Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Characterization of variable presentations of diabetic ketoacidosis based on blood ketone levels and major society diagnostic criteria: a new view point on the assessment of diabetic ketoacidosis
Authors Lee K , Park IB , Yu SH , Kim SK, Kim SH , Seo DH, Hong S , Jeon JY, Kim DJ , Kim SW , Choi CS, Lee DH
Received 25 March 2019
Accepted for publication 24 June 2019
Published 16 July 2019 Volume 2019:12 Pages 1161—1171
DOI https://doi.org/10.2147/DMSO.S209938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Kiyoung Lee,1,2 Ie Byung Park,1,2 Seung Hee Yu,2 Soo-Kyung Kim,3 So Hun Kim,4 Da Hea Seo,4 Seongbin Hong,4 Ja Young Jeon,5 Dae Jung Kim,5 Soo Wan Kim,6 Cheol Soo Choi,1,2 Dae Ho Lee1,2
1Department of Internal Medicine, Gachon University College of Medicine, Incheon, Republic of Korea; 2Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Republic of Korea; 3Department of Internal Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea; 4Department of Internal Medicine, Inha University School of Medicine, Incheon, Republic of Korea; 5Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, Republic of Korea; 6Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Republic of Korea
Aim: We aimed to evaluate the clinical utility of blood ketone measurement and to test the performance of the diagnostic criteria for diabetic ketoacidosis (DKA) issued by the American Diabetes Association, the Joint British Diabetes Societies, and the American Association of Clinical Endocrinologists and the American College of Endocrinology.
Methods: This retrospective analysis included 278 patients with suspected DKA who were hospitalized at 4 university hospitals and aged ≥16 years with a blood glucose level of >200 mg/dL and a blood ketone level of ≥1.0 mmol/L as well as other biochemical data. The patients were categorized into four subgroups (ketosis, typical DKA, atypical DKA, and DKA + lactic acidosis). Atypical DKA in each analysis was defined by our supplementary criteria if the biochemical data did not meet each set of diagnostic criteria from the aforementioned societies.
Results: Blood ketone levels in patients with diabetic ketosis and those with DKA varied widely, 1.05–5.13 mmol/L and 1.02–15.9 mmol/L, respectively. Additionally, there were significant discrepancies between the guidelines in the diagnosis of DKA. Thus, the proportion of patients with atypical DKA ranged from 16.5% to 42.4%. Notably, the in-hospital mortality was comparable between patients with typical and atypical DKA, with a very high mortality in patients with DKA + lactic acidosis (blood lactate >5 mmol/L).
Conclusions: Our results showed that considering variable presentations of DKA, blood ketone data need to be interpreted cautiously along with other biochemical data and suggested that a new system is required to better characterize DKA.
Keywords: Diabetic ketoacidosis, ketone bodies, diagnosis, lactic acidosis, acid base imbalance
Diabetic ketoacidosis (DKA) is an acute diabetic complication, characterized by hyperglycemia, increased blood ketone level, and metabolic acidosis, usually with a high anion gap (AG).1 Although mortality due to DKA is relatively low nowadays,2 it is still high in some DKA patients, such as older patients and those with recurrent DKA, and DKA is one of major causes of death in children and adolescents with type 1 diabetes.1,3,4 However, diagnostic criteria for DKA are not concordant between several guidelines which may create complexity in the early diagnosis of DKA.
A recent consensus statement from the International Society for Pediatric and Adolescent Diabetes added the cutoff level of blood ketone concentration to the previous diagnostic criteria for DKA: blood glucose >11 mmol/L (>200 mg/dL); venous pH <7.3 or serum bicarbonate <15 mmol/L; and ketonemia [β-hydroxybutyrate (BOHB) ≥3 mmol/L] or moderate or large ketonuria (≥2+).3,5 The criteria are similar to those in the UK national guidelines produced by the Joint British Diabetes Societies (JBDS), with the glycemic parameters being more flexible in the JBDS guideline (blood glucose >11 mmol/L or known diabetes mellitus).6 The diagnostic criteria for DKA in adults issued by the American Diabetes Association (ADA) do not suggest a cutoff level for blood ketone concentration: hyperglycemia (blood glucose >13.9 mmol/L [>250 mg/dL]); arterial pH ≤7.3; serum bicarbonate ≤18 mmol/L; blood AG >10 mmol/L; and positive serum or urine ketone on a semi-quantitative test.1 Another recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology (AACE/ACE) strongly recommended measuring blood BOHB and arterial pH to diagnose DKA with different cutoff levels for the biochemical parameters but no specific cutoff level for blood bicarbonate: arterial pH <7.3, BOHB ≥3.8 mmol/L in adults, AG >10 mmol/L, and hyperglycemia (≥250 mg/dL) or lower glycemia in specific cases.7 However, to the best of our knowledge, there is no study to evaluate the concordance of currently available diagnostic criteria for DKA produced from different societies or to find an algorithm to better define and characterize DKA.
With regard to the measurements of ketone bodies, there are several methods to measure blood ketones with their inherent strengths and limitations:8–10 the nitroprusside reaction method, gas chromatography–mass spectrometry (GC/MS), and enzymatic reaction methods. In addition, questions of which ketone molecule(s) [BOHB only or total ketones (BOHB and acetoacetate)] need to be measured and at what cutoff values of BOHB or total ketones DKA could be diagnosed are not clearly answered.11
In the present study, we aimed to test the performance of various DKA diagnostic criteria produced by major societies, by applying serum ketone levels and other classic parameters and to evaluate the clinical utility of blood ketone measurement. Additionally, we suggested new supplementary diagnostic criteria considering both the typical and atypical presentations of DKA, blood ketone levels, and other classic parameters.
Research design and methods
Study population and data extraction
We performed a retrospective analysis of patients who were suspected of having DKA and hospitalized between April 2014 and September 2018. We retrieved demographic, clinical, and laboratory data from electronic medical records of 381 consecutive patients at 4 university hospitals in Korea (Ajou University Hospital, CHA Bundang Medical Center, Gachon University Gil Medical Center, and Inha University Hospital). The study was approved by the institutional review board in each hospital. And, waiver of the requirement for informed consent was obtained under institutional review board regulation because of the retrospective nature of the study and the analysis used anonymous clinical data. This study followed the ethical guidelines of the Declaration of Helsinki. The data were retrieved from electronic health records of each hospital if the following conditions were met: i) clinical diagnosis of DKA or diabetes with hyperglycemia, ketosis, and symptoms suggestive of DKA; ii) simultaneous measurements of serum ketone body levels, arterial blood pH and HCO3−, and serum electrolytes at initial presentation. Blood glucose levels of >11 mmol/L (>200 mg/dL) and ketonemia (≥1.0 mmol/L whichever ketone was measured, regardless of urine ketone) were required to be included in the present study. Because there have been reports of differences in buffering capacity related to age, subjects aged less than 16 years were not included in the present study.11
Among the 381 patients with suspected DKA, 103 patients were excluded because of a lack of blood ketone data (n=77), other missing data related to acid-base or AG assessments (n=5), no hyperketonemia (n=4), relatively low glucose level (≤11.1 mmol/L) (n=8), or insulin and fluid therapy received just before presentation to the hospitals (n=9). The remaining 278 patients were analyzed in the present study. The biochemical data included blood glucose and hemoglobin A1C, arterial blood gas analysis, serum ketone body, blood lactate, serum creatinine and electrolytes, serum osmolality at initial presentation, and serum C-peptide levels during hospitalization. The AG and effective osmolality, delta (Δ) AG (AG - 12), delta HCO3− (24 - HCO3−) was calculated as recommended previously.1,12,13 The type of diabetes (1, 2, other types, or could not determine exactly) was also determined based on all clinical and laboratory information.
The assessment and classification of diabetic patients with ketosis
Serum total ketone body (BOHB + acetoacetate) measurement was performed by using an enzymatic method, Autokit Total Ketone Bodies from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) in two hospitals (Gil Medical Center and Inha University Hospital) or GC-MS (Clarus 680-600T, PerkinElmer Life and Analytical Sciences. Shelton, CT, USA) in the CHA Bundang Medical Center. Serum BOHB was measured by using FreeStyle Optium β Ketone Test Strips (Abbott Diabetes Care Inc., Berkshire, England) in the Ajou University Hospital. Because no established guidelines are available for Asian patients and no standardization or adjustment methods are available for the total ketone or BOHB levels measured by different methods, we used the same cutoff levels for both total ketone and BOHB levels in the present study (≥1 mmol/L for ketosis and ≥3.0 or 3.8 mmol/L for ketoacidosis).8,14
We used the ketosis and ketoacidotic criteria for serum ketone levels instead of semi-quantitative urine or serum ketone readings.8 Then, we classified 278 patients into 4 groups: i) ketosis, ii) typical DKA based on the ADA, JBDS, and AACE/ACE guidelines, iii) atypical DKA, which failed to meet at least one diagnostic criterion in each analysis by different guidelines but had evidence of DKA on cautious interpretation of the laboratory results, and iv) DKA + lactic acidosis (blood lactate >5 mmol/L). If we care about the ADA, JBDS, and AACE/ACE guidelines strictly as summarized in the introduction, some discrepancies in the diagnosis between guidelines would occur due to cutoff levels for bicarbonate (18 or, 15, or not using the parameter), AG (use or not use), the application of the criteria (pH “and” bicarbonate vs pH “and/or” HCO3−), and different cutoff levels for hyperglycemia or ketone body values.1,6,7 In addition, none of the three guidelines suggested a detailed interpretation of mixed acid-base disorders. Thus, we diagnosed as “atypical DKA” if patients with hyperglycemia (blood glucose >11 mmol/L) and ketosis (serum ketone ≥1.0 mmol/L) had two or more of the following biochemical results: i) ketoacidotic level of blood ketone (≥3.0 mmol/L);8 ii) change in AG suggesting metabolic acidosis or mixed acid base disorders (for examples, ΔAG >5 mmol/L, with a reference value of 12 mmol/L) or if ΔAG - ΔHCO3− is >+5.0 or <-5 mmol/L);13 and iii) pH ≤7.3 or HCO3− ≤18). Typical hyperglycemic hyperosmolar state (HHS) and alcoholic ketoacidosis were excluded from the study analysis.
Statistical analysis
Categorical variables are expressed as numbers and percentages, and continuous variables are expressed as the mean ± standard deviation (SD). Continuous data were compared using Student’s t-tests (two-tailed) or Mann–Whitney U tests as appropriate, and categorical variables were compared using chi-squared or Fisher exact tests. Spearman correlation coefficients were used to assess univariate relationships between continuous variables. To evaluate independent factors that may predict in-hospital mortality, multivariable logistic regression analyses were performed to calculate the odds ratio (OR) and corresponding 95% confidence intervals (CIs). P<0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics 23 (IBM Corp., Armonk, NY, USA).
Results
Characteristic categories of DKA by different guidelines
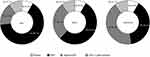
Based on the initial presentation patterns, 278 patients with suspected DKA were classified into 4 subgroups by the major guidelines and our supplementary criteria. Although ketosis (n=23) and significant lactic acidosis-combined DKA (n=28) were defined concordantly based on our strategy, there was a significant variation in the diagnosis of typical DKA by those guidelines if applied in a strict manner. Thus, many patients escaped an exact classification by the 3 major guidelines and were diagnosed with atypical DKA by applying our supplementary criteria (Figure 1).
Table 1 summarizes the 4 categories according to initial presentation by the ADA criteria and by our supplementary criteria. Accordingly, 23 patients (8.3%) were diagnosed with diabetic ketosis but not DKA (Table 1). The characteristics of the patients with typical and atypical presentations judged by the JBDS and AACE/ACE criteria are also presented in Tables S1 and S2, respectively.
The range of blood ketone levels in patients with ketosis was 1.05–5.13 mmol/L, showing a huge variation of blood ketone levels even at a simple ketotic state. Five patients (21.7%) among 23 ketotic patients had a ketone level of 3 mmol/L or more.
Careful interpretation of biochemical data is required to not miss DKA with atypical presentation
Based on the ketotic levels of blood ketone and other classic parameters, the strict application of the ADA criteria did not correctly define DKA in 46 (16.5%) patients among the 278 DKA-suspected patients. Two other sets of diagnostic criteria from JBDS and AACE/ACE did not define DKA in significant proportions among the 278 patients, 28.1% and 42.40%, respectively (Figure 1 and Tables S1 and S2). The proportions of missed patients (atypical DKA by our supplementary criteria) were significantly different between the three societies (P<0.01 in all sets of between-society comparisons using the chi-squared test) (Figure 1)
As shown in Table 1, S1 and S2, compared with patients with typical DKA, patients with an atypical DKA presentation were more likely to have lower ketone body levels and slightly less deranged acid-base homeostasis with a tendency to be type 2 diabetics and to have higher fasting C-peptide levels. However, the in-hospital mortality rate was comparable between the two groups across the three tabulations. Among the ketotic patients, one patient (72-year-old male patient) died during hospitalization due to an accidental fall and subsequent complications, suggesting that the actual ketosis mortality is zero in this subgroup (Table 1).
DKA combined with significant lactic acidosis requires early detection and intensive treatment
Blood lactate levels were available in 274 patients. When we categorized these patients into three groups according to the lactate level (≤2.0 mmol/L, from >2.0–5.0 mmol/L, >5.0 mmol/L). The in-hospital mortality rate was significantly higher only in the group with lactate levels >5 mmol: 3/121 (2.5%), 7/125 (5.6%), and 8/28 (28.6%), respectively (P<0.01). This subgroup with high risk did not escape from the definition regardless of which guidelines were applied to diagnose DKA (Figure 1 and Table 1). Importantly, this DKA + lactic acidosis group was associated with a mortality of more than 7 times the mortality of the combined typical and atypical DKA groups (28.6% vs 4.0%, P<0.01) (Table 2). Compared with the 2 combined DKA groups, this high risk group was more likely to be older and had a lower diastolic blood pressure; lower serum levels of sodium, potassium, and chloride ions; lower hemoglobin, platelet count, arterial pH, and hemoglobin A1c; and higher levels of the AG (Table 2).
Correlations between blood ketone and bicarbonate and other parameters
Blood ketone levels showed a negative correlation with blood bicarbonate level (r = −0.308, P<0.01), blood pH (r= −0.240, P<0.01), and serum fasting C-peptide (r = −0.195, P<0.01) and a positive correlation with blood AG (r =0.321, P<0.01). We also defined the total ketone values corresponding to HCO3− levels of 18 mmol/L by linear regression analysis. The values were between 4.5 and 5.8 mmol/L across analytic methods and the kind of ketone body measured.
Independent factors associated with mortality
The causes of death the were infection/sepsis (n=5), cerebrovascular accident (n=2), gastrointestinal bleeding (n=1), fall and related complications (n=1), and undifferentiated shock (n=9) among the 278 patients. Univariate analysis showed that age, systolic and diastolic blood pressures, serum creatinine and blood lactate were independent predictors of mortality. In multiple logistic regression analysis, age and blood lactate were independently associated with the in-hospital mortality: the ORs (95% CIs, P-values) were 1.040 (1.006–1.075, P<0.05) and 1.275 (1.132–1.435, P<0.01), respectively (Table 3).
 |
Table 3 Multivariate logistic regression analysis for the prediction of independent factors for in-hospital mortality in 278 patients hospitalized due to suspected DKA |
Discussion
In the present study, we found that a single cutoff level of blood ketone concentration could not precisely define diabetic ketosis or DKA in diabetic patients because of the wide variation of blood ketone levels. In addition, if each set of diagnostic criteria for DKA produced by the ADA, JBDS, and AACE/ACE were applied strictly and separately, 16.5–42.4% of patients with suspected DKA may escape from the diagnosis of DKA depending on initial presentation patterns and diagnostic criteria. We observed that the AACE/ACE diagnostic criteria might perform most poorly due to the omission of the bicarbonate criterion and the use of a higher cutoff level for BOHB in the diagnostic criteria.7 Although it is a seemingly milder form of DKA, patients with atypical DKA had similar in-hospital mortality rates as patients with typical DKA. In addition, the current ADA classification of mild, moderate, and severe categories of DKA is not easy to apply to DKA patients because significant proportions of patients presented with variable clinical characteristics, including increasing type 2 diabetes, beta-cell function, mixed acid-base disorders, SGLT2 inhibitor therapy, or others.15,16
The results suggest that more careful interpretation in patients with suspected DKA as well as in patients with apparent ketotic presentation are required to not miss the diagnosis of DKA. In contrast, DKA patients with significant lactic acidosis or patients with diabetic ketosis could be categorized concordantly in the present study by applying each society guideline and our supplementary criteria simultaneously.
Among the three ketone molecules (acetoacetate, BOHB, and acetone), BOHB is a principal ketone body.8 The BOHB/acetoacetate ratio is normally approximately 1, and the ratio is increased in various pathophysiologic states, most notably in DKA, up to 3~10:1.14 However, the measurement of both BOHB and acetoacetate may capture the clinical status of DKA more precisely and may be useful for both the diagnosis and follow-up of DKA. In the present study, the majority of our ketone data were based on the total ketone levels, except for 20 cases with BOHB data. Our results showed that diabetic ketosis patients have blood ketone levels of 1.05–5.13 mmol/L, with 21.7% of ketotic patients having a blood ketone level of 3 mmol/L or more. If all the DKA patients (typical, atypical, and lactic acidosis-combined, n=255) were analyzed together, blood ketone levels ranged from 1.02–15.9 mmol/L, with approximately 30% of patients having less than 3.0 mmol/L. Because the BOHB levels were measured in a hospital (n=20), we compared the total ketone levels from other 3 hospitals (n=235) and the BOHB levels from this hospital. The distributions of ketone body levels were similar: total ketone, median level 4.0 with interquartile range (IQR) 2.90–7.50 mmol/L; and BOHB median (IQR) 4.2 (2.8–5.82) mmol/L. Our study showed that simple mechanical application of a cutoff level of blood ketone body concentration for the diagnosis of DKA would be problematic. Additionally, it is becoming more popular to measure blood ketone bodies using point-of-care (POC) ketone meters, automatic enzymatic analyzers, or GC/MS in various clinical environments. Thus, ketone body measurement, including methods, types of ketones measured, and standardization, also needs to be studied further to better define DKA.
Although there have been several guidelines related to DKA, much of the information for guideline or diagnostic criteria development was from data on which studies were performed more than 20–30 years ago, when semi-quantitative blood or urine ketone measurements were the only or the usual measures to check the ketone body level.17 Few amendments to diagnostic criteria or few attempts to develop a unifying and updated guideline have been made until now, despite there have been many changes in the characteristics of DKA patients and technical advances in diabetes management and ketone measurement.15,18 In addition, there have been significant gaps between the definition of metabolic acidosis and the diagnostic criteria of DKA: for blood pH levels 7.35 vs 7.30, respectively; and for bicarbonate, usually 22 mmol/L vs 15 or 18 mmol/L, respectively,1,6,7,13,19 with the terms “and”, “or”, and “and/or” being used when interpreting pH and bicarbonate data. The AACE/ACE guideline recommends not using bicarbonate data for DKA diagnosis,7 while the JBDS guideline does not include the AG cutoff criterion, but sets a lower bicarbonate cutoff level.6 The ADA and AACE/ACE set the cutoff level of the AG at 10 mmol/L for DKA diagnosis.1 However, the reference ranges of the AG are wide, depending on the laboratory (3–12 mmol/L).13,20 In certain presentations with DKA, normal values for concentrations of bicarbonate, PaCO2, and pH level do not ensure the absence of an acid-base disturbance. An increase in the AG of >5 mmol per liter above the upper normal limit may then be the only clue to an underlying mixed acid-base disorder.12,13 Or ΔAG - ΔHCO3− may be calculated to identify a mixed acid-base disorder or normal AG metabolic acidosis as reviewed previously.13 Various medical conditions accompanying DKA may not allow simple interpretations of pH, bicarbonate, and the AG values, requiring more flexible application of the criteria and careful interpretation of the biochemical results; for example, a normal AG (diarrhea, maintained water and salt intake, or renal dysfunction) or metabolic alkalosis (vomiting, volume depletion, the use of diuretics, or hypokalemia).12,13,21
Lactic acidosis is frequently observed in DKA patients, with a variable impact on prognosis, depending on the definition.19 A previous study showed that increases in blood lactate levels of >2.5 mmol/L and >4 mmol/L were observed in more than two-thirds and in 40%, respectively, of DKA patients without a significant impact on mortality.22 However, our results showed that blood lactate >5 mmol/L in DKA was associated with a mortality rate more than 7 times higher than the mortality rate associated with a lactate level ≤5 mmol/L (28.6% vs 4.0%, respectively). Our results suggest that DKA patients should be routinely tested for blood lactate. Additionally, all patients with DKA and elevated lactate levels must have an estimation of relevant comorbid conditions and DKA triggers (renal function, infections, sepsis, and others).
Considering our findings of a broad range of blood ketone levels in DKA patients, the need for systematic interpretation of biochemical data, including the pH level, bicarbonate, and the AG, in patients with a mixed acid-base disorder, and a high mortality in patients accompanying significant lactic acidosis, we suggest a new algorithm for DKA diagnosis (Figure 2).
 |
Figure 2 Suggested diagnostic flow chart for DKA and differential diagnosis with other frequently combined disorders. ΔAG denotes an increase (delta) in the anion gap above the upper reference value (eg, 12 mmol per liter), Δ[HCO3−] the change (delta) in the concentration of bicarbonate ions from the lower reference value of bicarbonate ions (eg, 24 mmol per liter)13 and HHS hyperglycemic hyperosmolar state. Each laboratory has its own reference values of the AG due to differences in the laboratory methods.13 amay be euglycemic or moderately elevated in some cases. bΔAG - Δ[HCO3−]: >+5 suggests DKA combined with metabolic alkalosis; <−5 suggests DKA combined with normal AG metabolic acidosis. |
There are several limitations in the present study. First, the present study is a retrospective study based on electronic medical records. Second, although we measured BOHB and acetoacetate in the majority of patients, some patients have measured BOHB using POC ketone meters in a hospital. Notably, there are few data on the combined measurements of the sum of BOHB and acetoacetate using enzymatic automatic analyzers, although widely available. Third, although we have suggested supplementary criteria to detect DKA with mixed acid-base disorders, we did not prove the performance of our supplementary criteria using an independent DKA cohort. Fourth, we excluded patients with relatively low hyperglycemia (so-called euglycemic DKA or ketosis) to compare 3 different diagnostic criteria for our convenience. There were 7 patients with euglycemic DKA and 1 patient with ketosis, where sodium–glucose cotransporter 2 (SGLT2) inhibitors were not involved, except for one patient whose drug information was not available. Euglycemic DKA has been reported to be associated with various factors as well as with recently available SGLT2 inhibitors.23,24 Thus, this trait also needs to be studied further. Fifth, our study involved only a single population. There may be some inter-ethnic variation in the characteristics of DKA patients, including the degree of acidosis and type of diabetes.15,16
In conclusion, our results suggest that due to variable presentations and a wide range of blood ketone levels in DKA, a new system is required to better characterize DKA and to supplement current diagnostic criteria for DKA issued by major societies.
Summary
- The clinical parameters and criteria issued by major societies for the diagnosis of DKA are not concordant and blood ketone measurement is not standardized yet despite various analytic methods and options became available.
- We found that there were significant discrepancies between 3 major society guidelines in detecting DKA if applied strictly and that a single cutoff level of blood ketone could not be applied for the diagnosis of DKA because of wide variation of blood ketone levels and variable presentation of DKA.
- A new system is required to better characterize and diagnose DKA.
Acknowledgments
This study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (HI14C1135 and HI16C1997).
Disclosure
No potential conflicts of interest relevant to this article were reported by the authors.
References
1. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. doi:10.2337/dc09-9032
2. Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality - United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018;67(12):362–365. doi:10.15585/mmwr.mm6712a3
3. Wolfsdorf JI, Glaser N, Agus M, et al. Diabetic ketoacidosis and hyperglycemic hyperosmolar state: a consensus statement from the international society for pediatric and adolescent diabetes. Pediatr Diabetes. 2018;19(Suppl27):155–177. doi:10.1111/pedi.1270.
4. Mays JA, Jackson KL, Derby TA, et al. An evaluation of recurrent diabetic ketoacidosis, fragmentation of care, and mortality across chicago, illinois. Diabetes Care. 2016;39(10):1671–1676. doi:10.2337/dc16-0668
5. Dunger DB, Sperling MA, Acerini CL, et al. ESPE/LWPES consensus statement on diabetic ketoacidosis in children and adolescents. Arch Dis Child. 2004;89(2):188–194. doi:10.1136/adc.2003.044875
6. Savage MW, Dhatariya KK, Kilvert A, et al. Joint British diabetes societies guideline for the management of diabetic ketoacidosis. Diabet Med. 2011;28(5):508–515. doi:10.1111/j.1464-5491.2011.03246.x
7. Handelsman Y, Henry RR, Bloomgarden ZT, et al. American ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND American college of endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22(6):753–762. doi:10.4158/EP161292.PS
8. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15(6):412–426.
9. Guerci B, Benichou M, Floriot M, et al. Accuracy of an electrochemical sensor for measuring capillary blood ketones by fingerstick samples during metabolic deterioration after continuous subcutaneous insulin infusion interruption in type 1 diabetic patients. Diabetes Care. 2003;26(4):1137–1141. doi:10.2337/diacare.26.4.1137
10. Khan AS, Talbot JA, Tieszen KL, Gardener EA, Gibson JM, New JP. Evaluation of a bedside blood ketone sensor: the effects of acidosis, hyperglycaemia and acetoacetate on sensor performance. Diabet Med. 2004;21(7):782–785. doi:10.1111/j.1464-5491.2004.01233.x
11. Sheikh-Ali M, Karon BS, Basu A, et al. Can serum beta-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care. 2008;31(4):643–647. doi:10.2337/dc07-1683
12. Berend K. Diagnostic use of base excess in acid-base disorders. N Engl J Med. 2018;378(15):1419–1428. doi:10.1056/NEJMra1711860
13. Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid-base disturbances. N Engl J Med. 2014;371(15):1434–1445. doi:10.1056/NEJMra1003327
14. Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, et al. Medical aspects of ketone body metabolism. Clinical and Investigative Medicine Medecine Clinique Et Experimentale. 1995;18(3):193–216.
15. Tan H, Zhou Y, Yu Y. Characteristics of diabetic ketoacidosis in Chinese adults and adolescents – a teaching hospital-based analysis. Diabetes Res Clin Pract. 2012;97(2):306–312. doi:10.1016/j.diabres.2012.05.004
16. Nyenwe E, Loganathan R, Blum S, et al. Admissions for diabetic ketoacidosis in ethnic minority groups in a city hospital. Metabolism. 2007;56(2):172–178. doi:10.1016/j.metabol.2006.09.010
17. Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med. 1976;84(6):633–638. doi:10.7326/0003-4819-84-6-633
18. Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131–153. doi:10.2337/diacare.24.1.131
19. Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014;371(24):2309–2319. doi:10.1056/NEJMra1309483
20. Farwell WR, Taylor EN. Serum bicarbonate, anion gap and insulin resistance in the national health and nutrition examination survey. Diabet Med. 2008;25(7):798–804. doi:10.1111/j.1464-5491.2008.02471.x
21. Adrogue HJ, Eknoyan G, Suki WK. Diabetic ketoacidosis: role of the kidney in the acid-base homeostasis re-evaluated. Kidney Int. 1984;25(4):591–598.
22. Cox K, Cocchi MN, Salciccioli JD, Carney E, Howell M, Donnino MW. Prevalence and significance of lactic acidosis in diabetic ketoacidosis. J Crit Care. 2012;27(2):132–137. doi:10.1016/j.jcrc.2011.07.071
23. Munro JF, Campbell IW, McCuish AC, Duncan LJ. Euglycaemic diabetic ketoacidosis. Br Med J. 1973;2(5866):578–580. doi:10.1136/bmj.2.5866.578
24. Ireland JT, Thomson WS. Euglycemic diabetic ketoacidosis. Br Med J. 1973;3(5871):107. doi:10.1136/bmj.3.5871.107-a
Supplementary materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.