Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Childhood Cancer Survivors’ Adherence to Healthcare Recommendations Made Through a Distance-Delivered Survivorship Program
Authors Alchin JE , Signorelli C , McLoone JK, Wakefield CE, Fardell JE, Johnston K, Cohn RJ
Received 25 February 2022
Accepted for publication 27 June 2022
Published 12 August 2022 Volume 2022:15 Pages 1719—1734
DOI https://doi.org/10.2147/JMDH.S363653
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Joseph Elliot Alchin,1,2 Christina Signorelli,1,2 Jordana Kathleen McLoone,1,2 Claire Elizabeth Wakefield,1,2 Joanna Elizabeth Fardell,1,2 Karen Johnston,1 Richard J Cohn1,2
1Kids Cancer Centre, Sydney Children’s Hospital, Randwick, NSW, Australia; 2School of Women’s and Children’s Health,UNSW Medicine, University of New South Wales, Sydney, NSW, Australia
Correspondence: Christina Signorelli, Kids Cancer Centre, Level 1 South, Sydney Children’s Hospital, High St, Randwick, NSW, 2031, Australia, Tel +61 431 547 350, Fax +61-2-9382-1789, Email [email protected]
Purpose: Ongoing survivorship care allows childhood cancer survivors the opportunity to address treatment-related health problems and improve their quality of life. However, many survivors do not adhere to their healthcare professionals’ recommendations and the factors supporting their adherence remain unclear.
Patients and Methods: Long-term childhood cancer survivors completed the “Re-engage” program, which assessed survivors’ heath needs and provided individualised recommendations for health interventions and surveillance developed by an expert multi-disciplinary team (MDT). We measured survivors’ recall of, and adherence to, their individualised healthcare recommendations at one and six months post-intervention. We conducted a series of univariate negative binomial regressions to investigate factors associated with the total number of recommendations that were correctly recalled and adhered to.
Results: We analysed the data of 25 childhood cancer survivors who participated in Re-engage (mean age = 31.9 years). On average, survivors were provided with 6.6 recommendations (range = 1– 11). Survivors accurately recalled receiving 3.0 recommendations at one month post-intervention and 1.9 at six months. Survivors had adhered to an average of 1.3 recommendations by six-month follow-up. In total, 56% of participants reported that they did not adhere to any recommendations. By six-month follow-up, greater adherence to MDT recommendations was associated with having a history of a second cancer (B = 1.391; 95% confidence interval [CI], 0.686 to 2.097; p < 0.001) and reporting a greater level of worry about late effects (B = 1.381; 95% CI, 0.494 to 2.269; p = 0.002).
Conclusion: Survivors reported sub-optimal levels of adherence and demonstrated limited recall of their healthcare recommendations. Effective communication of recommendations and clear discussion of barriers limiting adherence, coupled with late effects education, may be critical to ensure that survivors engage with their recommendations, to improve their quality of life and health outcomes.
Trial Registration Number: ACTRN12618000194268.
Keywords: childhood cancer, survivorship, surveillance, adherence, model of care, late effects, recall
Plain Language Summary
The problem: Receiving regular survivorship care and following recommended surveillance schedules are known to help prevent and manage the late-effects of childhood cancer and its treatment. However, many survivors disengage from survivorship care in the years and decades after treatment completion and do not know/adhere to the guidelines that are recommended to maximise their health and quality of life.
What we need to learn: What are the factors which facilitate survivors’ adherence, or drive non-adherence, to healthcare recommendations.
What we did: We reviewed childhood cancer survivors’ current health and provided them with recommendations made by an expert, multidisciplinary panel of specialists. One year later, we asked them to recall their recommendations and whether they had adhered to them.
What we found: Our study found that childhood cancer survivors had limited recall of their recommendations and reported suboptimal levels of adherence. Adherence to recommendations was associated with a greater perceived risk of developing late-effects in the future and having already experienced a second cancer.
What we can do to improve outcomes for cancer survivors: To optimise survivors’ adherence to expert survivorship care recommendations follow-up support is needed. Survivors would benefit from education on the risks of late-effects. We suggest that this education could be delivered in conjunction with evidenced-based prevention strategies, so that survivors’ health concerns remain within a manageable range.
Introduction
The impact of childhood cancer extends well beyond its cure. Although approximately 80% of children survive 5 or more years after a cancer diagnosis,1 treatment-related health problems (ie, “late effects”) are prevalent after treatment completion in the ensuing survivorship period,2 which may span six or more decades. Late effects commonly include second primary cancers,3 cardiac toxicity,4 body weight and growth disorders,5,6 endocrinopathies,7 and neurological problems,8,9 with a subset experiencing psychosocial difficulties that can be related to physical health.10 Many late effects only develop after a long-latency period, becoming symptomatic in adulthood, and often remain poorly addressed in post-cancer management.8,11–13
The widespread prevalence and often debilitating impact of late effects after childhood cancer are increasingly reflected in healthcare guidelines, which now strongly encourage ongoing survivorship care and tailored surveillance for all childhood cancer survivors (CCS).12,14–16 Survivorship care provides opportunities to address survivors’ physical and emotional late-effects across all body systems to maximize their quality of life,7,17 whilst emphasizing the importance of preventative lifestyle behaviours through education.12 This may assist survivors to become engaged with their health as active drivers of their own healthcare as they age.
Among survivors of adult cancer, greater adherence to the American Cancer Society (ACS)18 and American Society of Clinical Oncology (ASCO)19 guidelines, recommending physical activity, maintaining a healthy diet, and engaging in follow-up care, has been associated with greater levels of physical20 and psychosocial functioning.21,22 In CCS, greater adherence to ACS guidelines18 has been associated with better health-related quality of life.23 Yet, many survivors display suboptimal adherence to healthcare professionals’ (HCP’s) medical recommendations for late-effect surveillance24–27 and follow-up guidelines,25,28,29 and many disengage during the transition from pediatric to adult care.30
“Adherence” encompasses the degree to which a survivor complies with advice about managing their physical and psychological conditions, participating in surveillance, or engaging in lifestyle-based recommendations, provided by HCPs.31 There is increased recognition of the importance of the patient’s role as an autonomous driver of, and an active participant in, the management of their own health.32 However, survivors’ adherence to individualized medical recommendations is complex and may be hindered by a multitude of survivor, provider and system barriers.33 Potential barriers include low health literacy and lack of awareness of late effects,34 a limited capacity to enact self-management, attend medical appointments or comply with stringent health-based guidelines,35 ageing out of pediatric care,33,36 living outside major cities,33 and a lack of evidence-based frameworks that would augment communication between survivors, primary care physicians (PCPs) and tertiary specialists.37
As few as 23% of childhood cancer survivors access comprehensive follow-up care.26,38,39 During survivorship, the role of HCPs, whether it be specialist oncologists or primary care physicians, can become less clear to survivors.40 Survivors may find the concept of risk-stratified follow-up care confusing and may not know how to access the healthcare they need, nor where the responsibility for their care lies.41 Poor adherence to standardised surveillance recommendations may allow late-effects to worsen or potential cancer recurrence to develop unchecked,42 making it imperative that survivors engage with their medical recommendations. Recent research has corroborated this, revealing that early implementation of breast cancer screening in survivors of childhood cancer (starting at age 25) can reduce mortality by half,43,44 in a cost-effective manner.43 More generally, poor adherence to medical advice can exacerbate physician frustration,45 survivors’ physical and emotional distress,46 and may ultimately be associated with compromised health and quality of life.47,48
Little is known about what factors drive survivors’ adherence to medical and surveillance follow-up recommendations. Previous studies have investigated factors relating to non-adherence to cancer treatment,49–51 with follow-up care,52,53 or adherence to specific clinic attendance (eg, cardiomyopathy screening),54 yet few studies have systematically addressed patient recall of, and adherence to, a full range of survivorship care plan recommendations, particularly among childhood cancer survivors. Extant literature has often focussed on adherence to single screening follow-up recommendations, such as echocardiograms or hearing tests,24 or examined engagement in survivorship care more generally.55 These studies suggest that younger age at the time of diagnosis and receipt of recommendations,24 and less time since treatment completion,52,55 are associated with greater adherence to medical and surveillance recommendations. Should survivors be asked to adhere to multiple screening follow-up recommendations simultaneously, as is common during survivorship follow-up appointments,12,56–58 it is unknown if this would overwhelm the survivor or decrease adherence.32 Further research is vital to expand our knowledge of the factors which contribute to recollection of, and adherence to, survivorship recommendations.
This paper builds upon the findings from our team’s pilot study of our novel survivorship program, “Re-engage”,57,59 a multidisciplinary, distance delivered intervention designed for survivors who have not received any cancer-related care in the past two years or longer. Our pilot showed Re-engage improved survivors’ health-related self-efficacy which is fundamental to survivors becoming autonomous drivers of their own cancer-related healthcare.59 In this paper, we explored Re-engage survivors’ adherence to their recommended follow-up schedule.
Specifically, we aimed to assess:
- The number of healthcare recommendations accurately recalled by survivors at one and six-months post-intervention.
- The number of healthcare recommendations survivors reported adhering to at one or six months post-intervention.
- The factors (eg, clinical and demographic) associated with survivors’ accurate recollection of recommendations and adherence to them by 6 months post-intervention.
Methods
Study Design
This study is a secondary analysis of data acquired from the Re-engage pilot,59 to assess survivors’ recall of and adherence to the healthcare recommendations delivered as part of Re-engage.
Participants
We recruited long-term survivors of childhood cancer who: (1) were over 16 years of age; (2) were diagnosed with cancer prior to 18 years of age; (3) were at least 5 years post-diagnosis at the time of study participation; (4) were treated for cancer at Sydney Children’s Hospital, Randwick, NSW, Australia; (5) had completed active cancer treatment; (6) were alive and in remission at the time of study participation; and (7) had not received cancer-related survivorship care for at least two years prior to study recruitment (ie, were considered “disengaged” from care).
Recruitment
We identified eligible survivors through the Sydney Children’s Hospital electronic medical database and confirmed eligibility through the clinic’s survivorship nurse and oncologist. We invited eligible participants by emailing or posting an information package consisting of an individualized invitation letter, study information sheet, consent form, and opt-in/opt-out cards. Survivors were considered uncontactable after two unsuccessful attempts to contact them through phone or email, and non-receipt of the opt-in/opt-out cards. We conducted recruitment and finalized data collection prior to the COVID-19 pandemic.
Re-Engage Intervention
Re-engage offers eligible survivors, a novel distance-delivered intervention to engage them back into essential survivorship care. Re-engage aims to support survivors to become autonomous drivers of their own healthcare, enhance their health-related self-efficacy and quality of life, and assist survivors in their adoption of a prevention-focussed healthy lifestyle. Re-engage is described in detail elsewhere.57,59 In brief, Re-engage involves i) an in-depth online/telephone nurse consultation to assess survivors’ medical and psychosocial health using a standardized health assessment tool, ii) a multidisciplinary team (MDT) review of each survivor’s case and risk level, iii) creation of a summary letter and individualized survivorship care plan sent to the survivor and their PCP, and iv) a second nurse consultation to review the recommendations made by the MDT, present the recommendations to the survivor, and review survivors’ barriers to care.
Outcomes and Measures (Table 1, Supplementary Table 1)
We collected data at baseline (before Re-engage, time-point 1; T1), one-month (time-point 2; T2) and six-months (time-point 3; T3) post-intervention, through online or paper questionnaires. This included participants’ demographic and clinical information, in addition to measures of quality of life and health-related self-efficacy. We also collected information on survivors’ level of anxiety or worry and perceived risk of late effects or cancer recurrence. We examined participants’ accuracy of recall of and adherence to their MDT recommendations. At one- and six-months post-intervention, participants reported which recommendations they recalled receiving from a list of 18 possible recommendations. Survivors were also provided with space to add any “other recommendations” they recalled receiving. Survivors were given three response options (response options: 0=“No”; 1=“Yes”; 2=“I don”t know’), dichotomised as “yes” (response option = 1) or “no” (response option = 0 or 2) for analysis. We assessed how accurately survivors recalled their MDT recommendations, by comparing the MDT recommendations with the recommendations recalled by survivors. All the accurately recalled recommendations were scored as “1”. Each time-point was assessed independently. To measure the extent to which survivors adhered to their MDT recommendations, participants were asked at both the one- and six-month follow-up time points to self-report, using the list of 18 options, which possible recommendations they had adhered to, with 3 response options: (0=“No”; 1=“Yes”; 2=“No, I”m planning to’). This was dichotomised as “yes” (response option = 1) or “no” (response option = 0 or 2) for analysis. As one-month may not have been sufficient time to fulfil all recommendations, we created a single total score using the one and six-month data to indicate whether participants had at any time over the past 6-months adhered to a recommendation that was made for them by the MDT (ie, if reported “adhered to” at one or six-months post-intervention, then interpreted as adhered to or as adherent and this was given a score of 1).
 |  |  |
Table 1 Demographic and Clinical Characteristics of Participating Childhood Cancer Survivors (N = 25) |
Data Analysis
We conducted a quantitative data analysis using IBM SPSS v26.0.60 We used descriptive statistics to summarize the demographic and clinical characteristics of the sample. To determine which demographic (eg, age), clinical (eg, time since treatment completion) and psychosocial factors (eg, perceived risk of late effects; see Supplementary Table 1 for full list) were independently associated with the total number of healthcare recommendations recalled at six-months post-intervention and adhered to by six-months post-intervention, we employed univariate negative binomial regressions (see Tables 2 and 3). Multivariate analysis was not appropriate due to the modest sample size at follow-up (N = 25), which limited the maximum number of possible variables that could be employed in the model. We considered results statistically significant if p<0.05 (two-tailed). We allocated each recommendation type to one of the four broader categories of healthcare, including referral to specialist-care, PCP, or allied health professional, and healthy lifestyle change (see Supplementary Table 1 recommendation category allocations). The natural log-transformed total number of recommendations provided by the MDT was included as an offset variable in all univariate negative binomial models, due to the close association with each dependent variable.
 |
Table 2 Univariate Negative Binomial Regression Analyses – Factors Associated with the Total Number of Correctly Recalled Recommendations at Six Months Post-Intervention (T3) |
 |
Table 3 Univariate Negative Binomial Regression Analyses – Factors Associated with the Total Number of Recommendations Adhered to at One and/or Six Months Post-Intervention |
Ethics Approval and Informed Consent
We obtained written consent from all participants. The study was approved by the South-Eastern Sydney Local Health District (SESLHD) Human Research Ethics Committee (Ref:16/366). A detailed protocol for the Re-engage pilot study has been published.57 The trial registration for the pilot study is ACTRN12618000194268.
Results
Demographics
For the Re-engage pilot, 36 survivors were identified as eligible to participate, of whom 27 were contactable and agreed to participate (75% response rate; Table 1). However, two participants did not report on our primary outcomes (recall or adherence data) and so were excluded from analyses. Sixty percent of participants were male. The median age of participants at study enrolment was 31 years (interquartile range: 27–39). According to the MDT’s risk ratings based on survivors’ psychosocial and medical needs, 48% of survivors were determined to be at high risk (n = 12), 28% were medium risk (n = 7) and 24% were lower risk (n = 6).
Healthcare Recommendations (Figure 1)
The average number of recommendations made for each survivor was 6.6 (range: 1–11; SD = 2.3). A large proportion of all the healthcare recommendations made were related to referrals for specialist care (60.6%, n=100/165 total recommendations). Ninety-two percent (n=23/25) of participants were advised to attend a survivorship clinic (the reason for which was detailed in each survivor’s survivorship care plan). Of those, 10/23 (40%) were advised to return for at least a once-off visit to the hospital-based survivorship clinic. The next most common recommendations were to see a PCP for review or surveillance (88%, n=22/25), to see a cardiologist or undergo a cardiology review (64%, n=16/25), to see an endocrinologist or undergo an endocrine review (52%, n =13/25), and to receive a skin check through a PCP or skin specialist (52%, n=13/25). Recommendations to see allied health professionals were also commonly provided (56%, n=14/25). This included recommendations for psychosocial support or assessment (48%, n=12/25), and vocational guidance (8%, n=2/25). The most common healthy-lifestyle change recommendations were related to increasing exercise (36%, n=9/25), improving diet (28%, n=7/25), and smoking cessation (12%, n=3/25).
 |
Figure 1 Frequency of recommendations provided by the multidisciplinary team and those recalled by participants. |
Survivors’ Recall of Healthcare Recommendations (Figure 1)
The average number of recommendations that survivors accurately recalled receiving at one and six-months post-intervention was 3 (SD = 2.53, range = 0-8) and 1.88 (SD = 2.51, range = 0-8), respectively (Figure 1). The total proportion of recommendations correctly recalled relatively to the number provided by the MDT (N = 165) was 45.5% at one-month post-intervention (75/165), decreasing to 28.5% at six-months post-intervention (47/165).
Survivors’ Adherence to Healthcare Recommendations (Figure 2)
The mean total number of recommendations that survivors correctly adhered to (ie, adhering to a recommendation from the MDT) by six-months post-intervention was 1.3 (n = 25; range=0-7; SD = 2.03). Out of a total of 165 recommendations provided by the MDT, survivors reported that they correctly adhered to 32 (19%). Survivors were most likely to report attending an appointment with a PCP as recommended (36%, 8/22), followed by specialists (23%, 23/100) and adhering to healthy lifestyle recommendations (16%, 3/19). Survivors were least likely to self-report adhering to recommendations to see allied healthcare professionals such as seeing a psychologist/social worker or receiving vocational guidance services (14%, 2/14).
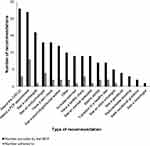 |
Figure 2 Frequency of total number of self-reported recommendations adhered to at one or six-months post-intervention. |
Adherence to recommendations was completed largely by the same survivors. For example, of the participants who reported adhering to at least one recommendation (n = 15), almost half (47%) reported adhering to more than 3 recommendations (n = 7). 56% of participants (n = 14) reported no adherence to any of the MDT recommendations they had received.
Factors Associated with Correctly Recalled Recommendations (Table 2)
At six-month follow-up, the total number of correctly recalled recommendations was statistically significantly negatively associated with a medium risk level assigned by the MDT relative to a lower risk level (B= −2.036; 95% CI, −3.768 to −0.304; p=0.021). There was no statistical significant difference in the predicted rate for the total number of recommendations correctly recalled at 6 months between survivors assigned as high versus lower risk by the MDT (B= −0.031; 95% CI, −1.041 to 0.978; p=0.952). No other demographic, clinical, or psychosocial factors were associated with the number of correctly recalled recommendations at six-month follow-up.
Factors Associated with Adherence (Table 3)
The number of recommendations adhered to at one or six-months post-intervention was statistically significantly associated with having had a second cancer (B=1.391; 95% CI, 0.686 to 2.097; p<0.001), and experiencing a greater level of worry or anxiety about possibility of late effects (B=1.381; 95% CI, 0.494 to 2.269; p=0.002). No other demographic, clinical, or psychosocial factors were associated with the number of recommendations adhered to at one or six-month follow-up.
Discussion
We investigated survivors’ recall of, and adherence to, healthcare recommendations made by an expert MDT as part of a survivorship care program named Re-engage. Overall, survivors’ accuracy when recalling their individualised healthcare recommendations was low (45% and 28% at 1- and 6-months post-intervention respectively).61–64 In addition, adherence (19%) was suboptimal, particularly regarding seeing allied health professionals (14%). At six-months post-intervention, survivors assigned as medium risk by the MDT reported recalling a higher number of recommendations correctly, compared to survivors categorised as lower risk. Greater adherence to MDT recommendations at one- or six-months post-intervention was associated with having had a second cancer and a greater level of worry about late effects.
Echoing previous research on the recall of survivorship care recommendations and advice,61–64 poor recall is commonly reported among individuals at risk of cancer65 and cancer patients.66 Poor recall may have been due to survivors’ low health literacy,67,68 or the potentially distressing nature of the information inhibiting recall.64,69,70 Prior research reports an association between poor recommendation recall and low awareness of the importance of or need for follow-up.71,72 Survivors commonly report experiencing “cognitive overload” if presented with large amounts of medical information,73 although survivors’ in our study were on average provided with 6.6 individualised MDT recommendations, which is comparably fewer than another study’s survivorship care plan (eg, n = 22),61 and more individualised than standardised guidelines.14,18 Low recall accuracy could also be attributed to survivors’ difficulty accurately recalling complex information.64,74 Given our survivors’ decline in recall overtime, improving survivors’ recall via regular reminders or booster sessions may be a critical prerequisite for improving their adherence to individualized healthcare recommendations.
In our study, lower recall of MDT recommendations was associated with being assigned to a medium risk level (relative to lower risk). Prior research has instead suggested that survivors more accurately recall survivorship care recommendations which address more severe late effects, compared to mild symptomatology.61 Survivors experiencing current symptoms, relative to survivors advised on prevention, have also been reported as more accurately recalling their recommendations.61 Other factors that may impact patients’ recall include patient anxiety,61 and greater perceived “stressfulness” or unanticipated nature of the recommendation.64 To aid recall, further emphasis could thus be placed on providing patient-tailored recommendations that closely account for both patient risk (according to treatment and current health) and pertinence of the survivor’s current health concerns. Further research should aim to pinpoint vulnerable demographic groups and investigate modifiable factors associated with improved survivorship recommendation recall.
Suboptimal adherence to recommendations in our study (19% by six-months post-intervention) reflects prior literature, demonstrating low adherence to surveillance and healthcare recommendations in childhood, adolescent and young adult cancer survivors.25,27,52,75 We expected adherence may be somewhat higher in our study given that the available evidence indicates that clear healthcare professional-patient discussion (eg, nurse consultation) in combination with written information (eg, recommendation letter) can be effective in improving survivors’ uptake of medical information.76 Yet, in our cohort, 56% of survivors did not adhere to any MDT recommendations at any time point, which is comparable to other studies reporting rates of non-adherence ranging from approximately 33%77 to 70%.52,77 Adherence to recommendations advising consultation with a specialist (23/100 total recommendations, 23%) or allied healthcare professional (14/100, 14%) was particularly low, echoed in other studies.24,26 Sub-optimal adherence may partly be explained by our short-medium follow-up timeframe (one- and six-months) which may not have been sufficient time to successfully organise and enact healthcare recommendations. Successful and timely adherence may also be influenced by logistical issues or local availability of services (eg, long waiting lists for specialists).33 Notably, only 14% of survivors who were recommended to see a psychologist or social worker had adhered to their recommendation. Access to care may have been more difficult for survivors in our cohort living rurally (28%), especially given that 83% of full-time psychologists are located in major Australian cities.78
Six months may also be inadequate for survivors to seek out and engage in surveillance screening or enact and maintain lifestyle behaviour change. Studies previously examining survivors’ adherence to screening have assessed adherence over 12 months or more24,26,76 and reported surveillance rates up to 74%.24,76 More time may be needed for specific recommendations such as biannual screening. However, given that recall declined by six months, this may suggest the need for a booster call, letter or other reminder modalities to maximise recall and adherence in the long term. Future longitudinal research is needed to more accurately understand survivors’ adherence to multiple recommendations for screening, healthcare, and health behaviours over a longer time period.
Survivors who reported having a second cancer and those who were more worried or anxious about the possibility of late effects displayed higher adherence to their recommendations. Consistent with our findings, the literature suggests that greater intention to engage in, and actual engagement in, follow-up care is associated with a higher perceived risk of late effects,79,80 perception of more severe late effects,81 greater worry80 and a belief that a medical recommendation might prevent a secondary cancer or recurrence.82,83 Whilst there is some evidence to suggest that more frequent health fears may deter some survivors from undergoing recommended surveillance,84 low to moderate anxiety related to late effects may motivate survivors’ desire to adopt protective health behaviours and screening.80 Health-related anxiety to a degree may motivate engagement with recommended medical practices in childhood cancer survivors, however further research should explore the association between health-related anxiety, the nature of survivors’ motivation, and adherence.
Greater recommendation adherence was also associated with a history of a second cancer.85 Survivors with second cancers have more contact with the medical system, including opportunities for further education, which might raise their awareness of their susceptibility to late health effects.86 Other factors highlighted in the literature that influence adherence to surveillance, healthcare and health-behaviour recommendations include younger age at diagnosis, higher education level,87 clear patient-provider discussion on risks and needs,76,81,88 a greater awareness of the benefits and barriers to care,89 and perceived appropriateness of the information relative to the survivors’ treatment stage and general state of health.90 This complex array of socio-demographic and clinical factors influencing survivors’ adherence to recommendations underscores the importance of providing tailored survivorship care for each survivor and the indispensable role of communication between the clinic, survivor and their PCP. Further research is necessary to understand whether greater adherence is more achievable in the long-term and to evaluate longitudinal predictors of adherence during the survivorship period.
We relied on self-report data, meaning that we were unable to verify survivors’ actual adherence to their recommendations.91 Future research should ascertain patterns of adherence to follow-up care through data-linkages with medical records to validate these measures. All participating survivors in our study were English speaking. Non-English speaking cancer survivors are known to face additional barriers to care (eg, poor communication),92 warranting further examination of their patterns of adherence in the future. It would also be valuable to compare adherence to healthcare recommendations provided through face-to-face clinic appointments with those provided through Re-engage’s remote delivery (including both written SCPs and telehealth). Our pilot study sample size was also small and potentially underpowered. Without a control group, we were unable to determine patterns of engagement with surveillance, healthcare and health behaviours without the Re-engage program. Further research should aim to systematically investigate patterns of, and factors associated with, augmented survivor recall and adherence to healthcare recommendations across a larger cohort, and delineate according to age groups (eg, adolescent and young adult, child or adult survivors).
Further development on the presentation of verbal and written recommendations is needed to ensure that survivors can easily understand and enact recommendations. It is also imperative that the longitudinal evaluation of survivorship programs takes into account factors which promote adherence and drive non-adherence, with opportunities to review the success of such programs as time progresses. Methodologically, this study highlights the importance of survivorship research reporting more than just participation in survivorship care. If the survivorship consult is not the point of care (eg, the point of care is a specific surveillance scan), this may allow us to develop a more accurate view of the quality of survivors’ care. As a result of the findings from our Re-engage pilot study, we have made a number of significant improvements to the program design to maximize the potential impact of the program on survivors’ adherence to their recommendations. In brief, we have substantially improved the program though i) the addition of a “booster” nurse-consult, three-months after receiving the MDT’s recommendations, ii) a more standardised structure for the nurse consultations, to ensure discussion of each recommendation and an opportunity for survivors to ask questions and address any barriers to care, and iii) improved the readability of the SCP given to survivors and their PCP, which now includes a concise and yet comprehensive summary of survivors’ cancer treatment(s), medical history, and survivorship needs.
Conclusion
High-quality survivorship care relies on expert recommendations being adhered to and implemented. In our study, we observed sub-optimal levels of survivor recall and adherence to expert healthcare recommendations, despite the provision of both written and verbal communication of the MDT’s recommendations. Clearly articulated recommendations conveyed in both verbal and written form, may not be sufficient to ensure adequate adherence. Survivors experience numerous barriers to care, and therefore, nurse-led follow-up to troubleshoot and increase motivation may be warranted. Our findings indicate that greater adherence to recommendations appears to be related to greater concerns regarding one’s risk of developing late effects in the future. Whilst perceived risk is a modifiable factor, increasing survivors’ concerns may be counterproductive and harmful. However, appropriate late effects education coupled with evidenced-based prevention strategies (eg, smoking cessation, sun protection and exercise) may result in survivors’ concerns remaining within the normal range, whilst potentially increasing adherence. Further research is imperative to identify effective ways of optimising survivors’ engagement with their healthcare recommendations.
Abbreviations
ARIA, Area of Remoteness Index Australia; CHFUC, Children’s Hospital Follow-up Clinic; CI, Confidence Interval; EQ QOL INDEX, summed composite score for the England EQ-5D-5L instrument; MDT, Multi-disciplinary team; PCP, Primary care physician; T1, baseline – before the Re-engage intervention; T2, one-month post-intervention; T3, six-months post-intervention.
Data Sharing Statement
The data that support the findings of this study are not publicly available due to privacy or ethical restrictions, and the full dataset is not able to be released due to ethical restrictions. Requests may be made to the authors.
Acknowledgments
We would like to acknowledge Mark Donoghoe for his advice regarding the statistical analyses. We are also grateful to the members of our Scientific Advisory Committee and Consumer Advisory Committee, who provided input into the design and ongoing conduct of the study. A special thanks to the survivors who participated in Re-engage.
Funding
Dr Christina Signorelli is supported by the Kids Cancer Alliance and a Cancer Institute NSW Early Career Fellowship (2020/ECF1144). Claire Wakefield is supported by an Investigator Grant from the National Health and Medical Research Council of Australia (APP1143767 and APP2008300). Dr Jordana McLoone is supported by Medical Research Future Fund (MRFF) (MRFBC000002) grant and a Cancer Clinical Academic Group (Cancer CAG) E/MCR Seed Grant. The Behavioural Sciences Unit (BSU) is proudly supported by the Kids with Cancer Foundation. The BSU’s survivorship research program is funded by The Kids’ Cancer Project and a Cancer Council NSW Program Grant PG16-02 with the support of the Estate of the Late Harry McPaul. These funding bodies did not have any role in the study, nor did they have a role in the writing of the manuscript or the decision to submit it for publication.
Disclosure
No author has any conflicts of interest to disclose in relation to this manuscript.
References
1. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics. CA Cancer J Clin. 2014;64:83–103.
2. Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–2634.
3. Youlden DR, Baade PD, Green AC, et al. Second primary cancers in people who had cancer as children: an Australian Childhood Cancer Registry population-based study. Med J Aust. 2020;212(3):458.
4. Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128(17):1927–1995.
5. Robien K, Ness KK, Klesges LM, Baker KS, Gurney JG. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30(11):815–822.
6. Chemaitilly W, Cohen LE. Diagnosis of endocrine disease: endocrine late-effects of childhood cancer and its treatments. Eur J Endocrinol. 2017;176(4):R183–R203.
7. Mittal N, Kent P. Long-term survivors of childhood cancer: the late effects of therapy. In: Wonders K, Stout B, editors. Pediatric Cancer Survivors: InTechOpen. 2017. doi:10.5772/67366
8. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582.
9. Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27(14):2339–2355.
10. Zebrack BJ, Gurney JG, Oeffinger KC, et al. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the Childhood Cancer Survivor StuDy. J Clin Oncol. 2004;22(6):999–1006.
11. Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381.
12. Signorelli C, Wakefield CE, Fardell JE, et al. The impact of long-term follow-up care for childhood cancer survivors: a systematic review. Crit Rev Oncol Hematol. 2017;114:131–138.
13. Oeffinger KC, Hudson MM. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J Clin. 2004;54(4):208–236.
14. Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 5.0. Monrovia, CA: Children’s Oncology Group; 2018.
15. Mulder RL, Hudson MM, Bhatia S, et al. Updated breast cancer surveillance recommendations for female survivors of childhood, adolescent, and young adult cancer from the International Guideline Harmonization Group. J Clin Oncol. 2020;38:35.
16. Armenian SHHM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16(3):e123–e36.
17. Grossi M. Management and long-term complications of pediatric cancer. Pediatr Clin North Am. 1998;45(6):1637–1658.
18. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274.
19. Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(7):961–965.
20. Koh D, Song S, Moon SE, et al. Adherence to the American Cancer Society Guidelines for Cancer Survivors and Health-Related Quality of Life among Breast Cancer Survivors. Nutrients. 2019;11(12):2924.
21. Song S, Hwang E, Moon HG, Noh DY, Lee JE. Adherence to Guidelines for Cancer Survivors and Health-Related Quality of Life among Korean Breast Cancer Survivors. Nutrients. 2015;7(12):10307–10319.
22. Boekhout AH, Maunsell E, Pond GR, et al. A survivorship care plan for breast cancer survivors: extended results of a randomized clinical trial. J Cancer Surviv. 2015;9(4):683–691.
23. Zhang FF, Hudson MM, Huang IC, et al. Lifestyle factors and health-related quality of life in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer. 2018;124(19):3918–3923.
24. Reppucci ML, Schleien CL, Fish JD. Looking for trouble: adherence to late-effects surveillance among childhood cancer survivors. Pediatr Blood Cancer. 2017;64(2):353–357.
25. Kagramanov D, Sutradhar R, Lau C, et al. Impact of the model of long-term follow-up care on adherence to guideline-recommended surveillance among survivors of adolescent and young adult cancers. Cancer Med. 2021;10(15):5078–5087.
26. Zabih V, Kahane A, O’Neill NE, Ivers N, Nathan PC. Interventions to improve adherence to surveillance guidelines in survivors of childhood cancer: a systematic review. J Cancer Surviv. 2019;13(5):713–729.
27. Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26(27):4401–4409.
28. Berdan CA, Tangney CC, Scala C, Stolley M. Childhood cancer survivors and adherence to the American Cancer Society Guidelines on Nutrition and Physical Activity. J Cancer Surviv. 2014;8:671–679.
29. Park SH, Knobf MT, Kerstetter J, Jeon S. Adherence to American Cancer Society Guidelines on Nutrition and Physical Activity in Female Cancer Survivors: results From a Randomized Controlled Trial (Yale Fitness Intervention Trial). Cancer Nurs. 2019;42(3):242–250.
30. Henderson TO, Friedman DL, Meadows AT. Childhood cancer survivors: transition to adult-focused risk-based care. Pediatrics. 2010;126(1):129–136.
31. Sabaté E. Adherence to long-term therapies: evidence for action. World Health Organization; 2003. Available from: https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf.
32. Hansen LA. Impact of nonadherence to cancer therapy. J Hematol Oncol Pharm. 2022;12:2154
33. Signorelli C, Wakefield CE, McLoone J, et al. Childhood cancer survivorship: barriers and preferences. BMJ Supportive Palliative Care. 2019;1:1–9.
34. Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors’ knowledge about their past diagnosis and treatment: childhood Cancer Survivor Study. JAMA. 2002;287(14):1832–1839.
35. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189–199.
36. Freyer DR. Transition of care for young adult survivors of childhood and adolescent cancer: rationale and approaches. J Clin Oncol. 2010;28:4810–4818.
37. Grunfeld E, Earle CC. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010;2010(40):25–30.
38. Miller KA, Wojcik KY, Ramirez CN, et al. Supporting long-term follow-up of young adult survivors of childhood cancer: correlates of healthcare self-efficacy. Pediatr Blood Cancer. 2017;64(2):358–363.
39. Wilson CL, Cohn RJ, Johnson KA, Ashton LJ. Tracing survivors of childhood cancer in Australia. Pediatr Blood Cancer. 2009;52(4):510–515.
40. Medicine IO, Council NR. From Cancer Patient to Cancer Survivor: lost in Transition. In: Hewitt M, Greenfield S, Stovall E, editors. Washington, DC: The National Academies Press; 2006:534 p. doi:10.17226/11468
41. Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101(8):1712–1719.
42. DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811.
43. Yeh JM, Lowry KP, Schechter CB, et al. Breast cancer screening among childhood cancer survivors treated without chest radiation: clinical benefits and cost-effectiveness. J Natl Cancer Inst. 2021;114(2):235–244.
44. Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation: a comparative modeling study. Ann Intern Med. 2020;173(5):331–341.
45. Delgado SV. Non-adherence to treatment: different rules for different patients. Arch Fam Med Gen Pract. 2016;1(1):12–17.
46. Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clin Epidemiol. 2001;54(Suppl 1):S57–60.
47. Blair CK, McDougall JA, Chiu VK, et al. Correlates of poor adherence to a healthy lifestyle among a diverse group of colorectal cancer survivors. Cancer Causes Control. 2019;30(12):1327–1339.
48. Khera N, Chow EJ, Leisenring WM, et al. Factors associated with adherence to preventive care practices among hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant. 2011;17(7):995–1003.
49. Puts MT, Tu HA, Tourangeau A, et al. Factors influencing adherence to cancer treatment in older adults with cancer: a systematic review. Ann Oncol. 2014;25(3):564–577.
50. Bender CM, Gentry AL, Brufsky AM, et al. Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol Nurs Forum. 2014;41(3):274–285.
51. Bouwman L, Eeltink CM, Visser O, Janssen J, Maaskant JM. Prevalence and associated factors of medication non-adherence in hematological-oncological patients in their home situation. BMC Cancer. 2017;17(1):739.
52. Devine KA, Viola A, Capucilli P, Sahler OJ, Andolina JR. Factors associated with noncompliance with long-term follow-up care among pediatric cancer survivors. J Pediatr Hematol Oncol. 2017;39(3):167–173.
53. Klosky JL, Cash DK, Buscemi J, et al. Factors influencing long-term follow-up clinic attendance among survivors of childhood cancer. J Cancer Surviv. 2008;2(4):225–232.
54. Marr KC, Agha M, Sutradhar R, et al. Specialized survivor clinic attendance increases adherence to cardiomyopathy screening guidelines in adult survivors of childhood cancer. J Cancer Surviv. 2017;11(5):614–623.
55. Devine KA, Viola AS, Coups EJ, Wu YP. Digital health interventions for adolescent and young adult cancer survivors. JCO Clin Cancer Inform. 2018;2:1–15.
56. Signorelli C, Wakefield CE, McLoone JK, et al. Models of childhood cancer survivorship care in Australia and New Zealand: strengths and challenges. Asia Pac J Clin Oncol. 2017;13(6):407–415.
57. Signorelli C, Wakefield CE, Johnston KA, et al. ‘Re-engage’ pilot study protocol: a nurse-led eHealth intervention to re-engage, educate and empower childhood cancer survivors. BMJ Open. 2018;8(4):e022269.
58. Yan AP, Chen Y, Henderson TO, et al. Adherence to surveillance for second malignant neoplasms and cardiac dysfunction in childhood cancer survivors: a childhood cancer survivor study. J Clin Oncol. 2020;38(15):1711–1722.
59. Signorelli C, Wakefield CE, Johnston KA, et al. Re-engage: piloting a novel nurse-led, distance-delivered, program for survivors of childhood cancer who are disengaged from cancer-related care. J Natl Compr Canc Netw. 2020;18(8):1067–1074.
60. IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp; 2014.
61. Salner AL, Walker D, Seltzer A, et al. Recall and uptake of survivorship care plan recommendations. J Clin Oncol. 2016;34(3suppl):48.
62. Syed IA, Klassen AF, Barr R, et al. Factors associated with childhood cancer survivors’ knowledge about their diagnosis, treatment, and risk for late effects. J Cancer Surviv. 2016;10(2):363–374.
63. Backhaus L, Reinke L, Au D, Zeliadt S, Edwards T. P2.08-004 The importance of patient recall within cancer survivorship care for improved post-treatment surveillance in lung cancer survivors. J Thorac Oncol. 2017;12(1):458.
64. Bober SL, Hoke LA, Duda RB, Tung NM. Recommendation recall and satisfaction after attending breast/ovarian cancer risk counseling. J Genet Couns. 2007;16(6):755–762.
65. Antill YC, Reynolds J, Young MA, et al. Screening behavior in women at increased familial risk for breast cancer. Fam Cancer. 2006;5:4.
66. Fisher A, Williams K, Beeken R, Wardle J. Recall of physical activity advice was associated with higher levels of physical activity in colorectal cancer patients. BMJ open. 2015;5:4.
67. Oeffinger KC, Mertens AC, Hudson MM, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2(1):61–70.
68. Mertens AC, Cotter KL, Foster BM, et al. Improving health care for adult survivors of childhood cancer: recommendations from a delphi panel of health policy experts. Health Policy (New York). 2004;69(2):169–178.
69. Nguyen MH, Smets EMA, Bol N, et al. Fear and forget: how anxiety impacts information recall in newly diagnosed cancer patients visiting a fast-track clinic. Acta Oncol. 2019;58(2):182–188.
70. Gorman JR, Malcarne VL, Roesch SC, Madlensky L, Pierce JP. Depressive symptoms among young breast cancer survivors: the importance of reproductive concerns. Breast Cancer Res Treat. 2010;123(2):477–485.
71. Ford JS, Chou JF, Sklar CA. Attendance at a survivorship clinic: impact on knowledge and psychosocial adjustment. J Cancer Survivorship. 2013;7:4.
72. Staba-Hogan MJ, Ma X, Kadan‐Lottick NS. New health conditions identified at a regional childhood cancer survivor clinic visit. Paediatr Blood Cancer. 2020;60(4):682–687.
73. Selove R, Foster M, Wujcik D, et al. Psychosocial concerns and needs of cancer survivors treated at a comprehensive cancer center and a community safety-net hospital. Support Care Cancer. 2017;25(3):895–904.
74. Ong L, de Haes J, Hoos A, Lammes F. Doctor-patient communication: a review of the literature. Soc Sci Med. 1995;40(7):478.
75. Yan AP, Chen Y, Henderson TO, et al. Adherence to surveillance for second malignant neoplasms and cardiac dysfunction in childhood cancer survivors: a Childhood Cancer Survivor Study. J Clin Oncol. 2020;38:15.
76. Steele JR, Wall M, Salkowski N, et al. Predictors of risk-based medical follow-up: a report from the childhood cancer survivor study. J Cancer Surviv. 2013;7(3):379–391.
77. Beaupin LM, Boldt A, Amato K. Come back: identifying targets to engage young adult survivors who have been lost to follow-up. J Clin Oncol. 2018;36(7 suppl):29.
78. Australia Institute of Health and Welfare. Mental health services in Australia. Australian Government: Australian Institute of Health and Welfare; 2020. Available from: https://www.aihw.gov.au/reports/mental-health-services/mental-health-services-in-australia/report-contents/mental-health-workforce/psychologist-workforce.
79. Signorelli C, Wakefield CE, Fardell JE, et al. Perceptions of future health and cancer risk in adult survivors of childhood cancer: implications for engagement in follow-up care. Cancer. 2019;125(16):1008–1009.
80. Mullens AB, McCaul KD, Erickson SC, Sandgren AK. Coping after cancer: risk perceptions, worry, and health behaviors among colorectal cancer survivors. Psycho-Oncol. 2004;13(6):367–376.
81. Cox CL, Oeffinger K, Montgomery M, et al. Determinants of mammography screening participation in adult childhood cancer survivors: results from the Childhood Cancer Survivor Study. Oncol Nurs Forum. 2009;36(5):335–344.
82. Rabin C, Pinto B. Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives. Psycho-Oncology. 2006;15(8):701–712.
83. Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345–2353.
84. Cox CL, Hudson M, Mertens A, et al. Medical screening participation in the Childhood Cancer Survivor Study. Arch Intern Med. 2009;169(5):454–462.
85. Sheen V, Tucker MA, Abramson DH, Seddon JM, Kleinerman RA. Cancer screening practices of adult survivors of retinoblastoma at risk of second cancers. Cancer. 2008;113(2):434–441.
86. Wilkins KL, Woodgate RL. Preventing second cancers in cancer survivors. Oncol Nurs Forum. 2008;35(2):E12–E22.
87. Rokitka DA, Curtin C, Heffler JE, et al. Patterns of loss to follow-up care among childhood cancer survivors. J Adolesc Young Adult Oncol. 2017;6(1):67–73.
88. Daniel CL, Kohler CL, Stratton KL, et al. Predictors of colorectal cancer surveillance among radiation-treated survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2015;121(11):1856–1863.
89. Lupatsch JE, Wengenroth L, Rueegg CS, et al. Follow‐up care of adolescent survivors of childhood cancer: the role of health beliefs. Paediatr Blood Cancer. 2016;63(2):318–325.
90. Orange ST, Gilbert SE, Brown MC, Saxton JM. Recall, perceptions and determinants of receiving physical activity advice amongst cancer survivors: a mixed‑methods survey. Supportive Care in Cancer. 2021;29:6369–6378.
91. Bhatia S, Armenian SH, Armstrong GT, et al. Collaborative research in childhood cancer survivorship: the current landscape. J Clin Oncol. 2015;33(27):3055–3064.
92. Tan L, Gallego G, Nguyen TTC, Bokey L, Reath J. Perceptions of shared care among survivors of colorectal cancer from non-English-speaking and English-speaking backgrounds: a qualitative study. BMC Fam Pract. 2018;19(1):1–10.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
