Back to Journals » International Journal of General Medicine » Volume 13
Chemokine Ligand 5 to Predict Optimal Cytoreduction in Ovarian Cancer
Authors Hidayat YM , Munizar, Harsono AB , Winarno GNA , Hasanuddin, Salima S
Received 9 September 2020
Accepted for publication 2 November 2020
Published 20 November 2020 Volume 2020:13 Pages 1201—1206
DOI https://doi.org/10.2147/IJGM.S280858
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yudi Mulyana Hidayat,1 Munizar,1 Ali Budi Harsono,1 Gatot Nyarumenteng Adhipurnawan Winarno,1 Hasanuddin,2 Siti Salima1
1Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia; 2Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Syiah Kuala, Aceh, Indonesia
Correspondence: Yudi Mulyana Hidayat Tel +62 8122024759
Email [email protected]
Purpose: The ultimate goal of cytoreduction surgery is the complete removal of all visible tumors (complete cytoreductive surgery) or tumor residues < 1 cm (optimal cytoreduction surgery). Following cytoreduction surgery in ovarian cancer, tumor residue is one of the most important prognostic factors. Oncologists strive to be able to predict the outcome of cytoreduction surgery during the presurgical period. The purpose of this study was to assess CCL5 as a modality for determining whether a patient could perform optimal cytoreduction surgery or not.
Materials and Methods: This was an observational, analytic, and cross-sectional study of patients with ovarian cancer who underwent surgery at the Dr. Hasan Sadikin Bandung from 2019 to 2020. All of the patients had stage I–IV disease based on the International Federation of Gynecology and Obstetrics (FIGO) score.
Results: In total, 72 patients were enrolled in this study, 31 of whom underwent suboptimal cytoreduction surgery and 41 underwent optimal cytoreduction surgery. The mean serum CCL5 level at suboptimal cytoreduction was 70,920.87 ± 36,362.966, while that at optimal cytoreduction was 43,244.95 ± 21,983.887. CCL5, as a predictor of suboptimal cytoreduction surgery, had a sensitivity of 61.3%, a specificity of 68.3%, and an accuracy of 65.7% (p = 0.012).
Conclusion: Preoperative CCL5 serum levels can predict suboptimal cytoreduction surgery outcomes in patients with ovarian cancer.
Keywords: ovarian cancer, CCL5, cytoreductive surgery
Introduction
Ovarian cancer is one of the most deadly malignancies of the female reproductive system. Statistically, 70–80% of all patients with ovarian cancer who come to the hospital have metastatic disease; however, diagnosis may be delayed given that specific symptoms are often absent at the early stages of the disease.1,2 Advanced-stage ovarian cancer has a grim 5-year survival rate. Popular treatments for ovarian cancer include cytoreduction surgery and adjuvant chemotherapy. Since the mid-1990s, primary cytoreduction surgery, followed by platinum and taxane combination chemotherapy, has become the standard treatment regimen for patients with advanced ovarian cancer.3
After cytoreduction surgery, tumor residue is one of the most important prognostic factors for increasing survival rates.4,5 The ultimate goal of cytoreduction surgery is the complete removal of all visible tumors (complete cytoreductive surgery) or tumor residues <1 cm (optimal cytoreduction surgery).
Our ability to predict optimal or suboptimal cytoreduction is important when treating patients with ovarian cancer, especially in the advanced stages. Several authors have used radiological modalities, CA125 tumor markers, laparoscopy, MRI, CT-Scan and peritoneal carcinomatosis index (PCI) as methods for predicting optimal surgical results.6–9 Unfortunately, these studies have produced mixed outcomes, and whether outcomes depend more on patient- or hospital-specific factors remain unknown.
Predicting surgical outcome for patients with advanced ovarian cancer is a dilemma. The gynecologic oncologist must decide between performing primary surgery and pursuing an alternative approach. Neoadjuvant chemotherapy and debulking interval surgery are valid alternatives in patients at higher risk for complications. One of the main objectives of surgical treatment is to predict whether the surgical team will be able to perform optimal cytoreduction.8
Higher CCL5 levels are associated with lower histological differentiation, higher tumor invasion depth, more frequent lymph node involvement, and higher tumor stage.10 CCL5 is highly expressed and secreted by tumor and tumor stromal cells. Evidence supporting the relationship between CCL5 and the progression of ovarian cancer has also been demonstrated by studies that analyzed chemokine levels in patients’ plasma at various disease stages.11 CCL5 appears to influence the optimality of ovarian cancer cytoreduction.
Materials and Methods
This study was an observational analytic study with a cross-sectional design. This study’s population was all patients with suspected ovarian cancer by a gynecological oncology consultant who would undergo surgery at Dr. Hasan Sadikin Bandung. The research sample is part of the affordable population that meets the inclusion criteria. All study subjects were females with stage I–IV ovarian cancer, based on the International Federation of Gynecology and Obstetrics (FIGO). All subjects underwent a planned primary cytoreduction surgery, were willing to follow the research, and provided written informed consent. Patients with blood disorders or infectious diseases and with incomplete examination results were excluded. The independent variable in this study was serum CCL5 levels, the dependent variable in this study was suboptimal and optimal cytoreduction surgery, while the confounding variables in this study were age, ASA, stage, and histopathology.
The researchers collected preoperative venous blood samples from patients with ovarian cancer and examined serum CCL5 levels. The gynecologic oncologist determined if the patient underwent suboptimal or optimal cytoreduction surgery before the surgery was over. If there were visible >1 cm residual tumors after surgery, then the surgery was deemed “suboptimal cytoreduction,” and if there were visible <1 cm residual tumors after surgery, then the surgery was considered to be an “optimal cytoreduction.” Tissue samples obtained during surgery underwent microscopic examination to determine the cell type and its spread. These histopathological results were used to diagnose ovarian cancer.
If ovarian cancer was confirmed by histopathological examination, CCL levels in presurgical serum samples were measured using Quantikine® Human CCL5/Rantes ELISA kit (catalog numbers DRN00B, SRN00B, PDR00B) in PRODIA laboratories. We compared CCL5 levels between the suboptimal and optimal cytoreduction groups.
For continuous data, the Kolmogorov–Smirnov test was used to test normality, and the normally distributed variables between two groups were compared using unpaired Student’s t-test. The comparison of categorical data was performed using the chi-square test. All statistical analyses were performed using SPSS version 24.0 for Windows and receiver operating curve12 analysis. P-values <0.05 indicated statistical significance.
Results
This research was completed during the period between August 2019 and March 2020 at the RS Dr. Hasan Sadikin Bandung. There were 72 patients with histopathologically confirmed ovarian cancer. The distribution of patients in this study is presented in Table 1. The ages at suboptimal cytoreduction surgery ranged from 18 to 68 years, while that of optimal cytoreduction surgery ranged from 22 to 84 years. No significant between-group differences were observed between the suboptimal and optimal cytoreduction surgery groups for age, ASA, and histopathology (p > 0.05).
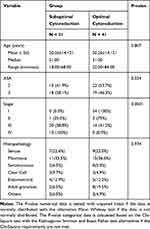 |
Table 1 Background Characteristics of the Study Population |
We found significant between-group differences in CCL5 serum levels in patients who underwent suboptimal and optimal cytoreduction surgery. The average of CCL serum levels in the suboptimal cytoreduction surgery group was 70,920.87 ± 36,362,966, while that in the optimal cytoreduction surgery group was 43,244.95 ± 21,983.887 (P-value = 0.0001; Figure 1). Serum CCL5 levels predicted suboptimal cytoreduction surgery with a sensitivity of 61.3%, a specificity of 68.3%, and an accuracy of 65.7% (Table 2).
 |
Table 2 Sensitivity and Specificity of CCL5 as a Predictor of Cytoreduction Surgery Outcomes in Patients with Ovarian Cancer |
 |
Figure 1 The level CCL5 between suboptimal and optimal cytoreduction. |
The ROC analysis revealed an area under the curve (AUC) of 73.2% with a P-value = 0.001. Therefore, as many as 73.2% of patients with serum levels greater than 53,170 were predicted to undergo suboptimal cytoreduction surgery (Figure 2).
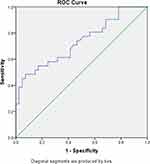 |
Figure 2 ROC curve showing the value of CCL5 with cytoreduction. |
Discussion
No prior research has linked the optimality of cytoreduction with CCL5 serum markers. Patients with ovarian cancer often demonstrate elevated levels of serum CCL5.13 Long et al mentioned that there was evidence to support the relationship between CCL5 and progression in patients with ovarian cancer.11 Similar results were obtained by Wang et al (2016) in their retrospective study of 105 patients with gastric cancer. Here, increased levels of CCL5 were associated with more advanced stages of T and N, poorer histological types, peritoneal metastases, higher tumor residuals, and shorter survival.14 Patients who underwent suboptimal cytoreduction surgery showed higher CCL5 levels than those who underwent optimal cytoreduction surgery.
In a meta-analysis of 14 studies including patients with stage III–IV ovarian cancer, Kang et al found that CA-125 as a predictor of suboptimal cytoredutcion has sensiivity value 68.9% and specificity value 63.3%.15 In addition, several studies have also evaluated HE-4 as a predictive marker in cytoreduction surgery; a study by Tang et al found that a cut-off HE-4 value of 473 pmol/L had a sensitivity value 81.0 % and specificity value of 56%.16 Chemokine C-C ligand 5 (CCL5) is a chemokine that has promoted tumor development by changing the tumor microenvironment in some in vivo and in vitro studies. In addition to modulating the recruitment and activity of immune cells, CCL5 also aids in angiogenesis, which depends on vascular endothelial growth factors (VEGFs).17,18 CCL5 can promote cancer cell growth, stimulate cell proliferation by inducing the mTOR pathway and the subsequent rapid upregulation of cyclin D1, c-Myc, and Dad-1 expression or by increasing glucose uptake followed by increased ATP production and glycolysis.19
Tumor-associated macrophages (TAM) are a heterogeneous population of myeloid cells that contribute to immunosuppression, tumor formation, persistence, and metastasis.20 CCL5 plays an active role in the recruitment of leukocytes, such as T cells, macrophages, eosinophils, and basophils, to the site of inflammation. Together with specific cytokines released by T cells, such as interleukin-2 and IFN-, CCL5 also induces the activation and proliferation of specific natural killer cells to produce C-C chemokine-activated killer cells.21
Angiogenesis is a prerequisite for tumor growth and invasion. CCL5 exerts a proangiogenic role by promoting endothelial cell migration, spread, new vessel formation, and VEGF secretion. Additionally, in response to CCL5 stimulation, tumor cells can produce VEGF or, through CCL5 secretion, can recruit TAM expressing CCR5. In turn, TAM can induce angiogenesis by secreting VEGF.20
Chemokine binding to G-protein-coupled receptors activates a series of downstream effects that facilitate receptor internalization and signal transduction, leading to integrin activation (adhesion) and polarization of the actin cytoskeleton. The consequences are directional sensing, cell polarization, accumulation of Rac/Cdc42 GTPase signaling and activation of phosphoinositide 3-kinase, polymerization of actin, and formation of F-actin. These changes lead to the contraction of actomyosin, retraction of the ends of filaments, and, ultimately, cell migration. More specifically, in lung cancer, CCL5 contributes to αvβ3 integrin activation and cell migration via phosphoinositide 3-kinase/AKT, which in turn activates IKKα/β and NF-κB.22
In ovarian cancer, CCL5 can induce the secretion of matrix metalloproteinase-9 by monocytes, causing matrix degradation and allowing extravasation of tumor cells.23 In gastric cancer, higher levels of CCL5 are associated with lower histologic differentiation, a higher depth of tumor invasion, more frequent lymph node involvement, and advanced tumor stage.10
A recent retrospective analysis of 105 patients with gastric cancer found that elevated serum CCL5 levels were correlated with advanced T and N stages, poor histological type, peritoneal metastasis, higher residual tumor, and shorter survival. The group with high CCL5 levels also had stronger CCL5 immunohistochemical staining in tumor tissues and metastatic lymph nodes.21,24,25 Thus, high serum CCL5 levels, together with strong CCL5 immunohistochemical staining and poor differentiation, can be used to predict peritoneal spread and worse prognosis. CCL5 is also a useful genetic marker that can be utilized to predict chemotherapy efficacy for better prognosis and survival outcomes in gastric cancer.23
The serum CCL5 cutoff point was 53,170 (Table 2). Serum CCL5 values >53,170.00 indicate a greater likelihood of suboptimal cytoreduction surgery. In contrast, when serum CCL5 values were < 53,170.0, optimal cytoreduction surgery was predicted. This cutoff value had a sensitivity of 61.3%, a specificity of 68.3%, a positive predictive value of 59.4%, a negative estimated value of 70%, and an accuracy of 65.7% (Table 2).
The ROC curve is shown in Figure 2. The AUC value on CCL5 as a predictor in suboptimal cytoreduction operations is 61.4% (P < 0.001). Serum CCL5 levels can accurately predict cytoreduction surgery outcomes in 53 out of 72 patients.
Measurement of CCL5 levels to predict cytoreduction surgery can be utilized to provide counseling and to determine the most appropriate therapeutic management in patients with ovarian cancer patients. Furthermore, CCL5 levels can be determined in healthcare facilities with limited access to radiological imaging devices such as CT, MRI, and PET-SCAN.
This study has several limitations. We did not examine all histological subtypes of ovarian cancer or patients with stage I–IV cancer [which, incidentally, is possible in the early stages with complete surgical staging (complete cytoreduction)] and were therefore unable to distinguish whether CCL5 is directly related to high stage or histological subtype. Importantly, higher levels of CCL5 have been shown to be associated with lower histologic differentiation, higher depth of tumor invasion, more frequent lymph node involvement, and advanced tumor stage in gastric cancer.10 Other studies have also found that plasma CCL5 levels are higher in patients with ovarian cancer than in those with ovarian cysts; CCL5 levels have also been shown to be higher in stage III–IV ovarian cancer than in stage I–II ovarian cancer.13 In addition, our hospital ceased performing cytoreduction in response to the global COVID-19 pandemic. Future studies should include a larger patient cohort.
Conclusion
Levels of CCL5 can be a factor in the occurrence of suboptimal cytoreduction surgery to be considered in determining the management of therapy in ovarian cancer patients. However, this also suggests that CCL5 is not strong enough to be a major predictor of determining cytoreduction surgery with accuracy.
Abbreviations
CCL5, chemokine ligand 5; ROC, receiver operating characteristic; AUC, area under the curve; AC, accuracy classification; PPV, positive predictive value; NPV, negative predictive value.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
The study was approved by the Research Ethics Committee, Faculty of Medicine Padjadjaran University/Dr. Hasan Sadikin Hospital, Bandung, Indonesia, No LB.02.01/X.6.5/295/2019 all study participants gave informed consent, patients consent to participate was written. All authors hereby declare that all patients have been examined in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Acknowledgments
The researchers to thank the Department of Obstetrics and Gynecology, RSUP Dr. Hasan Sadikin, Universitas Padjajaran Bandung, PRODIA Laboratories for their support of this study, and Aisyah Shofiatun Nisa, MD as appreciation in technical assistance.
Funding
All authors declare no funding resources.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Lupia M, Cavallaro U. Ovarian cancer stem cells: still an elusive entity? Mol Cancer. 2017;16(1):64.
2. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–1388. doi:10.1016/S0140-6736(13)62146-7
3. Coward JI, Middleton K, Murphy F. New perspectives on targeted therapy in ovarian cancer. Int J Women’s Health. 2015;7:189. doi:10.2147/IJWH.S52379
4. Chang S-J, Hodeib M, Chang J, Bristow RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol. 2013;130(3):493–498. doi:10.1016/j.ygyno.2013.05.040
5. Eisenkop SM, Spirtos NM, Friedman RL, Lin W-CM, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003;90(2):390–396. doi:10.1016/S0090-8258(03)00278-6
6. Ibeanu OA, Bristow RE. Predicting the outcome of cytoreductive surgery for advanced ovarian cancer: a review. Int J Gynecol Cancer. 2010;20(S1):S1–S11. doi:10.1111/IGC.0b013e3181cff38b
7. Engbersen MP, Van’ t Sant I, Lok C, et al. MRI with diffusion-weighted imaging to predict feasibility of complete cytoreduction with the peritoneal cancer index (PCI) in advanced stage ovarian cancer patients. Eur J Radiol. 2019;114:146–151. doi:10.1016/j.ejrad.2019.03.007
8. Llueca A, Serra A, Rivadulla I, Gomez L, Escrig J; Group MW. Prediction of suboptimal cytoreductive surgery in patients with advanced ovarian cancer based on preoperative and intraoperative determination of the peritoneal carcinomatosis index. World J Surg Oncol. 2018;16(1):37. doi:10.1186/s12957-018-1339-0
9. Low RN, Barone RM, Lucero J. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol. 2015;22(5):1708–1715. doi:10.1245/s10434-014-4041-7
10. Kim HK, Song K, Park Y, et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003;39(2):184–191. doi:10.1016/S0959-8049(02)00596-8
11. Long H, Xie R, Xiang T, et al. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem‐like cells via NF‐κB‐mediated MMP‐9 upregulation. Stem Cells. 2012;30(10):2309–2319. doi:10.1002/stem.1194
12. MacKintosh ML, Rahim R, Rajashanker B, et al. CT scan does not predict optimal debulking in stage III–IV epithelial ovarian cancer: a multicentre validation study. J Obstet Gynaecol. 2014;34(5):424–428. doi:10.3109/01443615.2014.899330
13. Tsukishiro S, Suzumori N, Nishikawa H, Arakawa A, Suzumori K. Elevated serum RANTES levels in patients with ovarian cancer correlate with the extent of the disorder. Gynecol Oncol. 2006;102(3):542–545. doi:10.1016/j.ygyno.2006.01.029
14. Wang T, Wei Y, Tian L, et al. CC motif chemokine ligand 5 (CCL5) levels in gastric cancer patient sera predict occult peritoneal metastasis and a poorer prognosis. Int J Surg. 2016;32:136–142. doi:10.1016/j.ijsu.2016.07.008
15. Kang S, Kim TJ, Nam BH, Seo SS, Kim BG, Bae DS, et al. Preoperative serum CA-125 levels and risk of suboptimal cytoreduction in ovarian cancer: A meta-analysis. J Surg Oncol.. 2010;101(1):13–7.
16. Tang Z, Chang X, Ye X, Li Y, Cheng H, Cui H, et al. Usefulness of human epididymis protein 4 in predicting cytoreductive surgical outcomes for advanced ovarian tubal and peritoneal carcinoma. Chin J Cancer Res.. 2015;27(3):309–17.
17. Liu G-T, Chen H-T, Tsou H-K, et al. CCL5 promotes VEGF-dependent angiogenesis by down-regulating miR-200b through PI3K/Akt signaling pathway in human chondrosarcoma cells. Oncotarget. 2014;5(21):10718. doi:10.18632/oncotarget.2532
18. Wang S-W, Liu S-C, Sun H-L, et al. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis. 2015;36(1):104–114. doi:10.1093/carcin/bgu218
19. Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014;2014:1–12. doi:10.1155/2014/292376
20. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399.
21. Cao Z, Xu X, Luo X, et al. Role of RANTES and its receptor in gastric cancer metastasis. J Huazhong Univ Sci Technol. 2011;31(3):342–347. doi:10.1007/s11596-011-0378-3
22. Huang C-Y, Fong Y-C, Lee C-Y, et al. CCL5 increases lung cancer migration via PI3K, Akt and NF-κB pathways. Biochem Pharmacol. 2009;77(5):794–803.
23. Aldinucci D, Casagrande N. Inhibition of the CCL5/CCR5 axis against the progression of gastric cancer. Int J Mol Sci. 2018;19(5):1477. doi:10.3390/ijms19051477
24. Sima AR, Sima HR, Rafatpanah H, et al. Serum chemokine ligand 5 (CCL5/RANTES) level might be utilized as a predictive marker of tumor behavior and disease prognosis in patients with gastric adenocarcinoma. J Gastrointest Cancer. 2014;45(4):476–480. doi:10.1007/s12029-014-9652-5
25. Ding H, Zhao L, Dai S, Li L, Wang F, Shan B. CCL5 secreted by tumor associated macrophages may be a new target in treatment of gastric cancer. Biomed Pharmacother. 2016;77:142–149. doi:10.1016/j.biopha.2015.12.004
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
