Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Characterization and Treatment of Inflammation and Insulin Resistance in Obese Adipose Tissue
Received 9 July 2020
Accepted for publication 1 September 2020
Published 1 October 2020 Volume 2020:13 Pages 3449—3460
DOI https://doi.org/10.2147/DMSO.S271509
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Zhenhua Lu, Yao Li, Jinghai Song
Department of General Surgery, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China
Correspondence: Jinghai Song
Department of General Surgery, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, No. 1 DaHua Road, Dong Dan, Beijing 100730, People’s Republic of China
Tel +8619800315020
Email [email protected]
Abstract: Adipose tissue is the largest energy storage and protection organ. It is distributed subcutaneously and around the internal organs. It regulates metabolism by storing and releasing fatty acids and secreting adipokines. Excessive nutritional intake results in adipocyte hypertrophy and proliferation, leading to local hypoxia in adipose tissue and changes in the release of adipokines. These lead to recruit of more immune cells into adipose tissue and release of inflammatory signaling factors. Excess free fatty acids and inflammatory factors interfere with intracellular insulin signaling. In this review, we summarize the characteristics of obese adipose tissue and analyze how its inflammation causes insulin resistance. We further discuss the latest clinical research progress on the control of insulin resistance and inflammation resulting from obesity through anti-inflammatory therapy and bariatric surgery. Our review shows that targeted anti-inflammatory therapy is of great significance for obese patients with insulin resistance.
Keywords: obesity, adipose tissue, inflammation, insulin resistance, anti-inflammatory therapy
Introduction
Over the past 40 years, the incidence of overweight and obesity has risen in both developed and developing countries due to unbalanced diets, inadequate physical activity, chronic stress, certain drug intake, and environmental pollutants.1–4 There are over 1.9 billion overweight adults worldwide, and more than 650 million were classified as obese in 2016. The world’s obesity rate has almost tripled since 1975.5,6 Obesity may cause many chronic diseases, including cardiovascular and cerebrovascular diseases, diabetes, and some cancers.7–12 In particular, non-insulin-dependent diabetes (type 2 diabetes) is closely related to obesity.13,14 Obesity is defined as “abnormal or excessive fat accumulation that may impair health” by the World Health Organization.5 Adipose tissue (AT) remodeling occurs during obesity, resulting in hypertrophy, hypoxic necrosis, immune cell infiltration, release of adipokines, and changes in inflammatory signaling.15 All these factors lead to AT dysfunction and chronic sterile inflammation. By discussing the inflammatory changes in obese AT, we will further review the effects of anti-inflammatory treatments and bariatric surgery on insulin resistance.
Adipose Tissue Classification
In humans, multiple types of AT are distributed throughout the body. White AT (WAT) includes subcutaneous AT (SAT) and visceral AT (VAT). Both types have an important role in regulating metabolism.16 During energy supplementation, excess non-esterified fatty acids (NEFA) become esterified into triacylglycerols (TAGs). White adipocytes store TAGs in cytosolic lipid droplets (LDs). During exercise and fasting, TAGs are mobilized by hormones, releasing fatty acids via lipolysis.17 Compared with SAT, VAT is less sensitive to fatty acid synthesis by insulin, but has a higher sensitivity to catecholamines, which promote lipolysis.18 Higher lipolysis in VAT can lead to metabolic complications related to visceral obesity. Klein et al found that omental AT removal can significantly reduce insulin resistance, but subcutaneous liposuction has no such effect.19 Hocking et al found that SAT transplantation can improve insulin sensitivity in mice, especially when transplanting them into VAT.20 In obesity, these regulatory functions are impaired, mainly due to the decrease in esterification and the increase of lipolysis.
Brown AT may be present in the clavicle, perirenal, paravertebral, and other parts of the human body.21 It is characterized by more lipid droplets and mitochondria, giving it a brown appearance.22 Muscle cells and brown adipocytes are derived from Myf5+ cells and they uniquely express uncoupling protein 1 (UCP-1) which can regulate the conversion of energy to heat by uncoupling ATP in mitochondrial respiration.23 Brown adipocytes maintain body temperature through non-shivering heat production. They are abundant in human neonates, gradually decreasing in adults, and decreasing further in obese people.21 Specialized white adipocytes called beige adipocytes have the shape and high metabolic activity of brown fat cells.24 Because of their high metabolic functions, increasing the number of beige or brown adipocytes may be an effective strategy to reduce obesity and insulin resistance. More characteristics of different adipose tissues and adipocytes are summarized in Table 1.
 |
Table 1 The Characteristics of Different Adipose Tissues |
Immune Cells in Obese Adipose Tissue Inflammation
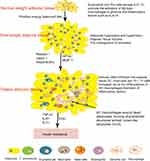
Adipocytes account for about 90% of human AT by volume. However, in terms of cell diversity, approximately 4 million other types of cells exist in one gram of AT compared to 1–2 million adipocytes per gram.18 Other cells include various immune cells, endothelial cells, pre-adipocytes, and pericytes. Immune cells are roughly divided into lymphocytes and bone marrow cells. Lymphocytes include T and B cells, while myeloid cells include eosinophils, basophils, dendritic cells (DCs), macrophages, neutrophils, mast cells, and so on.25 During the development of obesity, adipocytes secrete adipokines, gradually changing the balance of immune cells from anti-inflammatory to pro-inflammatory. This process leads to chronic inflammation of AT and insulin resistance.
Macrophages have different functions depending on environmental stimuli. In acute inflammation and injury, macrophages kill infected cells by phagocytosis. In chronic inflammation caused by obesity, the production of anti-inflammatory macrophages can be insufficient, leading to extracellular matrix (ECM) fibrosis.26 In healthy AT, M2 macrophages are widely distributed and have anti-inflammatory effects. They express IL-4, IL-10, TGF-β, and other anti-inflammatory factors.27 IL-10 can antagonize the effect of TNF-α and promote insulin sensitivity.28 Additionally, M2 macrophages and eosinophils can assist in the production of beige adipocytes.29 In obesity, more adipokines like MCP-1 are secreted from hypertrophic adipocytes, inducing monocytes to infiltrate AT and differentiate into macrophages.30,44 Macrophages account for only 5% of healthy AT but can account for 50% of obese AT.31 In obese AT, type I interferon, LPS, TLR4, saturated FFA, and ceramide activation can induce M1 macrophages to gather around necrotic adipocytes to form “crown-like structures” (CLS)28,32 (Figure 1). Unlike M2 macrophages, activated M1 type macrophages express CD11c and produce proinflammatory mediators such as resistin, IL-6, IL −1β,TNF-α, and NO.33 These mediators further induce adipocyte death and downregulate the expression of peroxisome proliferator-activated receptor (PPAR-γ), which normally promotes adipose synthesis.34 A decrease in PPAR-γ activity contributes to insulin resistance.35
In obese AT, the ratio of CD4 + and CD8 + type T cells changes.36 Inflammatory factors such as IL-4, and IL-6 can stimulate CD4 + T cells to differentiate into Th1, Th2, Treg, and Th17 cells. These types of cells participate in the inflammatory response. In healthy AT, Th2 cells secrete anti-inflammatory cytokines such as IL-4 and IL-13 that can activate M2 macrophages to secrete IL-10 and promote insulin sensitivity.37 As body weight increases, Th2 cells and Tregs are gradually polarized into Th1, Th17, and CD8 + T cells. These cells produce pro-inflammatory cytokines.38–40 In mice with a high-fat diet, Treg decreased by 50% and CD8 + T cells doubled. After a normal diet, body weight and adipocyte size normalized, but the content of CD8 cells and Treg cells in AT did not. Insulin resistance did not improve, which indicates that immune cell memory may be the main cause of insulin resistance.41 By transferring Th2 cells to diet-induced obese mice with immunodeficiency, weight gain and insulin resistance can be reversed. Short-term treatment with CD3 specific antibodies or F(ab’)2 fragments can reduce Th1 cells, and It can reduce insulin resistance caused by high-fat diet.37 In recent years, it has been discovered that mucosal-associated invariant T cells (MAIT) induce polarization of M1 macrophages in obese AT, which promotes inflammation and intestinal microbiota disorders, leading to insulin resistance.42
A relative low number of B cells in healthy visceral fat resist bacterial infections from the peritoneal cavity.43 However, B cells increase in obese AT, promoting the activation of other immune cells such as T cells and M1 macrophages, which affect the metabolic state.40,44 B cells produce cytokines and the antibody IgG2c, which may also directly interact with adipocytes and affect insulin sensitivity.45 Treatment with CD20 antibody depletes B cells and can reduce insulin resistance and inflammation.38
Eosinophils are related to helminth immunity and allergy. They are reduced in obese AT and recover during intermittent fasting.46 Eosinophils can express IL-4 and IL-5, activate alternatively activated macrophages (AAMs) and exert anti-inflammatory effects.47 In worm-infected mice, eosinophils increased, while AT and blood sugar decreased.47 Additionally, eosinophils can activate beige fat cells by secreting certain factors such as KLF3, thereby reducing obesity-related disease.48
Neutrophils are often the first immune cells to reach the site of inflamed tissue. The production of leukotriene B 4 (LTB 4) in AT promotes the accumulation of neutrophils which express IL-1β through the NF-κB pathway to cause chronic inflammation.49 Other studies have also shown that, when exposed to saturated fatty acids, macrophages release nucleotides through pannexin-1. This may promote the recruitment of neutrophils into obese AT.50 These results indicate that in diet-induced obesity, neutrophils quickly infiltrate the abdominal AT and cause chronic inflammation.
Dendritic cells are the most effective antigen presenting cells (APC) in the immune system, they can play an important role in the transition from innate immunity to adaptive immunity by initiating differentiation of CD4 + helper T cells into Th1 and Th17. The increase of DCs in the AT of obese patients promotes the differentiation of Th17, which in turn leads to insulin resistance.51 In diet-induced obesity, DCs increase significantly in both the liver and in AT which promotes macrophage infiltration.52
Mast cells (MCs) can secrete many immune factors that are closely related to human allergic diseases. These cells induce obesity and insulin resistance by producing IL-6 and interferon-γ (IFN-γ).54 In diet-induced obese humans and mice, the number of mast cells increases and knocking out mast cells can reduce body weight and inflammation.53
We summarize the above immune cell changes in Figure 1. During the development of obesity, the immune cells in AT are transformed from anti-inflammatory immune cells (eosinophils, Th2 cells, and Tregs) to pro-inflammatory immune cells (neutrophils, B cells, CD8 T cells, DC, Th1 cells, and mast cells), leading to the occurrence of chronic sterile inflammation of AT, which in turn leads to insulin resistance.
Adipokine Dynamics in Obese Adipose Tissue
In addition to their role in energy regulation, adipocytes and AT immune cells secrete adipokines, biologically active peptides and proteins that regulate metabolism. Various proteomics methods have been used to study adipokines, and more than 600 potential adipokines have been identified.55 Cells secrete different types of adipokines depending on AT type and BMI levels. Adipokines can be roughly divided into two types: anti-inflammatory and pro-inflammatory. Unregulated expression of adipokines may be related to obesity, which further causes adipocyte dysfunction, chronic inflammation, and systemic insulin resistance. This review summarizes well-studied adipokines and the dysfunctions resulting from obesity.
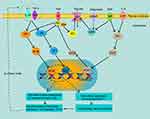
Leptin is a peptide hormone that is produced by differentiated adipocytes in subcutaneous WAT.56,57 Leptin-deficient mice will progress to obesity with an unrestricted diet, which shows that leptin may suppress the appetite via the central nervous system.58 Related studies have shown that leptin binds to the leptin receptor (LepR or LRb)(Figure 2), and directly inhibits the feeding center by activating signal transducers and transcription activators (STAT).59,60 Leptin can also increase fatty acid oxidation and insulin sensitivity by activating AMP protein kinase (AMPK).61 As body weight increases, the level of leptin in the body also increases. In obese patients, increased leptin does not suppress appetite, which may be due to leptin resistance. This may be due to down-regulation of leptin signaling by stimulating tyrosine phosphorylation on leptin receptor and suppression of cytokine signaling 3(SOCS3).62
Adiponectin is an insulin-sensitive adipokine that is highly expressed in AT. It exists in the blood in three main forms: high molecular weight, hexamer, and trimer.63 Adiponectin mRNA expression level is inversely proportional to BMI and is lower in VAT than in SAT.64,65 Adiponectin promotes insulin sensitivity by activating AMPK and peroxisome proliferator-activated receptor (PPAR)-α pathways (Figure 2). These pathways inhibit hepatic gluconeogenesis and increase the oxidation of FFA.28 Adiponectin can also inhibit the adhesion of monocytes and vascular endothelial cells and alleviate inflammation in AT.29
Resistin is so named because it can induce insulin resistance by reducing the expression of insulin receptor substrate (IRS) and AMPK (Figure 2).66 Resistin increases significantly in obese and diabetic people.67 Resistin can also directly damage endothelial cells by inducing the expression of MCP-1 and vascular cell adhesion molecules (VCAM-1).66 Mouse resistin is mainly expressed in AT, but in humans it is mainly secreted by monocytes and macrophages.68 Resistin also increases the expression of inflammatory factors such as IL-6 and TNF-α in AT.69
Omentin is a novel adipokine synthesized mainly in visceral stromal vascular cells. In adipocytes, omentum enhances the phosphorylation of Akt in insulin signaling and improves insulin sensitivity.70 In obese and diabetic patients, omentum decreases.71 Omentin can also inhibit the expression of endothelial cell adhesion molecules, and thus play a protective role in cardiovascular diseases.72,73
Tumor necrosis factor-α (TNF-α) is an adipokine that can be secreted by both adipocytes and immune cells.74 Adipose tissue TNF-α increases in overweight individuals. Compared with lean humans adipose tissue, TNF-α expression is 2.5 times higher in obesity. There is a strong positive correlation with hyperinsulinemia.75 TNF-α can increase lipolysis by increasing the level of cAMP.76 TNFα can also increase FFA release by directly activating hormone-sensitive lipase (HSL), which in turn promotes insulin resistance in the liver and skeletal muscle. TNFα inhibits the phosphorylation of insulin receptor substrate 1 (IRS-1) by activating c-Jun N-terminal kinase (JNK) and IκB kinase (IKK), thereby preventing insulin signal transduction (Figure 2).17 In human obesity, TNF-α can also accelerate atherosclerosis by inducing vascular cell adhesion molecule 1 (VCAM1).77
IL-6 directly stimulates lipolysis.78 IL-6 in AT can stimulate the liver to produce C-reactive protein (CRP), which is an important cardiovascular risk factor.79 Omentum AT releases 2–3 times IL-6 than subcutaneous AT. IL-6, like leptin, can suppress appetite via STAT3 signaling in the central nervous system. In addition, IL-6 can inhibit the phosphorylation of IRS-1 in adipocytes and liver cells by increasing the expression of suppressor of cytokine signaling 3 (SOCS3) (Figure 2). This inhibits insulin conduction and leads to insulin resistance.80,81
Monocyte chemoattractant protein-1 (MCP-1/CCL2) is a CC chemokine family member.82 During the development of obesity, macrophages and adipocytes secrete MCP-1. It binds to monocytes in the blood, causing them to accumulate in AT. These monocytes differentiate into M1 macrophages that secrete proinflammatory factors, accelerating AT inflammation and systemic Insulin resistance.83,84 Palmitate (PA) induces MCP-1 secretion of macrophages through the MAPK/TLR4 signaling pathway.85 BMI and obesity are positively correlated with the expression level of MCP-1. Weight loss causes a decrease in MCP-1 expression.86
In recent years, many other adipokines have been found which may be related to obesity metabolism. They are summarized in Table 2. In obese patients, the levels of these adipokines and inflammatory signals undergo changes. These changes are closely related to obesity-related conditions such as insulin resistance, cardiovascular disease, and cancer.
 |
Table 2 Some Other Adipokines Related to Obesity Metabolism |
Anti-Inflammatory Therapeutic Effect of Some Drugs
We already know that chronic sterile inflammation of obese AT leads to insulin resistance. Can it be targeted to prevent the transmission of inflammation signals and improve insulin sensitivity? Many studies have confirmed that this is feasible. Anti-inflammatory treatment has visible potential. Biological inhibitors of classical inflammatory molecules (including TNF-α, IL-6 and IL-1) are being used in the clinical treatment of rheumatoid arthritis (RA), and there are several prospective clinical trials for RA patients with insulin resistance. The results have shown that the use of anti-TNF drugs such as infliximab, etanercept, and other treatments can increase AKT phosphorylation and can significantly improve insulin sensitivity.87 After 3–6 months of anti-TNF treatment in patients with rheumatoid arthritis and insulin resistance, insulin sensitivity and β-cell function were significantly improved.88 Additionally, the non-steroidal anti-inflammatory drug aspirin can also inhibit TNF-α levels and NF-κB activation to improve insulin sensitivity, with 8 weeks of aspirin treatment in diabetic rats, insulin resistance and inflammatory factors reduced.89 However, some related studies indicate of TNF-α inhibitors that have not replicated these findings.90 The latest systematic analysis of retrospective studies concluded that anti-TNF therapy can improve insulin sensitivity.91 Other studies also provided promising evidence that anakinra, an IL-1 receptor antagonist, can significantly improve insulin resistance and related inflammation in RA and T2D participants.92 The IL-6 inhibitor, tocilizumab, produces a rapid beneficial effect on insulin sensitivity.93 The JAK inhibitor, tofacitinib, can reduce insulin resistance and hyperglycemia in T2D patients.94 Erythropoietin (EPO) has been found in recent years to not only promote erythropoiesis, but also activate phosphatidylinositol 3-kinase (PI3K)/AKT pathway and promote PPAR-γ transcription. EPO can promote fat synthesis and glucose transport, so it is a potential drug for the treatment of insulin resistance.95,96 At present, these targeted therapies to inhibit inflammatory signaling have achieved limited success for insulin-resistant patients without other complications, and further studies are needed. The harmful immunosuppressive effects and possible harmful side effects of any anti-inflammatory treatment must be carefully examined.
Anti-Inflammatory Therapeutic Effect of Bariatric Surgery
In 1991, the National Institutes of Health established the initial surgical intervention standard for obesity. Patients with a BMI ≥ 35 kg/m2 with comorbidities, such as cardiovascular disease, diabetes, arthritis, respiratory barriers, reproductive disorders, etc., or those with BMI ≥40 kg/m2 are suitable candidates for bariatric surgery.97,98 Compared with lifestyle changes and medication management, bariatric surgery can lead to sustained weight loss (20% to 30%). Type 2 diabetes remission rates range from 23% to 60%, and the risk of surgery is as low as ordinary appendectomy and cholecystectomy.99 Bariatric surgery aims to physically limit the intake or absorption of food. It can produce lasting weight loss and health benefits by changing metabolism and reducing appetite.100 In 2018, the number of bariatric surgeries in the United States reached 250,000. Sleeve gastrectomy (SG) was the most common operation at 61.4%, followed by Roux-en-Y gastric bypass (RYGB) at 17.0%, laparoscopic adjustable gastric banding (LAGB) at 1.1%; biliopancreatic diversion at 0.8%, and a modified surgery, bioenterics intragastric balloon and vagal blockade.101 Sjostrom’s study showed that the adjusted mortality rate of bariatric surgery was 30.7% lower than that of non-surgical group.102 Jouan et al found that chemerin may play a key role in inflammation caused by obesity. In addition, there was a significant correlation between weight loss and improvement of inflammatory parameters. After surgery, weight loss reached (39.5±13.8 kg), and pro-inflammatory markers (IL-6, CRP, leptin, and resistin) were significantly reduced. The anti-inflammatory markers (IL-10 and adiponectin) increased.103 In another prospective observational study, the levels of inflammation markers like high-sensitivity CRP and soluble urokinase gradually decreased, and the secretion of pro-inflammatory interleukins (1, 6, and 8) decreased within one year after RYGB surgery.104 Many clinical studies have also shown that inflammatory factors and TLR receptors are significantly reduced after surgery, but adipokines like leptin and adiponectin have not shown consistent results.105–107 Some bariatric surgery patients regained weight after weight loss, but the inflammatory factors continued to decrease, indicating that bariatric surgery may have a long-term effect on inflammation control.108
Conclusion
In conclusion, AT is a multifunctional organ with complex energy regulation and immune functions throughout the body. White AT regulates metabolism through esterification and lipolysis, while brown and beige AT utilize fatty acids for heat production and energy consumption via UCP-1. In healthy individuals, adipocytes can resist the lipotoxicity of non-esterified fatty acids through hyperplasia and hypertrophy. They also secrete adipokines such as leptin and resistin to regulate appetite and fatty acid oxidation. However, in obese individuals, with excessive energy intake, adipocytes proliferate and hypertrophy, blood supply decreased, AT secretes more hypoxia factors and adipokines such as HIF-1, MCP-1, leptin, resistin, etc. These dysregulated adipokines attract more pro-inflammatory cells such as M1 macrophages, neutrophils, Th1, and Th17 cells, into the AT. These pro-inflammatory cells will secrete more inflammatory signals such as TNF-α, IL- 6, which will increase lipolysis and decrease synthesis. AT releases more FFA and proinflammatory adipokines will interfere with the insulin-glucose transport pathway, which will lead to the occurrence of insulin resistance. Therefore, for obese people with insulin resistance, anti-inflammatory therapy has great potential.
Future Perspectives
In recent years, there have been more and more clinical studies on drugs and surgery for obese individuals. We also summarized the effects of anti-inflammatory and surgical treatments on inflammation and insulin resistance. The results show that both anti-inflammatory treatments and surgical treatments benefit insulin resistance and reduce inflammatory factors in circulation. Therefore, controlling AT inflammation may be an effective approach to treat obesity and insulin resistance. However, these studies are still in the early stages. In the future, researchers may focus on finding inhibitors of specific inflammatory signals without the immunosuppressive side effects of existing anti-inflammatory drugs.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Siegel RL, Torre LA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69(2):88–112.
2. Andersen LB, Mota J, Di Pietro L. Update on the global pandemic of physical inactivity. Lancet. 2016;388(10051):1255–1256. doi:10.1016/S0140-6736(16)30960-6
3. Tomiyama AJ. Stress and obesity. Ann Rev Psychol. 2019;70(1):703–718. doi:10.1146/annurev-psych-010418-102936
4. Yang C, Kong APS, Cai Z, Chung ACK. Persistent organic pollutants as risk factors for obesity and diabetes. Curr Diab Rep. 2017;17(12).
5. WHO. Obesity and overweight fact sheet; 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
6. Murtagh E. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642.
7. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. doi:10.1056/NEJMsr1606602
8. Gadde KM, Martin CK, Berthoud H-R, Heymsfield SB. Obesity pathophysiology and management. J Am Coll Cardiol. 2018;71(1):69–84. doi:10.1016/j.jacc.2017.11.011
9. Greco F, Mallio CA, Grippo R, et al. Increased visceral adipose tissue in male patients with non-clear cell renal cell carcinoma. Radiol Med. 2020;125(6):538–543. doi:10.1007/s11547-020-01146-6
10. Greco F, Quarta LG, Grasso RF, Beomonte Zobel B, Mallio CA. Increased visceral adipose tissue in clear cell renal cell carcinoma with and without peritumoral collateral vessels. Br J Radiol. 2020;93(1112):20200334. doi:10.1259/bjr.20200334
11. Matrone A, Ferrari F, Santini F, Elisei R. Obesity as a risk factor for thyroid cancer. Curr Opin Endocrinol Diabetes Obes. 2020;27(5):358–363. doi:10.1097/MED.0000000000000556
12. Silveira EA, Kliemann N, Noll M, Sarrafzadegan N, de Oliveira C. Visceral obesity and incident cancer and cardiovascular disease: an integrative review of the epidemiological evidence. Obes Rev. 2020. doi:10.1111/obr.13088
13. Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world–a growing challenge. N Engl J Med. 2007;356(3):213–215. doi:10.1056/NEJMp068177
14. Jiang G, Luk AO, Tam CHT, et al. Obesity, clinical, and genetic predictors for glycemic progression in Chinese patients with type 2 diabetes: a cohort study using the Hong Kong diabetes register and Hong Kong diabetes biobank. PLoS Med. 2020;17(7):e1003209. doi:10.1371/journal.pmed.1003209
15. Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59(6):1075–1088. doi:10.1007/s00125-016-3933-4
16. Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology. 2013;154(9):2992–3000. doi:10.1210/en.2013-1403
17. Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22.
18. Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34(1):1–11. doi:10.1016/j.mam.2012.10.001
19. Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350(25):2549–2557. doi:10.1056/NEJMoa033179
20. Hocking SL, Stewart RL, Brandon AE, et al. Subcutaneous fat transplantation alleviates diet-induced glucose intolerance and inflammation in mice. Diabetologia. 2015;58(7):1587–1600. doi:10.1007/s00125-015-3583-y
21. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. doi:10.1056/NEJMoa0810780
22. Ravussin E, Galgani JE. The implication of brown adipose tissue for humans. Annu Rev Nutr. 2011;31(1):33–47. doi:10.1146/annurev-nutr-072610-145209
23. Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125(2):478–486. doi:10.1172/JCI78362
24. Sepa-Kishi DM, Ceddia RB. White and beige adipocytes: are they metabolically distinct? Horm Mol Biol Clin Investig. 2018;33(2). doi:10.1515/hmbci-2018-0003
25. Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842(3):446–462. doi:10.1016/j.bbadis.2013.05.017
26. Oishi Y, Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol. 2018;30(11):511–528.
27. Aron-Wisnewsky J, Tordjman J, Poitou C, et al. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94(11):4619–4623. doi:10.1210/jc.2009-0925
28. Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155(4):407–417. doi:10.1111/imm.13002
29. Qiu Y, Nguyen KD, Odegaard JI, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–1308. doi:10.1016/j.cell.2014.03.066
30. Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120(10):3466–3479. doi:10.1172/JCI42845
31. Ferrante AW
32. Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. doi:10.1194/jlr.M500294-JLR200
33. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi:10.1172/JCI29881
34. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830.
35. Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications–a review. Nutr J. 2014;13(1):17. doi:10.1186/1475-2891-13-17
36. Oestreich KJ, Weinmann AS. Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors. Nat Rev Immunol. 2012;12(11):799–804. doi:10.1038/nri3321
37. Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921–929. doi:10.1038/nm.2001
38. Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17(5):610–617. doi:10.1038/nm.2353
39. Ahmed M, Gaffen SL. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 2010;21(6):449–453. doi:10.1016/j.cytogfr.2010.10.005
40. DeFuria J, Belkina AC, Jagannathan-Bogdan M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110(13):5133–5138. doi:10.1073/pnas.1215840110
41. Blaszczak AM, Bernier M, Wright VP, et al. Obesogenic memory maintains adipose tissue inflammation and insulin resistance. Immunometabolism. 2020;2(3).
42. Toubal A, Kiaf B, Beaudoin L, et al. Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat Commun. 2020;11(1):3755. doi:10.1038/s41467-020-17307-0
43. Khan S, Tsai S, Winer DA. Adipose tissue B cells come of age: the AABs of fat inflammation. Cell Metab. 2019;30(6):997–999. doi:10.1016/j.cmet.2019.11.007
44. Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113(9):1009–1023.
45. Frasca D, Diaz A, Romero M, Thaller S, Blomberg BB. Secretion of autoimmune antibodies in the human subcutaneous adipose tissue. PLoS One. 2018;13(5):e0197472. doi:10.1371/journal.pone.0197472
46. Bolus WR, Kennedy AJ, Hasty AH. Obesity‐induced reduction of adipose eosinophils is reversed with low‐calorie dietary intervention. Physiol Rep. 2018;6(22):22. doi:10.14814/phy2.13919
47. Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–247. doi:10.1126/science.1201475
48. Knights AJ, Vohralik EJ, Houweling PJ, et al. Eosinophil function in adipose tissue is regulated by Krüppel-like factor 3 (KLF3). Nat Commun. 2020;11(1):1–12. doi:10.1038/s41467-020-16758-9
49. Watanabe Y, Nagai Y, Honda H, et al. Bidirectional crosstalk between neutrophils and adipocytes promotes adipose tissue inflammation. FASEB J. 2019;33(11):11821–11835.
50. Tam TH, Chan KL, Boroumand P, et al. Nucleotides released from palmitate-activated murine macrophages attract neutrophils. J Biol Chem. 2020;295(15):4902–4911. doi:10.1074/jbc.RA119.010868
51. Bertola A, Ciucci T, Rousseau D, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61(9):2238–2247. doi:10.2337/db11-1274
52. Stefanovic-Racic M, Yang X, Turner MS, et al. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 2012;61(9):2330–2339.
53. Zhou Y, Yu X, Chen H, et al. Leptin deficiency shifts mast cells toward anti-inflammatory actions and protects mice from obesity and diabetes by polarizing M2 macrophages. Cell Metab. 2015;22(6):1045–1058. doi:10.1016/j.cmet.2015.09.013
54. Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15(8):940–945. doi:10.1038/nm.1994
55. Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012;6(1‐2):91–101.
56. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JMJN. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi:10.1038/372425a0
57. Hube F, Lietz U, Igel M, et al. Difference in leptin mRNA levels between omental and subcutaneous abdominal adipose tissue from obese humans. Horm Metab Res. 1996;28(12):690–693. doi:10.1055/s-2007-979879
58. Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295.
59. Leshan RL, Bjornholm M, Munzberg H, Myers MG
60. Pan W, Allison MB, Sabatini P, et al. Transcriptional and physiological roles for STAT proteins in leptin action. Mol Metab. 2019;22:121–131. doi:10.1016/j.molmet.2019.01.007
61. Minokoshi Y, Kim Y-B, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–343. doi:10.1038/415339a
62. Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70(1):537–556. doi:10.1146/annurev.physiol.70.113006.100707
63. Mai S, Walker GE, Vietti R, et al. Acute vitamin D(3) supplementation in severe obesity: evaluation of multimeric adiponectin. Nutrients. 2017;9(5):459. doi:10.3390/nu9050459
64. Lihn AS, Bruun JM, He G, Pedersen SB, Jensen PF, Richelsen BJM. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol Cell Endocrinol. 2004;219(1–2):9–15. doi:10.1016/j.mce.2004.03.002
65. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–26749. doi:10.1074/jbc.270.45.26746
66. Leal VdO MD. Adipokines in obesity. Clin Chim Acta. 2013;419:87–94.
67. Liu W, Zhou X, Li Y, et al. Serum leptin, resistin, and adiponectin levels in obese and non-obese patients with newly diagnosed type 2 diabetes mellitus: a population-based study. Medicine. 2020;99(6):e19052. doi:10.1097/MD.0000000000019052
68. Tsiotra PC, Tsigos C, Anastasiou E, et al. Peripheral mononuclear cell resistin mRNA expression is increased in type 2 diabetic women. Mediators Inflamm. 2008;2008:892864. doi:10.1155/2008/892864
69. Nagaev I, Bokarewa M, Tarkowski A, Smith U. Human resistin is a systemic immune-derived proinflammatory cytokine targeting both leukocytes and adipocytes. PLoS One. 2006;1(1):e31. doi:10.1371/journal.pone.0000031
70. Yang R-Z, Lee M-J, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290(6):E1253–E1261. doi:10.1152/ajpendo.00572.2004
71. Elsaid NH, Sadik NA, Ahmed NR, Fayez SE, Mohammed NAE-G. Serum omentin-1 levels in type 2 diabetic obese women in relation to glycemic control, insulin resistance and metabolic parameters. J Clin Transl Endocrinol. 2018;13:14–19. doi:10.1016/j.jcte.2018.05.003
72. Zhong X, Li X, Liu F, Tan H, Shang DJB. Omentin inhibits TNF-α-induced expression of adhesion molecules in endothelial cells via ERK/NF-κB pathway. Biochem Biophys Res Commun. 2012;425(2):401–406.
73. Chen Y, Liu F, Han F, Lv L, Tang CE, Luo F. Omentin-1 ameliorated free fatty acid-induced impairment in proliferation, migration, and inflammatory states of HUVECs. Cardiol Res Pract. 2020;2020:3054379. doi:10.1155/2020/3054379
74. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi:10.1126/science.7678183
75. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–2415. doi:10.1172/JCI117936
76. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377.
77. Stanley TL, Zanni MV, Johnsen S, et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96(1):E146–150. doi:10.1210/jc.2010-1170
78. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW
79. Maachi M, Pieroni L, Bruckert E, et al. Systemic low-grade inflammation is related to both circulating and adipose tissue TNF α, leptin and IL-6 levels in obese women. Int J Obes. 2004;28(8):993–997. doi:10.1038/sj.ijo.0802718
80. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–139. doi:10.1016/j.mce.2009.08.018
81. Timper K, Denson JL, Steculorum SM, et al. IL-6 improves energy and glucose homeostasis in obesity via enhanced central IL-6 trans-signaling. Cell Rep. 2017;19(2):267–280. doi:10.1016/j.celrep.2017.03.043
82. Ray I, Mahata SK, De RK. Obesity: an immunometabolic perspective. Front Endocrinol (Lausanne). 2016;7:157. doi:10.3389/fendo.2016.00157
83. Nishimoto S, Fukuda D, Higashikuni Y, et al. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci Adv. 2016;2(3):e1501332.
84. Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–124. doi:10.1172/JCI24335
85. Wang X, Jiang X, Deng B, Xiao J, Jin J, Huang Z. Lipopolysaccharide and palmitic acid synergistically induced MCP-1 production via MAPK-meditated TLR4 signaling pathway in RAW264.7 cells. Lipids Health Dis. 2019;18(1):71. doi:10.1186/s12944-019-1017-4
86. Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes. 2005;29(1):146–150. doi:10.1038/sj.ijo.0802839
87. Stagakis I, Bertsias G, Karvounaris S, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14(3):R141.
88. van den Oever IAM, Baniaamam M, Simsek S, et al. The effect of anti-TNF treatment on body composition and insulin resistance in patients with rheumatoid arthritis. Rheumatol Int. 2020. doi:10.1007/s00296-020-04666-6
89. Sun X, Han F, Yi J, Han L, Wang B. Effect of aspirin on the expression of hepatocyte NF-kappaB and serum TNF-alpha in streptozotocin-induced type 2 diabetic rats. J Korean Med Sci. 2011;26(6):765–770. doi:10.3346/jkms.2011.26.6.765
90. Ferraz-Amaro I, Arce-Franco M, Muniz J, et al. Systemic blockade of TNF-alpha does not improve insulin resistance in humans. Horm Metab Res. 2011;43(11):801–808. doi:10.1055/s-0031-1287783
91. Leporini C, Russo E. Insulin-sensiting effects of tumor necrosis factor alpha inhibitors in rheumatoid arthritis: a systematic review and meta-analysis. Rev Recent Clin Trials. 2018;13(3):184–191. doi:10.2174/1574887113666180314100340
92. Ruscitti P, Masedu F, Alvaro S, et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): a multicentre, open-label, randomised controlled trial. PLoS Med. 2019;16(9):e1002901. doi:10.1371/journal.pmed.1002901
93. Castaneda S, Remuzgo-Martinez S, Lopez-Mejias R, et al. Rapid beneficial effect of the IL-6 receptor blockade on insulin resistance and insulin sensitivity in non-diabetic patients with rheumatoid arthritis. Clin Exp Rheumatol. 2019;37(3):465–473.
94. Bako HY, Ibrahim MA, Isah MS, Ibrahim S. Inhibition of JAK-STAT and NF-kappaB signalling systems could be a novel therapeutic target against insulin resistance and type 2 diabetes. Life Sci. 2019;239:117045. doi:10.1016/j.lfs.2019.117045
95. Ge Z, Zhang P, Hong T, et al. Erythropoietin alleviates hepatic insulin resistance via PPARgamma-dependent AKT activation. Sci Rep. 2015;5(1):17878. doi:10.1038/srep17878
96. Zhang H, Ge Z, Tang S, Meng R, Bi Y, Zhu D. Erythropoietin ameliorates PA-induced insulin resistance through the IRS/AKT/FOXO1 and GSK-3beta signaling pathway, and inhibits the inflammatory response in HepG2 cells. Mol Med Rep. 2017;16(2):2295–2301. doi:10.3892/mmr.2017.6810
97. Wolfe BM, Kvach E, Eckel RH. Treatment of obesity: weight loss and bariatric surgery. Circ Res. 2016;118(11):1844–1855. doi:10.1161/CIRCRESAHA.116.307591
98. Panel NIoHCD. Gastrointestinal surgery for severe obesity. Ann Intern Med. 1991;115:956–961.
99. Hanipah ZN, Schauer PR. Bariatric surgery as a long-term treatment for type 2 diabetes/metabolic syndrome. Annu Rev Med. 2020;71:1–15.
100. Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018;39(2):79–132.
101. English WJ, DeMaria EJ, Hutter MM, et al. American society for metabolic and bariatric surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis. 2020;16(4):457–463. doi:10.1016/j.soard.2019.12.022
102. Sjostrom L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes. 2008;32(Suppl 7):S93–97. doi:10.1038/ijo.2008.244
103. Jouan Y, Blasco H, Bongrani A, Couet C, Dupont J, Maillot F. Preoperative chemerin level is predictive of inflammatory status 1 year after bariatric surgery. Obes Surg. 2020;30(10):3852–3861. doi:10.1007/s11695-020-04584-3
104. Faramia J, Ostinelli G, Drolet-Labelle V, Picard F, Tchernof A. Metabolic adaptations after bariatric surgery: adipokines, myokines and hepatokines. Curr Opin Pharmacol. 2020;52:67–74. doi:10.1016/j.coph.2020.06.005
105. Sala P, Torrinhas R, Fonseca DC, et al. Intestinal expression of toll-like receptor gene changes early after gastric bypass surgery and association with type 2 diabetes remission. Nutrition. 2020;79–80:110885. doi:10.1016/j.nut.2020.110885
106. Salman MA, Salman AA, Nafea MA, et al. Study of changes of obesity-related inflammatory cytokines after laparoscopic sleeve gastrectomy. ANZ J Surg. 2019;89(10):1265–1269. doi:10.1111/ans.15427
107. Min T, Prior SL, Dunseath G, Churm R, Barry JD, Stephens JW. Temporal effects of bariatric surgery on adipokines, inflammation and oxidative stress in subjects with impaired glucose homeostasis at 4 years of follow-up. Obes Surg. 2020;30(5):1712–1718. doi:10.1007/s11695-019-04377-3
108. Kerr AG, Andersson DP, Rydén M, Arner P, Dahlman I. Long-term changes in adipose tissue gene expression following bariatric surgery. J Intern Med. 2020;288(2):219–233. doi:10.1111/joim.13066
109. Lee Y-H, Hsiao H-F, Yang H-T, Huang S-Y, Chan WP. Reproducibility and repeatability of computer tomography-based measurement of abdominal subcutaneous and visceral adipose tissues. Sci Rep. 2017;7(1):40389. doi:10.1038/srep40389
110. Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes. 2011;2011:490650. doi:10.1155/2011/490650
111. Shen W, Wang Z, Punyanita M, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res. 2003;11(1):5–16. doi:10.1038/oby.2003.3
112. Cuevas-Ramos D, Almeda-Valdes P, Aguilar-Salinas CA, Cuevas-Ramos G, Cuevas-Sosa AA, Gomez-Perez FJ. The role of fibroblast growth factor 21 (FGF21) on energy balance, glucose and lipid metabolism. Curr Diabetes Rev. 2009;5(4):216–220. doi:10.2174/157339909789804396
113. Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020. doi:10.1038/s41574-020-0386-0
114. Alessi MC, Peiretti F, Morange P, Henry M, Nalbone G, Juhan-Vague I. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes. 1997;46(5):860–867. doi:10.2337/diab.46.5.860
115. Hosaka S, Yamada T, Takahashi K, et al. Inhibition of plasminogen activator inhibitor-1 activation suppresses high fat diet-induced weight gain via alleviation of hypothalamic leptin resistance. Front Pharmacol. 2020;11:943. doi:10.3389/fphar.2020.00943
116. Zheng Z, Nakamura K, Gershbaum S, et al. Interacting hepatic PAI-1/tPA gene regulatory pathways influence impaired fibrinolysis severity in obesity. J Clin Invest. 2020;10–1172.
117. Liu M, Zhu H, Dai Y, et al. Zinc-α2-glycoprotein is associated with obesity in chinese people and HFD-induced obese mice. Front Physiol. 2018;9:62. doi:10.3389/fphys.2018.00062
118. Severo JS, Morais JBS, Beserra JB, et al. Role of zinc in zinc-α2-glycoprotein metabolism in obesity: a review of literature. Biol Trace Elem Res. 2020;193(1):81–88. doi:10.1007/s12011-019-01702-w
119. Rychter AM, Skrzypczak-Zielińska M, Zielińska A, et al. Is the retinol-binding protein 4 a possible risk factor for cardiovascular diseases in obesity? Int J Mol Sci. 2020;21(15):5229. doi:10.3390/ijms21155229
120. Kilicarslan M, de Weijer BA, Simonyté Sjödin K, et al. RBP4 increases lipolysis in human adipocytes and is associated with increased lipolysis and hepatic insulin resistance in obese women. FASEB J. 2020;34(5):6099–6110. doi:10.1096/fj.201901979RR
121. Kelly KR, Kashyap SR, O’Leary VB, Major J, Schauer PR, Kirwan JP. Retinol-binding protein 4 (RBP4) protein expression is increased in omental adipose tissue of severely obese patients. Obesity (Silver Spring). 2010;18(4):663–666. doi:10.1038/oby.2009.328
122. Bozaoglu K, Bolton K, McMillan J, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148(10):4687–4694. doi:10.1210/en.2007-0175
123. Karczewska-Kupczewska M, Nikołajuk A, Stefanowicz M, Matulewicz N, Kowalska I, Strączkowski M. Serum and adipose tissue chemerin is differentially related to insulin sensitivity. Endocr Connect. 2020;9(5):360–369. doi:10.1530/EC-20-0084
124. Heo YJ, Choi SE, Jeon JY, et al. Visfatin induces inflammation and insulin resistance via the NF-κB and STAT3 signaling pathways in hepatocytes. J Diabetes Res. 2019;2019:4021623.
125. Nourbakhsh M, Nourbakhsh M, Gholinejad Z, Razzaghy-Azar M. Visfatin in obese children and adolescents and its association with insulin resistance and metabolic syndrome. Scand J Clin Lab Invest. 2015;75(2):183–188. doi:10.3109/00365513.2014.1003594
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.