Back to Journals » Infection and Drug Resistance » Volume 12
Characterization Of The Interaction Between Subviral Particles Of Hepatitis B Virus And Dendritic Cells – In Vitro Study
Authors Farag MMS , Peschel G, Müller M, Weigand K
Received 29 June 2019
Accepted for publication 28 August 2019
Published 7 October 2019 Volume 2019:12 Pages 3125—3135
DOI https://doi.org/10.2147/IDR.S221294
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Mohamed MS Farag,1 Georg Peschel,2 Martina Müller,2 Kilian Weigand2
1Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt; 2Department of Gastroenterology, Endocrinology, Rheumatology and Infectious Diseases, University Hospital Regensburg, Regensburg 93053, Germany
Correspondence: Mohamed MS Farag
Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo 11884, Egypt
Tel +20 2 100 670 4553
Fax +202 22611418
Email [email protected]
Background: During an infection with hepatitis B virus (HBV), infectious particles (Dane particles) can be detected in addition to aggregates of the subviral particles (SVP) which is considered an immune escaping mechanism for the virus. Dendritic cells (DCs) are a specialized type of antigen-presenting cell (APC) that can activate native T-cells to prime an immune response controlling HBV infection. The aim of this study was to characterize the interaction between HBVsvp and DCs in vitro.
Methods: HBVsvp that comprises surface and core proteins were produced in vitro by HepG2.2.15 as a culturing system; DCs derived from the bone marrow of mice were pulsed by HBVsvp. A different pattern of cytokines secreted by bone-marrow-derived dendritic cells from C56BL/6 mice pulsed with HBVsvp were analyzed. The interactions between HBVsvp and DCs were characterized using FACS analysis, protein assay, Western blot, and immunofluorescence staining.
Results: Pulsation of DCs with HBVsvp resulted in strong activation and higher secretion of DC cytokines including INF-α, INF-γ, TNF-α, IL-1α, IL-10, and IL-12; but not for IL-1β, IL-2, IL-6, and IL-15. The production of CXCL-10/IP-10 was increased during the observation period and reached the maximal secretion after 24 hrs (p < 0.001). In total protein assay, we found significantly higher protein concentration in HBVsvp stimulated DC groups compared to not activated DCs (p < 0.001). Both 24 kDa small surface antigen (HBVs) and the 21 kDa core protein (HBVc) were detected in activated DCs. For DCs immunofluorescence staining, our data showed clear differences in the morphology of DCs between negative control and those pulsed with HBVsvp.
Conclusion: Result demonstrates a significant complex interaction between HBVsvp and DCs, in vitro.
Keywords: dendritic cell-based therapy, cytokines, hepatitis B virus subviral particles
Introduction
Infection by hepatitis B virus (HBV) in adults results in acute hepatitis followed by clearance of HBV from the body.1–3Acute infection recovery depends on T-cell and B-cell responses.2,4,5 Globally, HBV infection was estimated at approximately 250 million patients with a chronic infection that eventually progresses to hepatic fibrosis, cirrhosis, and finally hepatocellular carcinoma.6 HBV transmission rates from infected moms to their infants were found to be high, nearly 90%.3,6 Resolving of HBV infections is required for cell-based immunity.7–9 Electron microscopy of HBV antigen (HBsAg) positive serum reveals three morphologic forms: the most numerous (1000 to 100,000-fold excess) spherical particles (25 nm in diameter), filaments (22 nm in diameter), and less frequently spherical infectious Dane particles (42 nm).3,10–12 Subviral particles (SVP) are immunogenic and used as HBV vaccine.13 The excess of SVP detected in patients and its biological function is unexplained and not understood at present. SVP might bind to the host neutralizing antibodies and increase the ability of the Dane particles to reach liver cells.14,15 SVP might contribute to a state of immune tolerance that led to highly productive chronic infection.10 Previous study with duck HBV reported that SVP could increase infection when found at low multiplicity. They were found to be inhibitory at their highest quantity.16,17
DCs are the most effective APC that launch adaptive immune responses after contact with HBsAg. DCs stimulate naive CD4+ differentiation into T helper cells to regulate immune responses including cytotoxic T cells, B-cells, and natural killer cells that clear the infection to eventually produces IL-12 and IFN-α cytokines,18–22 and has ability to induce immunity against HBV.20,23 However, chronic infection of HBV revealed impaired DC function,24,25 while the frequency of DC remains unchanged in some infected people.24–27 Reduction of DC numbers in peripheral blood resulted in weak cytokine production and a disruption of antigen presentation in patients who cannot clear the virus.28,29 This may be reflected by the weakness of both virus-specific immune responses in chronic HBV patients.
However, the interaction of DCs with HBV is not determined yet. DCs function and T-cell responses are iterated in patients who clear the virus after chronic infection or due to therapy.30,31 Therefore, DCs stimulation and reactivation probably can restore specific immune responses against HBV. Several approaches using DCs based therapy have been investigated.32–36 However, there are no sufficient explanations for clinically relevant impairment of DCs function in patients becoming chronically infected. INF-α production by DCs, for example, is reduced in chronic HBV patients but correlates to ALT levels in these patients and from not to HBV viral load.29,37
In this study, HBVsvp were delivered from HepG2.2.15 cell line38 to activate bone-marrow-derived DCs from C56BL/6 mice in vitro, to measure the direct interaction between HBVsvp and DCs. We focused therefore on changes in cytokine and chemokine production and on uptake and distribution of the HBV proteins into the DCs.
Methods And Materials
Production Of HBVsvp
HBVsvp were prepared as described previously.36 In brief, the human hepatoma cell line HepG2.2.15 cells were generously provided by Dr. Stefan Urban. The cells are commercially available, and the use was approved by the review board of the University Hospital Regensburg. The cells were cultured in Williams medium supplemented with 10% (v/v) FCS, 2 mL glutamine, 100 U of penicillin/mL, 100 µg of streptomycin/mL (all from Invitrogen), 5µg/mL of hydrocortisone, and 5µg/mL insulin (Sigma-Aldrich). HepG2.2.15 cells were cultured at 37°C in a CO2 incubator. This resulted in rather high production of HBsAg with a mean level of 939.2 IU/mL. Then, HBVsvp were concentrated as described previously by PEG. Briefly, HBVsvp containing supernatant was incubated with PEG (40%) at 4°C for overnight and then centrifuged for 1 hr at 8000 rpm at 4°C. Pellets were resuspended in 25% FCS-PBS followed by incubation for overnight at 4°C. HBVsvp were harvested and centrifuged for 20 mins at 4000 rpm at 4°C. Finally, HBVsvp were quantified by ELISA for HBsAg, collected and stored at −80°C.
Generation Of Bone-Marrow-Derived DCs (BMdDCs)
For bone-marrow cells (BM) preparation, 8–10-week-old naïve, wild type, C56BL/6 mice were purchased from Charles River Breeding Laboratories (Sulzfeld, Germany) and were maintained under specific pathogen-free conditions and handled according to international guidelines. Mice were euthanized using carbon dioxide. Tibia and femur bones were used to prepare BM cells. All procedures followed the University Regensburg guidelines for care and use of laboratory animals. BM cells were used for generation of DCs as described previously.39 Post 7 days of BM incubation; non-adherent cells were collected and confirmed by FACS analysis. Then, cells were cultured as 1.5 M cells per well in 1 mL RPMI 1640 medium supplemented with 10% FCS, 100 U of penicillin/mL, 100 µg of streptomycin/mL, 2 mM glutamine, and 50 μM 2-mercaptoethanol (all from Invitrogen, UK). To drive cell differentiation towards the DCs line, we added 20 ng/mL GM-CSF and IL-4 (R&D System, Wiesbaden-Norderstedt).
BMdDCs Detection By FACS
For detection of BMdDCs, FACS (BD FACSCalibur™ cytometer) was used to measure different surface molecules are expressed on DCs. FACS data were analyzed with Cell Quest Pro software (Beckton Dickinson). Around 2×105 cells were resuspended into FACS buffer (0.5% BSA-PBS) and stained with either 1 µL of specific antibodies or the corresponding isotype control [R-PE CD11c, FITC MHCII (I-Ad), APC CCR-7, FITC CD 86] (Bioscience, Heidelberg) for 30 mins on ice in dark. The cells were pelleted for 3 mins at 2000 rpm and washed with FACS buffer twice. Individual fluorescent probes were used first for setup the instrument settings and compensation of all used colors. Unstained cells were used for determination of the fluorescence baseline. Unspecific binding was gated away by using isotype controls. FACS analyses indicated 75–85% mature DCs at day 7, which were cultured with a concentration of 1.5 M cells per mL.
Activation Of DCs By HBVsvp
The DCs were activated via 1 day co-culture with either 75 μg/mL of HBVsvp, 1 μg/mL lipopolysaccharide (LPS), a mixture of HBVsvp and LPS or nothing (negative control). Then, cells were harvested, washed extensively, and analyzed by FACS for assessing the upregulation of activation markers. Another DC fraction was processed for BCA-protein-assay and Western blot analysis.
Protein Assay
For protein assay, DCs were generated from bone-marrow followed by culture for 7 days in the presence of IL-4 and GM-CSF. To these cultures, control RPMI supernatant, HBVsvp alone, and HBVsvp plus LPS were added, respectively. DCs were harvested 24 hrs later and lysed using lysis buffer (RIPA or NP-40) (Sigma-Aldrich, Munich) to differentiate between cytoplasmic and total (including nuclear) HBV proteins within the DCs. We have included one more assay for DCs with partial lysis and kept the nucleus intact for all groups examined. Protein assay was performed as the following: 10 µL from each probe were placed into 96-well microliter plates, followed by working reagent (200 µL each well). The plates were incubated shaking at RT in dark for 30 mins, then were read in an automated reader at 560 nm (EMAX; Molecular Devices, MWG-Biotech).
Western Blot
Western blotting of HBVs and HBVc proteins was done as described previously.37 Briefly, concentrated supernatant of cell lysates was run on SDS-PAGE (12%) and transferred to nitrocellulose membranes. After blocking, the membranes were incubated with primary antibodies specific to HBVs and HBVc proteins at 4°C for overnight followed by secondary antibody. Finally, membranes were analyzed by molecular imager (ChemiDocTM XRS+ with Image Lab software).
Immunofluorescence Staining
For immunofluorescence staining, DCs were activated by adding HBVsvp or nothing (negative control) for 24 hrs. Then, cells were fixed by 3.7% Formalin-PBS for 20 mins at RT. After washing with 0.3% Triton-PBS, the cells were blocked with blocking buffer (1% BSA- 0.3% Triton-PBS) for 1 hr. The cells were incubated with primary antibodies specific to HBs or HBc at 4°C for overnight followed by secondary antibody (Alexafluor® 594 goat anti-mouse, Invitrogen, USA). Stained cells were observed by fluorescence microscope.
Detection Of Cytokines Produced By DCs
The DCs were co-cultured with nothing, LPS, HBVsvp, or HBVsvp and LPS. Supernatants were collected 1 day later and were analyzed for the production of a wide range of cytokines (TNF-α, INF-γ, INF-α, IL-1α, IL-1β, IL-2, IL-6, IL-10, CXCL-10/IP-10, IL-12, and IL-15) by using ELISA via the Ready-SET-Go kits from (eBioscience).
Results
Dendritic Cells Are Strongly Activated By HBVsvp In Vitro
Figure 1 shows that HBVsvp strongly activated DCs in vitro. In control group, non-activated DCs present CD86 in 26.5%, MHCII in 33.0%, and CCR7 in 9.5% of the cell population. Incubation with LPS only led to a mild upregulation of CD86, MHCII, and CCR7. Incubation of DCs with HBVsvp led to a significant upregulation of all these markers; CD86 was 59.0%, MHCII was 74.0%, and CCR7 was 48.9% of the cell population. Finally, activation by HBVsvp and LPS led to a significant upregulation and highly increased to be 68.0% for CD86, 75.5% for MHC II, and 51.5% for CCR7 of the cell population.
Enhanced Cytokine Production By DCs
Stimulation of DCs with HBVsvp alone and/or LPS showed significantly higher cytokine production in the supernatant. These cytokines included INF-α, INF-γ, TNF-α, IL-12, IL-1α, IL-10, and CXCL-10/IP-10. We found five- to eight-fold increases of these factors in the activated DCsgroups compared to the negative control groups (DCs alone or DCs with LPS alone) (Figure 2). The increase was statistically significant (p < 0.001) for all these cytokines. Production of IL-1β, IL-2, IL-6, and IL-15 was not significantly increased. Thus, pulsation of DCs with HBVsvp leads to a distinguished change of the DCs cytokine production.
Cytokine Production Increases Over Time
We have included a comparison assay for the production of CXCL-10/IP-10 at different incubation times from activation. CXCL-10/IP-10 production was determined over time. The production of CXCL-10/IP-10 increased during the observation period, reaching significant levels after 2 to 4 hrs (p < 0.05). The maximal secretion of CXCL-10/IP-10 was reached after 24 hrs (p < 0.001) (Figure 3).
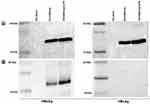
HBVs And HBVc Antigen Proteins Were Highly Detectable In Activated DCs And Distributed Differentially Between Cytoplasm And Nucleus
In total protein assay, we found significantly higher protein concentration in HBVsvp stimulated DC groups compared to not activated DCs (p < 0.001). For RIPA buffer lyses, the total protein was 549.5µg/mL, 1047µg/mL, and 1080.75µg/mL for negative control, DCs activated by HBVsvp alone and DCs activated by HBVsvp and LPS, respectively (Figure 4A). For partial DCs lyses, we have found the total protein was much less than a complete lyses of DCs. Again, DCs activated by SVP or SVP plus LPS were significantly (p < 0.001, p < 0.05) detectable for more proteins than negative control group. Here the total protein was 320.75µg/mL, 730.75µg/mL, and 755.75µg/mL for negative control, DCs activated by HBVsvp alone and DCs activated by HBVsvp and LPS, respectively (Figure 4B).
To determine whether the increase in total protein was due to uptake of HBV proteins, Western blot of the lysate fractions was performed. In complete lysed HBVsvp treated DCs, both 24 kDa small surface antigen (HBVs) and the 21 kDa core protein (HBVc) were detected, referring to a different intracellular distribution pattern of the viral proteins within DCs (Figure 5A). Interestingly, in partial lysed HBVsvp treated DCs, only HBVs were detected and HBc protein was not detected referring to the presence of HBc protein in the nucleus (Figure 5B).
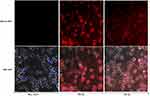
For DCs immunofluorescence staining, our data showed clear differences in the morphology of DCs between negative control and those pulsed with HBVsvp. Both HBs and HBc antigen proteins were found in cytoplasm of pulsed DCs. DCs were positive for HBVs and HBVc antigen proteins compared with negative control with and without nucleus staining (Figure 6).
Discussion
The present study explores the pattern of cytokines produced by DCs pulsed with HBVsvp or HBVsvp coupled with LPS in vitro. Our data demonstrated that DCs are strongly activated by HBVsvp in vitro, measured by increased activation markers and production of several cytokines related to T cell maturation (INF-α, INF-γ, IL-12) or related to T cell priming (TNF-α and IL-1α). Our findings agree with previous studies which showed the expression of IL-1α mRNA within peripheral blood DCs and Langerhans cells;40–43 the current study supports our previous studies which showed that DCs pulsed with Hepatitis C Virus Pseudo-particles produced large array of cytokines in vitro.44 However, this is in contrast to earlier publications demonstrating decreased INF-α level in chronic HBV patients.28,29,37,45 In these studies, INF-α levels were correlated to ALT levels but not viral load. However, HBsAg and HBcAg levels have not been correlated so far. Other studies reported a negative correlation between ALT levels and IFN-α production.46 This may be due to different stimuli of DCs. Th1 differentiation through induction of IFN-γ promoted by IL-12 which is a heterodimer molecule produced by APCs.47
In addition, we found high levels of IFN-γ-inducible protein 10 (IP-10, CXCL10) after stimulation of DCs with HBVsvp. IP-10 is a chemo-attractant for activated T cells, secreted from cells stimulated with type I and II IFNs. IP-10 expressions are found in many Th1-type inflammatory diseases, playing an important role in recruiting activated T cells into sites of inflammation tissues.40,48 Along with the increased TNF-α production, this suggests strong activation of anti-HBV immune responses through DCs activation. Higher production of TNF-α demonstrates the upregulated ability of DCs to begin an adaptive immune response.
In contrast, HBVsvp-activated DCs were also found to produce IL-10, a key immunomodulator that is included in controlling T cell activation levels;49 IL-10 has the ability to act on T cells to initiate allergy.50 This indicates a particular role of DCs in the priming of naive T cells in HBV patients. Moreover, effects of IL-10 on monocytes,51 it has been suggested the production of TNF-α, GM-CSF, IL-1α, IL-1β, IL-6, and IL-8 by DCs may be down-regulate by endogenous IL-10.
Here, DCs did not express IL-1β, IL-2, IL-6, and IL-15 when pulsed with HBVsvp in vitro. Our results confirm a previous study which reported that IL-2 expression by human DCs was downregulated52 and was in agreement with our previous studies which demonstrated that IL-2 and IL-6 were unexpressed by DCs pulsed with pseudo-particles of Hepatitis C Virus in virto.44 This could explain the different results in earlier publications. While in our study, DCs are activated via HBVsvp stimulation resulting in cytokine production to stimulate TH1 and TH2 cells, inflammation seems to be downregulated as well as Treg-cells. Liver inflammation, however, is necessary to eradicate HBV in infected patients.
We and others reported that DCs can be activated in vitro specifically against HBV and initiate immune responses.53–55 We were able to demonstrate that DCs based immunization can break down the tolerance in HBV transgenic mice, which may also be applicable in chronic HBV patients.36 We showed that HBVsvp stimulate DCs to produce different cytokines. The strategies reported were mainly based on different HBV antigens representing for certain epitopes on HBV. Furthermore, inappropriate epitopes lead to Th1/Th2 disequilibrium or could interfere with the communication of immunoactive cells.56,57 Therefore, we decided to use SVP with complete viral particles containing HBs and HBc as antigens to pulse DCs in vitro. In our previous studies, we were able to demonstrate that DCs can effectively be activated against HBVsvp and induce strong and specific immune responses in different mouse lines.53 Our results demonstrated that this DC-based-treatment was followed by effective Th1, Th2, CTL, and B-cell responses.55 DCs were allowed to select their epitopes by presenting structural proteins of HBV in their natural conformation. Furthermore, HBVsvp could be produced for different genotypes. We suggest that the empty spheres represent the empty HBs spheres in vivo.
At present, there is a little knowledge about the ability of DCs to process and present HBV antigens.46 We found that after pulsation, the HBV core and surface proteins distributed throughout the DCs as shown by fluorescence staining. Further analysis by fractionated Western blot showed that HBs was found in different intracellular sites while HBVc seemed to concentrate into the cell nucleus. How HBVc is taken up into the nucleus and if it is completely directed to the nucleus or is degraded within the cytoplasmic compounds must be open at present. However, the different distribution patterns hint to specific functions of HBVs and HBVc within the DCs. It is therefore likely that the outcome of a primary immune response will be affected not only by the subset of DCs involved but also by the activation signal engaged during the initiation phase of the response. This interaction could play an important and clinically relevant role in the impairment of DCs function after HBV infection.
Ethical Statement
We would like to confirm that this material is the authors’ own original work, which has not been previously published elsewhere. The paper is not currently being considered for publication elsewhere. The paper reflects the authors’ own research and analysis in a truthful and complete manner. The paper properly credits the meaningful contributions of co-authors and co-researchers. The results are appropriately placed in the context of prior and existing research. All authors have been personally and actively involved in substantive work leading to the manuscript and will hold themselves jointly and individually responsible for its content.
Abbreviations
APC, antigen-presenting cells; CCR7, chemokine receptor type 7; CD11c, cluster differentiation 11 c; CD86, cluster differentiation 86; CXCL-10/IP-10, CXC motif chemokine ligand 10/Interferon gamma-induced protein 10; FCS, fetal calf serum; GM-CSF, granulocyte/macrophage colony-stimulating factor; HBVsvp, hepatitis B virus subviral particles; HepG2.2.15, human hepatoma cell line; INF-α, interferon alfa; INF-γ, interferon gamma; IL-1α, interleukin one alfa; IL-1β, interleukin one beta; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor.
Acknowledgments
We thank University Hospital Regensburg routine laboratory for running the ELISA on the secreted HBs antigen. We would like to thank Prof. Stefan Urban and his group for providing the HepG2.2.15 cell line. The abstract of this paper was presented at German Association for the Study of the Liver (GASL) Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Z. Gastroenterol Journal: Hyperlink with DOI: 10.1055/s-0034-1386087.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87(6):1439–1449. doi:10.1099/vir.0.81920-0
2. Rehermann B, Fowler P, Sidney J, et al. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995;181(3):1047–1058. doi:10.1084/jem.181.3.1047
3. Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med. 2004;350(11):1118–1129. doi:10.1056/NEJMra031087
4. Maini MK, Boni C, Ogg GS, et al. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117(6):1386–1396. doi:10.1016/s0016-5085(99)70289-1
5. Webster GJ, Reignat S, Maini MK, et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000;32(5):1117–1124. doi:10.1053/jhep.2000.19324
6. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45(2):705–739. doi:10.1002/hep.21513
7. Guidotti LG, Rochford R, Chung J, et al. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284(5415):825–829. doi:10.1126/science.284.5415.825
8. Thimme R, Wieland S, Steiger C, et al. CD8 (+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77(1):68–76. doi:10.1128/jvi.77.1.68-76.2003
9. Menne S, Roneker CA, Roggendorf M, Gerin JL, Cote PJ, Tennant BC. Deficiencies in the acute-phase cell-mediated immune response to viral antigens are associated with development of chronic woodchuck hepatitis virus infection following neonatal inoculation. J Virol. 2002;76(4):1769–1780. doi:10.1128/jvi.76.4.1769-1780.2002
10. Gerlich WH, Kann M. Hepatitis B. In: Mahy BWJ, terMeulen V, editors. Topley and Wilson’s Microbiology and Microbial Infections. Vol. 2. Washington (DC): ASM Press; 2005:1226–1268.
11. Glebe D, Urban S. Viral and cellular determinants involved in hepadnaviral entry. World J Gastroenterol. 2007;13(1):22–38. doi:10.3748/wjg.v13.i1.22
12. Gudima S, He Y, Meier A, et al. Assembly of hepatitis delta virus: particle characterization including ability to infect primary human hepatocytes. J Virol. 2007;81(7):3608–3617. doi:10.1128/JVI.02277-06
13. Blumberg BS. Hepatitis B virus and the control of hepatocellular carcinoma. IARC Sci Publ. 1984;63:243–261.
14. Ganem D. Assembly of hepadnaviral virions and subviral particles. Curr Top Microbiol Immunol. 1991;168:61–83.
15. Seeger C, Zoulim F, Mason WS. Hepadnaviruses. In: Knipe DM, editor. Fields Virology.
16. Bruns M, Miska S, Chassot S, Will H. Enhancement of hepatitis B virus infection by noninfectious subviral particles. J Virol. 1998;72(2):1462–1468. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC124627/.
17. Klingmuller U, Schaller H. Hepadnavirus infection requires interaction between the viral pre-S domain and a specific hepatocellular receptor. J Virol. 1993;67(12):7414–7422. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC238206/.
18. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi:10.1038/32588
19. Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat Immunol. 2001;2(6):487–492. doi:10.1038/88678
20. Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106(3):263–266. doi:10.1016/S0092-8674(01)00455-X
21. Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2(12):957–964. doi:10.1038/nri956
22. Op Den Brouw ML, Binda RS, van Roosmalen MH, et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126(2):280–289. doi:10.1111/j.1365-2567.2008.02896.x
23. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi:10.1146/annurev.immunol.21.120601.141040
24. Beckebaum S, Cicinnati VR, Dworacki G, et al. Reduction in the circulating pDC1/pDC2 ratio and impaired function of ex vivo-generated DC1 in chronic hepatitis B infection. Clin Immunol. 2002;104(2):138–150. doi:10.1006/clim.2002.5245
25. Beckebaum S, Cicinnati VR, Zhang X, et al. Hepatitis B virus-induced defect of monocyte-derived dendritic cells leads to impaired T helper type 1 response in vitro: mechanisms for viral immune escape. Immunology. 2003;109(4):487–495. doi:10.1046/j.1365-2567.2003.01699.x
26. Zhang Z, Chen D, Yao J, et al. Increased infiltration of intrahepatic DC subsets closely correlate with viral control and liver injury in immune active pediatric patients with chronic hepatitis B. Clin Immunol. 2007;122(2):173–180. doi:10.1016/j.clim.2006.09.006
27. Koumbi LJ, Papadopoulos NG, Anastassiadou V, et al. Dendritic cells in unifected infants born to hepatitis B virus-positive mothers. Clin Vaccine Immunol. 2010;17(7):1079–1085. doi:10.1128/CVI.00074-10
28. Duan XZ, Zhuang H, Wang M, et al. Decreased numbers and impaired function of circulating dendritic cell subsets in patients with chronic hepatitis B infection (R2). J Gastroentero Hepatol. 2005;20:234–242. doi:10.1111/j.1440-1746.2004.03529.x
29. van der Molen RG, Sprengers D, Binda RS, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40(3):738–746. doi:10.1002/hep.20366
30. Boonstra A, Woltman AM, Janssen HL. Immunology of hepatitis B and hepatitis C virus infections. Best Pract Res Clin Gastroenterol. 2008;22(6):1049–1061. doi:10.1016/j.bpg.2008.11.015
31. Shi M, Fu J, Shi F, et al. Viral suppression correlates with dendritic cell restoration in chronic hepatitis B patients with autologous cytokine-induced killer cell transfusion. Liver Int. 2009;29(3):466–474. doi:10.1111/j.1478-3231.2008.01861.x
32. Akbar SM, Horiike N, Onji M. Immune therapy including dendritic cell based therapy in chronic hepatitis B virus infection. World J Gastroenterol. 2006;12(18):2876–2883. doi:10.3748/wjg.v12.i18.2876
33. Cohen S, Haimovich J, Hollander N. Dendritic cell-based therapeutic vaccination against myeloma: vaccine formulation determines efficacy against light chain myeloma. J Immunol. 2009;182(3):1667–1673. doi:10.4049/jimmunol.182.3.1667
34. Ma XJ, Tian DY, Xu D, et al. Uric acid enhances T cell immune responses to hepatitis B surface antigen-pulsed-dendritic cells in mice. World J Gastroenterol. 2007;13(7):1060–1066. doi:10.3748/wjg.v13.i7.1060
35. Qiu SJ, Lu L, Qiao C, et al. Induction of tumor immunity and cytotoxic t lymphocyte responses using dendritic cells transduced by adenoviral vectors encoding HBsAg: comparison to protein immunization. J Cancer Res Clin Oncol. 2005;131(7):429–438. doi:10.1007/s00432-004-0616-1
36. Farag MM, Tedjokusumo R, Flechtenmacher C, et al. Immune tolerance against HBV can be overcome in HBV transgenic mice by immunization with dendritic cells pulsed by HBVsvp. Vaccine. 2012;30(42):6034–6039. doi:10.1016/j.vaccine.2012.07.057
37. Woltman AM, Op den Brouw ML, Biesta PJ, et al. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One. 2011;6(1):e15324. doi:10.1371/journal.pone.0015324
38. Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci USA. 1987;84(4):1005–1009. doi:10.1073/pnas.84.4.1005
39. Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–1702. doi:10.1084/jem.176.6.1693
40. Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5(8):919–923. doi:10.1038/11360
41. Zhou LJ, Tedder TF. A distinct pattern of cytokine gene expression by human CD831 blood dendritic cells. Blood. 1995;86(9):3295–3301.
42. Schreiber S, Kilgus O, Payer E, et al. Cytokine pattern of Langerhans cells isolated from murine epidermal cell cultures. J Immunol. 1992;149(11):3524–3534.
43. Granucci F, Girolomoni G, Lutz MB, et al. Modulation of cytokine expression in mouse dendritic cell clones. Eur J Immunol. 1994;24(10):2522–2526. doi:10.1002/eji.1830241039
44. Farag MM. Production large array of cytokines by mouse dendritic cells pulsed with hepatitis C virus pseudo-particles. Int J Virol Mol Biol. 2014;3(1):9–18. doi:10.5923/j.ijvmb.20140301.02
45. Shi B, Ren G, Hu Y, et al. HBsAg inhibits IFN-α production in plasmacytoid dendritic cells through TNF-α and IL-10 induction in monocytes. PLoS One. 2012;7(9):e44900. doi:10.1371/journal.pone.0044900
46. Gehring AJ, D’Angelo JA. Dissecting the dendritic cell controversy in chronic hepatitis B virus infection. Cell Mol Immunol. 2015;12(3):283–291. doi:10.1038/cmi.2014.95
47. Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180(1):211–222. doi:10.1084/jem.180.1.211
48. Loetscher MB, Gerber P, Loetscher SA, et al. Chemokine receptor specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184(3):963–969. doi:10.1084/jem.184.3.963
49. Caux C, Massacrier C, Vanbervliet B, Barthelemy C, Liu YJ, Banchereau J. Interleukin-10 inhibits T cell alloreaction induced by human dendritic cells. Int Immunol. 1994;6(8):1177–1185. doi:10.1093/intimm/6.8.1177
50. Groux H, Bigler M, de Vries JE, Roncarolo MJ. Interleukin-10 induces a long-term antigen-specific anergic state in human CD41 T cells. J Exp Med. 1996;184(1):19–29. doi:10.1084/jem.184.1.19
51. De Waal Malefijt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174(5):1209–1220. doi:10.1084/jem.174.5.1209
52. Saint-Vis B, Fugier-Vivier I, Massacrier C, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160(4):1666–1676.
53. Farag MM, Hoyler B, Encke J, Stremmel W, Weigand K. Dendritic cells can effectively be pulsed by HBVsvp and induce specific immune reactions in mice. Vaccine. 2010;29(2):200–206. doi:10.1016/j.vaccine.2010.10.056
54. Oka Y, Akbar SM, Horiike N, Joko K, Onji M. Mechanism and therapeutic potential of DNA-based immunization against the envelope proteins of hepatitis B virus in normal and transgenic mice. Immunology. 2001;103(1):90–97. doi:10.1046/j.1365-2567.2001.01202.x
55. Huang Y, Chen Z, Jia H, Wu W, Zhong S, Zhou C. Induction of Tc1 response and enhanced cytotoxic T lymphocyte activity in mice by dendritic cells transduced with adenovirus expressing HBsAg. Clin Immunol. 2006;119(3):280–290. doi:10.1016/j.clim.2006.01.015
56. Jiang WZ, Fan Y, Liu X, et al. Therapeutic potential of dendritic cell-based immunization against HBV in transgenic mice. Antiviral Res. 2008;77(1):50–55. doi:10.1016/j.antiviral.2007.08.004
57. Chaiken IM, Williams WV. Identifying structure-function relationships in fourhelix bundle cytokines: towards de novo mimetics design. Trends Biotechnol. 1996;14(10):369–375. doi:10.1016/0167-7799(96)10050-0
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.