. coli BL21 (DE3)
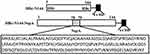
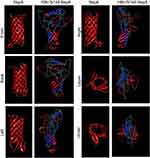
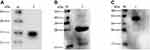
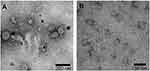
To express HBc-N144-NspA and HBc-N144 VLPs in E. coli BL21 (DE3), we inserted the NspA sequence into the MIR of truncated HBc-N144 (Figure 1), and the plasmid constructs were transformed into prokaryotic cells, as described in the section “Materials and methods.” Ideographs generated using Chimera 2.1, showing the predicted structures of NspA and HBc-N144-NspA, are presented in Figure 2. IPTG was used to induce chimeric protein expression from the pET28a-HBc-N144-NspA (37 kDa) and pET28a-HBc-N144 (16 kDa) plasmids, each of which had His tags at the C-terminus to facilitate purification (Figure S1). Western blotting revealed that the recombinant proteins could be detected using an anti-His-tag antibody (Figure 3). Spherical self-assembling VLPs of 120 nm in diameter were observed by TEM after refolding of purified HBc-N144-NspA protein, while TEM imaging of HBc-N144 revealed a diameter of 30 nm (Figure 4).
Immune responses to the chimeric proteins
ELISA was applied to conduct serological tests on mice following immunization with HBc-N144-NspA/F, rNspA/F, and HBc-N144-NspA. The results demonstrated that HBc-N144-NspA/F stimulated the highest specific antibody titer, relative to the other proteins. Further, we quantified the ratio of IgG1 to IgG2a, to determine the type of immune response elicited. Immunization with HBc-N144-NspA/F, HBc-N144-NspA, and rNspA/F all led to IgG1/IgG2a ratios >1, which were higher than those of control groups. Further, immunization with HBc-N144-NspA induced total levels of IgG almost identical to those detected in the rNspA/F group, including higher levels of IgG1 than IgG2a, which indicates that VLPs were able to stimulate the production of NspA-specific antibodies without assistance from an adjuvant. Immunization with HBc-N144-NspA led to a superior gradual increase in antibody titers, particularly after three immunizations.
We also investigated secretory IgA (sIgA) by testing vaginal secretion samples. Interestingly, although the mice were immunized intramuscularly, immunization with VLPs showed protective efficacy, even in the absence of adjuvant (Figure 5).
Activation of multiple cell types, including B and T cells, is required to elicit an effective immune response. Moreover, the humoral immune response has a vital role in defending against NMB. Thus, fluorescence-activated cell sorting (FACS) was conducted after stimulation of splenocytes harvested from immunized mice with PMA for 5 hrs. The results showed that immunization with HBc-N144-NspA, even without adjuvant, could elicit a higher percentage of NspA-specific IL-4-producing CD4+ T cells than any of the other immunogens tested (Figure 6). Since IL-4-producing T cells have a key role in controlling and clearing MenB in infected individuals, our results indicate that HBc-N144-NspA VLPs can evoke the potent NspA-specific humoral responses that are important for MenB clearance.
Cytokine secretion by rNspA- and HBc-N144-NspA-stimulated T cells
Cytokine levels secreted by rNspA- and HBc-N144-NspA-stimulated spleen cells were measured using cytokine ELISAs. Figure 7 shows that levels of IL-4 and IL-17A rapidly increased in mice following the first immunization with the recombinant VLP protein, HBc-N144-NspA. Levels of secreted IFN-γ were lower than those of IL-4 in the HBc-N144-NspA and rNspA groups. Immunizations with either HBc-N144 or PBS did not induce any significant difference in levels of IL-4 and IFN-γ (Figure 7). Overall, immunization with HBc-N144-NspA and rNspA induced an evident humoral immune response, which is crucial for the clearance of MenB.
MenB challenge
To investigate the effectiveness of the protection stimulated using the chimeric protein, HBc-N144-NspA, mice immunized with different antigens were administered intraperitoneal injections of the same dose of MC58. Mice exhibited few symptoms of infection after 24 hrs; however, animals immunized with PBS and HBC-N144 showed weak immunity to virulent MenB. The mortality of infected mice immunized with PBS and HBC-N144 was approximately 100% after 72 hrs, whereas mice immunized with rNspA showed an 80% survival rate, and immunization with VLPs induced a 90% survival rate, even in the absence of adjuvant, during the 2 weeks of observation (Figure 8).
In vitro SBA
To further test the efficacy of HBc-N144-NspA against MenB, SBA was conducted by collecting serum samples from each immunization group. We cultivated diluted MenB (MC58) on chocolate agar plates to obtain the appropriate bacterial concentration for investigation. The data showed a 1:2 bacterial killing titer after the first immunization in all groups; however, a ratio of 1:16 after three injections with HBc-N144-NspA mixed with Freund’s adjuvant. Immunization with HBc-N144-NspA was effective, with titers up to 1:8, even without adjuvant, which were higher than those of rNspA with adjuvant, while negative controls reached titers of only 1:2 (Table 1). As SBA is the gold standard for measuring defense against MenB, these data demonstrate that immunization with VLPs is a potential strategy for developing a vaccine against this bacterium.
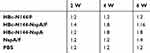 |
Table 1 Bactericidal antibody titers of the immune serum in vitro, the serum samples from the immunized mice were used for evaluating the serum bactericidal activity |
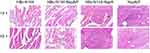
Analysis of local inflammatory reactions
Safety must be considered in immunization programs of approved vaccines, including the induction of weak or limited local inflammatory reactions. Therefore, we collected tissue from the injection sites for biochemical and pathological analyses in the pathology laboratory. Figure 9 shows that vaccination with VLPs without adjuvant generated low numbers of infiltrating inflammatory cells in all groups.
Discussion
A high incidence of epidemic infectious meningitis has been reported worldwide over the past three decades, and current meningococcal vaccines mostly face issues due to the wide variety of meningococcal strains.20 A vaccine currently available against meningococcal serogroup B is the four-component meningococcal serogroup B vaccine (4CMenB), comprising four outer membrane proteins. Further, two licensed vaccines, Bexsero and Trumenba, have been applied for the prevention of MenB, demonstrating high efficacy in vaccine-eligible infants in the UK since 2015.21 Nevertheless, problems with these vaccines have persisted, including the effectiveness of protection, variation in meningococcal strains, and the selection of potential new antigens.22 One important measure for developing a new generation vaccine against NMB is the introduction of a novel carrier that can better transport the antigen, as well as eliciting an immune response. In our study, we selected NspA as a potential vaccine candidate, as well as investigating the efficiency of a new carrier and the effects of the fusion protein, HBc-N144-NspA, on bacterial killing activity in mice.23 NspA is a vital target antigen for the development of vaccines.24 To date, approximately 90% of Neisseria strains have been shown to express this highly conserved outer membrane protein, which was developed in a Phase I trial.13 The characteristics of NspA are undoubtedly advantages for the development of new generation meningococcal serogroup B vaccines; however, NspA is also a human-specific ligand of FH, as inferred from its specific structure.24 A similar situation was reported for the outer surface protein, CspZ, of the spirochete, Borrelia burgdorferi. Modification of CspZ by fusion with VLPs eliminated binding to FH and enhanced immune protection.15 In our study, one purpose of fuzing HBc-N144 with NspA was to evaluate the consequent differences in the structure of the resulting recombinant protein. Therefore, we predicted the three-dimensional structure of HBc-N144-NspA and compared it with that of HBc-like particles. Moreover, we conducted a preliminary investigation of the microscopic structure of HBc-N144-NspA via TEM.
Observation of the predicted configuration of HBc-N144-NspA from different perspectives revealed a sophisticated structure; the fused HBc-N144-NspA protein presented a hairpin structure, consistent with previous investigation of the HBV capsid protein, which can easily assemble into particles. Further, microscopic evaluation of the purified HBc-N144-NspA chimeric protein revealed VLPs. Overall, we infer that HBc-like particles can modify NspA structure, resulting in the formation of HBc-N144-NspA particles.
To investigate the specific effects of NspA modification, we evaluated mice immunized with HBc-N144-NspA and found that injection of this protein yielded the highest levels of total specific IgG generated by any tested immunogen, even in the absence of adjuvant. Indeed, fusion of NspA and the truncated highly repetitive HBc-N144 molecule altered the stereochemical structure of the resulting chimeric protein HBc-N144-NspA, improving the presentation of NspA to antigen-presenting cells and boosting immunity by enhancing bactericidal activity.25 Immune responses are regulated by epitope density and B cell costimulatory thresholds.26,27 Trun cated HBc preferentially provokes Th2 immune responses, which are promoted by the distribution of antigen on the highly repetitive particle surface; we hypothesize that this process enhances the density of NspA, leading to induction of a potent immune response.28
Another interesting finding of this study is that immunization with VLPs can generate efficient mucosal immune responses. Potent mucosal immune responses can only be elicited by oral vaccination,29,30 while N. meningitidis invariably colonizes the nasal mucosa. In our study, mice immunized with HBc-N144-NspA produced high levels of sIgA after i.m.. The mucosa-associated lymphoid tissue is an immune tissue consisting of numerous lymph nodes. HBc-N144-NspA formed into 120 nm diameter particles, which is a suitable size for draining into lymph vessels. Such drainage could explain the increased levels of sIgA 2 weeks after the final immunization.31,32 These findings suggest that HBc-like particles may enable antigen-mediated elimination of colonized pathogenic bacteria. Further, our data demonstrate that HBc-N144-NspA immunization can generate an efficient mucosal immune response, which has a vital role in the elimination of N. meningitidis.
Another vital factor in N. meningitidis clearance involves Th2 immune responses. The results of FCM, as well as the ratio of IgG1 to IgG2a, show that the immune response to HBc-N144-NspA mixed with Freund’s adjuvant tended to be a clear Th2 type immune reaction in all groups, conferring protection against MC58. These results provide a foundation for further investigation of the Th2 immune response, as it is established that this response is crucial for SBA in vitro.33 The SBA is the current gold standard for determining resistance after VLP vaccination, and the serum bacterial killing titer of HBc-N144-NspA mixed with Freund’s adjuvant reached 1:16, proving that the immunized mice generated potent-specific antibodies, while vaccination with HBc-N144-NspA in the absence of adjuvant also generated SBA of approximately 1:8, mediated by human serum complement, which far exceeds the results reported from the previous Phase I clinical trial.34 Although the immune responses elicited by VLPs are weaker than those induced by the four-component vaccine, the purpose of this study was to screen for novel suitable vectors and potential antigens for the development of a new generation vaccine after structural modification.
The other crucial cytokine on the Neisseria meningitidis clearance is IL-17A, which acts as a critical mediator in inflammatory diseases, and it mediates activation by combining with a heterodimeric complex of receptors composed of IL-17RA and IL-17RC for downstream cell signaling. It is now known that Th17 cells are not the only IL-17A-producing cells; other lineages of T cells, such as cytotoxic CD8+ T cells (Tc17), IL-17-producing γδ T cells (γδ-17), and natural killer T cells (NKT-17), also secrete IL-17A. The secreted IL-17A mainly provoke the intense mucosal immune response that assists the clearance of NMB in the host mucosal surface, on the other hand, it can inhibit the secreting of Th2 cytokine IL-4 for slight inflammatory reaction. Following this evidence, we speculate that the increase in IL-17A may be caused by HBc-N144-NspA, which suggests the potential appearance of a balanced immune response and therapy. In addition, IL-17A production activates the early inflammatory response, which efficiently mediates cell adhesion, corresponding to the function of NspA. Thus, the IL-17A secretion after VLP immunization in our study indicated a significant adaptive immune reaction.
In this study, the application of Freund’s adjuvant led to stimulation of a highly effective immune response and high levels of serum bactericidal titer. During the immunization process, Freund’s adjuvant had a vital role and considerably influenced our results, which is a limitation of our study, since the use of Freund’s adjuvant is restricted in humans, as it can cause severe side effects; however, in the present study, this classical adjuvant was used to facilitate evaluation of the effectiveness of VLPs. In contrast, a previous study reported that aluminum adjuvant results in bias toward Th1 immune responses.12 Further, Yin et al demonstrated that HBc-N144 can generate almost identical levels of antibody as aluminum adjuvant;19 therefore, we chose Freund’s adjuvant for immunization of mice, to eliminate the effector response, and allow us to focus our evaluation of the immune response.
Overall, immunization with VLPs preferentially enhanced specific Th2 cytokine-mediated IgG1 responses to the meningococcal outer membrane protein, NspA, as well as significantly increasing the serum bacterial activity titer. In addition, our results demonstrate the suitability of the HBV capsid protein as a vector for full-length proteins, and determination of the complex 3-D configuration of the particles revealed details of their structure. These findings will facilitate further exploration of the mechanism involved in VLP packaging as a vaccine formula, as well as the modification of the NspA structure by incorporation in HBc-like particles.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under grant 81172890, by the Hunan Provincial Key Laboratory for Special Pathogens Prevention, and by the Hunan Province Cooperative Innovation Center for Molecular Target New Drug Study (2015-351). The authors thank Mr. Ting Yan (Air Force Medical University) for his excellent technical assistance and advice.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hedari CP, Khinkarly RW, Dbaibo GS. Meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine: a new conjugate vaccine against invasive meningococcal disease. Infect Drug Resist. 2014;2014(default):85–99.
2. Stork M, Grijpstra J, Bos MP, et al. Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLoS Pathog. 2013;9(10):e1003733. doi:10.1371/journal.ppat.1003733
3. Wang L, Tan Y, Wei C, et al. A preliminary study on the application of PspA as a carrier for group A meningococcal polysaccharide. PLoS One. 2019;14(7):e0218427. doi:10.1371/journal.pone.0218427
4. Sharkey K, Beernink PT, Langley JM, et al. Anti-factor H antibody reactivity in young adults vaccinated with a meningococcal serogroup B vaccine containing factor H binding protein. mSphere. 2019;4(4). doi:10.1128/mSphere.00393-19.
5. Martinon-Torres F, Nolan T, Toneatto D, Banzhoff A. Persistence of the immune response after 4CMenB vaccination, and the response to an additional booster dose in infants, children, adolescents, and young adults. Hum Vaccin Immunother. 2019;1–12. doi:10.1080/21645515.2019.1627159
6. Wa?ko I, Hong E, De Paola R, et al. High predicted strain coverage by the multicomponent meningococcal serogroup B vaccine (4CMenB) in Poland. Vaccine. 2016;34(4):510–515. doi:10.1016/j.vaccine.2015.11.070
7. Martin D. Highly conserved neisseria meningitidis surface protein confers protection against experimental infection. J Exp Med. 1997;185(7):1173. doi:10.1084/jem.185.7.1173
8. Lewis LA, Rice PA, Ram S. The role of gonococcal Neisserial surface protein A (NspA) in serum resistance and comparison of its factor H binding properties with that of its meningococcal counterpart. Infect Immun. 2019;87(2). doi:10.1128/IAI.00658-18
9. Halperin SA, Langley JM, Smith B, et al. Phase 1 first-in-human studies of the reactogenicity and immunogenicity of a recombinant meningococcal NspA vaccine in healthy adults. Vaccine. 2007;25(3):450–457. doi:10.1016/j.vaccine.2006.08.001
10. Marcinkiewicz AL, Ilva L, Svetlana K, et al. Eliminating factor H-binding activity of borrelia burgdorferi CspZ combined with virus-like particle conjugation enhances its efficacy as a lyme disease vaccine. Front Immunol. 2018;9:181. doi:10.3389/fimmu.2018.00181
11. Pearson L, Roy P. Genetically engineered multi-component virus-like particles as veterinary vaccines. Immunol Cell Biol. 1993;71(Pt 5):381. doi:10.1038/icb.1993.44
12. Cubas R, Zhang S, Kwon S, et al. Virus-like Particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J Immunother. 2009;32(2):118. doi:10.1097/CJI.0b013e31818f13c4
13. Godi A, Panwar K, Haque M, et al. Durability of the neutralizing antibody response to vaccine and non-vaccine HPV types 7 years following immunization with either Cervarix(R) or Gardasil(R) vaccine. Vaccine. 2019;37(18):2455–2462. doi:10.1016/j.vaccine.2019.03.052
14. Janitzek CM, Peabody J, Thrane S, et al. A proof-of-concept study for the design of a VLP-based combinatorial HPV and placental malaria vaccine. Sci Rep. 2019;9(1):5260. doi:10.1038/s41598-019-41522-5
15. Dhanasooraj D, Kumar RA, Mundayoor S. Subunit protein vaccine delivery system for tuberculosis based on hepatitis B Virus Core VLP (HBc-VLP) particles. Methods Mol Biol. 2016;1404:377.
16. Sun C, Ding F-X, Wang F, et al. Screen of multifunctional monoclonal antibodies against hepatitis B core virus-like particles. Microbiol Immunol. 2010;53(6):340–348. doi:10.1111/j.1348-0421.2009.00135.x
17. Qiao L, Zhang Y, Chai F, Tan Y, Huo C, Pan Z. Chimeric virus-like particles containing a conserved region of the G protein in combination with a single peptide of the M2 protein confer protection against respiratory syncytial virus infection. Antiviral Res. 2016;131:131–140. doi:10.1016/j.antiviral.2016.05.001
18. Ryan EP, Malboeuf CM, Bernard M, Rose RC, Phipps RP. Cyclooxygenase-2 inhibition attenuates antibody responses against human papillomavirus-like particles. J Immunol. 2006;177(11):7811. doi:10.4049/jimmunol.177.11.7811
19. Yin Y, Zhang J, Dong D, et al. Chimeric hepatitis B virus core particles carrying an epitope of anthrax protective antigen induce protective immunity against Bacillus anthracis. Vaccine. 2008;26(46):5814–5821. doi:10.1016/j.vaccine.2008.08.031
20. Li YW, Wooldridge KG, Javed MA, Tang CM, Ala’Aldeen DAA. Secreted proteins of Neisseria meningitidis protect mice against infection. Vaccine. 2009;27(17):0–2325. doi:10.1016/j.vaccine.2009.02.034
21. Folaranmi T, Rubin L, Martin SW, Patel M, Macneil JR. Use of serogroup B Meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(22):608–612.
22. Oviedo-Orta E, Ahmed S, Rappuoli R, Black SJV. Prevention and control of meningococcal outbreaks: The emerging role of serogroup B meningococcal vaccines. Vaccine. 2015;33(31):3628–3635. doi:10.1016/j.vaccine.2015.06.046
23. Li G, Jiao H, Jiang G, et al. Neisseria gonorrhoeae NspA induces specific bactericidal and opsonic antibodies in mice. Clin Vaccine Immunol. 2011;18(11):1817. doi:10.1128/CVI.05245-11
24. Cadieux N, Plante M, Rioux CR, et al. Bactericidal and cross-protective activities of a monoclonal antibody directed against neisseria meningitidis NspA outer membrane protein. Infect Immun. 1999;67(9):4955–4959.
25. Trujillo JA, Gras S, Twist KA, et al. Structural and functional correlates of enhanced antiviral immunity generated by heteroclitic CD8 T cell epitopes. J Immunol. 2014;192(11):5245–5256. doi:10.4049/jimmunol.1400111
26. Jegerlehner A, Storni T, Lipowsky G, Schmid M, Pumpens P, Bachmann MF. Regulation of IgG antibody responses by epitope density and CD21‐mediated costimulation. Eur J Immunol. 2015;32(11):3305–3314. doi:10.1002/1521-4141(200211)32:11<3305::AID-IMMU3305>3.0.CO;2-J
27. Zlotnick A, Cheng N, Conway JF, et al. Dimorphism of hepatitis B virus capsids is strongly influenced by the C-terminus of the capsid protein. Biochemistry. 1996;35(23):7412. doi:10.1021/bi9604800
28. Sominskaya I, Skrastina D, Dislers A, et al. Construction and immunological evaluation of multivalent hepatitis B virus (HBV) core virus-like particles carrying HBV and HCV epitopes. Clin Vaccine Immunol. 2010;17(6):1027–1033. doi:10.1128/CVI.00468-09
29. Lehner T, Bergmeier LA, Tao L, et al. Targeted lymph node immunization with simian immunodeficiency virus p27 antigen to elicit genital, rectal, and urinary immune responses in nonhuman primates. J Immunol. 1994;153(4):1858.
30. Jackson EM, Herbst-Kralovetz MM. Intranasal vaccination with murabutide enhances humoral and mucosal immune responses to a virus-like particle vaccine. PLoS One. 2012;7(7):e41529. doi:10.1371/journal.pone.0041529
31. Buonaguro L, Devito C, Tornesello ML, et al. DNA-VLP prime-boost intra-nasal immunization induces cellular and humoral anti-HIV-1 systemic and mucosal immunity with cross-clade neutralizing activity. Vaccine. 2007;25(32):5968–5977. doi:10.1016/j.vaccine.2007.05.052
32. Bretscher PA. On the mechanism determining the Th1/Th2 phenotype of an immune response, and its pertinence to strategies for the prevention, and treatment, of certain infectious diseases. Scand J Immunol. 2014;79(6):361–376. doi:10.1111/sji.12175
33. Ulanova M, Tarkowski A, Hahnzoric M, Hanson LA. The common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect Immun. 2001;69(2):1151–1159. doi:10.1128/IAI.69.2.1151-1159.2001
34. Pajon R, Buckwalter CM, Johswich KO, Gray-Owen SD, Dan MGJV. A native outer membrane vesicle vaccine confers protection against meningococcal colonization in human CEACAM1 transgenic mice. Vaccine. 2015;33(11):1317–1323. doi:10.1016/j.vaccine.2015.01.057
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.