Back to Journals » Journal of Asthma and Allergy » Volume 16
Characteristics of Severe Non-Eosinophilic Asthma: Analysis of Data from 1075 Patients Included in the FASE-CPHG Study
Authors Portel L, Fabry-Vendrand C, Texier N, Schwartz D, Capdepon A, Thabut G, Debieuvre D
Received 19 May 2022
Accepted for publication 10 December 2022
Published 4 January 2023 Volume 2023:16 Pages 9—21
DOI https://doi.org/10.2147/JAA.S375325
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Laurent Portel,1 Caroline Fabry-Vendrand,2 Nathalie Texier,3 Déborah Schwartz,3 Audrey Capdepon,2 Gabriel Thabut,2 Didier Debieuvre4
1Service de Pneumologie, Centre Hospitalier Robert Boulin, Libourne, France; 2Astra Zeneca, Courbevoie, France; 3Kappa Santé, Paris, France; 4Service de Pneumologie, Hôpital Émile Muller, Groupe Hospitalier de la Région Mulhouse Sud-Alsace (GHRMSA), Mulhouse, France
Correspondence: Laurent Portel, Service de Pneumologie, Centre Hospitalier Robert Boulin, 112 Avenue de la Marne, BP 199, Libourne, 33505, France, Tel +33 557 553 560, Fax +33 557 553 431, Email [email protected]
Purpose: Data on severe non-eosinophilic asthma are scarce. Moreover, as compared with eosinophilic asthma, non-eosinophilic asthma less frequently benefits from the latest therapeutic advances. This study aimed to highlight differences between non-eosinophilic and eosinophilic asthma as they may help the development of new therapeutic agents.
Patients and Methods: Data from 1075 adult patients with severe asthma (GINA treatment: 4/5) collected during the cross-sectional non-interventional FASE-CPHG study were analyzed. Two groups of patients (EOS-/EOS+) were constituted based on blood eosinophil counts (cutoff value: 300 G/l). Characteristics of EOS- (N = 500) and EOS+ (N = 575) patients were described; EOS- patients were also described according to their allergic profile based on skin allergy or allergen-specific immunoglobulin E (IgE) assays (cutoff value: 150 IU/mL).
Results: Percentages of patients with obesity (29%), allergen sensitization (57%), or ≥ 2 annual exacerbations in the last 12 months (68%) were similar in both groups. As compared with EOS+ patients, EOS- patients less frequently reported chronic rhinitis (41.1% vs 50.5%, p < 0.01) or nasal polyposis (13.6% vs 27.5%, p < 0.01), and more frequently reported GERD (45.2% vs 37.1%, p < 0.01), anxiety (45.5% vs 38.1%, p = 0.01), or depression (18.3% vs 13.3%, p = 0.02). EOS- patients had lower serum total IgE levels (median: 158 vs 319 IU/mL, p < 0.01) and were less frequently treated with long-term oral corticosteroid therapy (16.0% vs 23.7%; p < 0.01). Their asthma was more frequently uncontrolled (48% vs 40%, p < 0.01). Similar results were found with a cutoff value for blood eosinophil counts at 150 G/l. EOS- patients with allergic profile less frequently reported high serum IgE levels (35.6% vs 57.9%, p < 0.01). EOS- and EOS+ patients treated with long-term oral corticosteroids had similar profiles.
Conclusion: In our patients with severe asthma, EOS- asthma was approximately as frequent as EOS+ asthma; EOS- asthma was frequently poorly controlled or uncontrolled, confirming the need for a better management. Allergy did not appear to worsen clinical profile.
Keywords: asthma, eosinophils, France, hospital, observational study
Introduction
Worldwide, asthma affects over 260 million people.1 In this population, a limited but significant group has severe asthma.2 Although the European Respiratory Society/American Thoracic Society (ERS/ATS) and the Global Initiative for Asthma (GINA) define severe asthma differently, both use medication levels to distinguish severe asthma.2–4 Using GINA treatment steps 4 and 5 to define severe asthma, the West Sweden Asthma Study (WSAS) recently estimated the prevalence of severe asthma at 1.1% in the adult population and 9.5% in the asthma population.2 In France, about 4 million people have asthma according to the French national public health agency (https://www.santepubliquefrance.fr) and the estimated prevalence of severe asthma in adults is 5%.5,6
Severe asthma impacts the quality of life of patients and affects their emotional and mental health.4,6 Patients experience a heavy burden of symptoms, exacerbations, and medication side effects that frequently interfere with their daily life. Severe asthma also increases the burden of asthma on health care systems (medications, physician visits, hospitalizations).4,5 Consequently, severe asthma is recognized as a major unmet need and is under constant research.6
Asthma, including severe asthma, is a heterogenous disease with diverse clinical presentations or phenotypes, and several classifications have been proposed.7 Classifications are based on different parameters such as underlying mechanisms (allergic vs non-allergic) or histological background (eosinophilic vs non-eosinophilic). Approximately half of all patients with asthma had elevated eosinophil levels leading to classify their asthma as eosinophilic.8 Finally, within each phenotype, asthma can be further differentiated. For example, patients with non-eosinophilic asthma can be differentiated according to the presence of neutrophils in sputum or positive results to skin prick or allergy blood tests (allergic vs non-allergic asthma).
Although the heterogeneity of asthma is present across the whole spectrum of severity, it is particularly relevant in severe asthma as identification by phenotype is becoming crucial with the development of precision medicine and the recommendations of selective therapies for patients suffering from severe asthma.6,9 New, effective therapeutic approaches, such as targeted biologic add-on treatments, have been more frequently developed in the treatment of severe eosinophilic than non-eosinophilic asthma.7,10 Non-eosinophilic asthma is known to poorly respond to standard asthma treatments, especially to inhaled corticosteroids.8 Smoking cessation and removal from exposure to some occupational agents reduce neutrophilic inflammation in severe non-eosinophilic asthma.9 Regarding drug therapy, preliminary studies on off-label use of licensed drugs in severe non-eosinophilic asthma suggest that macrolides are effective in non-smokers,9 while theophylline and peroxisome proliferator-activated receptor gamma (PPARγ) agonists may benefit smokers. Novel small molecules targeting neutrophilic inflammation, such as chemokine receptor 2 (CXCR2) antagonists, reduce neutrophils, but to date, their benefit has not been evidenced in clinical studies.11,12 Inhaled phosphodiesterase (PDE) inhibitors, dual PDE3 and PDE4 inhibitors, p38MAPK (mitogen-activated protein kinase) inhibitors, tyrosine kinase inhibitors and PI (phosphoinositide) 3 kinase inhibitors are under development and these compounds may be of benefit in non-eosinophilic inflammation.11 Recently, tezepelumab, a human monoclonal antibody that blocks thymic stromal lymphopoietin (TSLP), an epithelial-cell-derived cytokine implicated in the pathogenesis of asthma, has shown efficacy in reducing exacerbation rates among patients with severe, uncontrolled asthma, regardless of the blood eosinophil counts.7
The goal of asthma treatment is to achieve good control of symptoms, reduce exacerbations, and improve quality of life. This goal is still far from being achieved for severe non-eosinophilic asthma. A better knowledge of severe non-eosinophilic asthma and its difference with eosinophilic asthma could favor the development of new therapeutic agents. Therefore, a secondary analysis of the data from the FASE-CPHG study which included adult patients with severe asthma was performed focusing on patients with non-eosinophilic asthma and comparing their characteristics and their management to those of patients with eosinophilic asthma.
Materials and Methods
This study was a secondary data analysis of the existing FASE-CPHG database. FASE-CPHG was an observational, cross-sectional, prospective, multicentric study conducted in France by respiratory physicians working in non-academic hospitals on a large population of adult patients with severe asthma. Its primary objective was to assess the characteristics of patients with severe non-eosinophilic asthma and the burden of severe non-eosinophilic asthma. Its secondary objectives were to compare data from patients with severe eosinophilic and non-eosinophilic asthma and describe the profile of patients with severe non-eosinophilic asthma according to their allergic profile.13–15
Data from 1502 adult (>18 years) patients with severe asthma were collected from May 2016 to June 2017 in France by 104 respiratory physicians in non-academic hospitals. All patients received information about the study and gave their informed consent before being included in the FASE-CPHG study.
Patients were informed during a regular visit by their physicians. The physicians documented patients’ and disease characteristics (including age, sex, body mass index, comorbidities, skin allergy test results, total serum Immunoglobulin E (IgE) levels, number of exacerbations within the last 12 months, and ongoing asthma treatment at the time of the visit. They based their diagnosis of severe asthma on GINA criteria: asthma was severe if it required GINA treatment step 4 or 5 to be controlled or if it remained uncontrolled despite this treatment. Patients completed the Asthma Control Test (ACT). Exacerbations were defined based on oral corticosteroids (OCS) use or increased dose of inhaled corticosteroids (ICS).
The FASE-CPHG study was approved by the local ethics committee (Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé, CCTIRS) and was conducted according to the French law and guidelines on epidemiological and descriptive studies. Further details may be found in previous published articles.13–15 Current analyses were performed using data that were deidentified.
Only data from patients with severe asthma as diagnosed by the physicians and with reported data for blood eosinophil (EOS) counts were included in the present study. For data analysis, two groups of patients (EOS+ and EOS-) were constituted based on blood EOS counts. The threshold value for EOS+ asthma was primarily set at 300 giga (G)/l, and secondarily at 150 G/l. EOS- asthma was defined by blood EOS count below the threshold. For total serum IgE level, the threshold was set at 150 international unit per milliliter (IU/mL). Skin allergy or allergen-specific IgE assays (when performed) allowed to differentiate patients with (ALL+) from without (ALL-) allergic asthma.
Statistical analyses were carried out using SAS software (SAS institute, 9.4 version, North Carolina, USA). No hypothesis was tested for the study which was descriptive. Missing data were not replaced, and analysis was based on available data, considering missing data as non-indicative. Briefly, descriptive analyses included mean with standard deviation (SD), median with interquartile range (IQR) for quantitative variables and sample size (N) and frequency (%) per category for qualitative and ordinal variables. Tests (Student’s t-test or Wilcoxon-Mann–Whitney nonparametric test) were performed to compare independent groups. Homogeneity of variances was tested by Levene’s test. The normal distribution was based on the overall shape of the histogram and the result of the Shapiro–Wilk test if required. Association between blood eosinophil counts and total serum IgE levels was assessed with Pearson’s Chi-square or Fisher’s exact test. The significance level was always set at 5%.
The sample size was focused on the precision of the estimations, measured as the range of their 95% confidence intervals (CIs). A 240-patient sample size permitted to obtain a precision of at least ±6.5% for the analysis.
Results
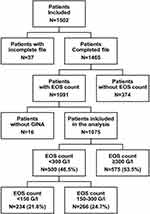
Of the 1502 included patients in the database, 1075 patients with severe asthma according to GINA treatment criteria and a blood EOS count were recorded and included in the present analysis. Of these 1075 patients, 234 had an EOS count <150 G/l (21.8%), 266 an EOS count between 150 and 300 G/l (24.7%), and 575 an EOS count ≥300 G/l (53.5%). When the sociodemographic and clinical characteristics of the patients considering either the 150 G/l or the 300 G/l thresholds were compared, they were very similar overall (data available in Supplementary Table 1 and Supplementary Table 2). The selected threshold was 300 G/l, and the EOS- and EOS+ groups therefore included 500 (46.5%) and 575 (53.5%) patients, respectively (Figure 1).
Sensitization to allergens, treatment with OCS, and nasal polyposis were reported for 616 (57.3%), 216 (20.1%) and 215 (20%) of the 1075 patients included in the study analysis, and 321 (52.4%), 136 (63.0%), and 151 (70.2%) of the 575 patients with EOS+ asthma, respectively.
Severe Non-Eosinophilic Asthma
EOS- patients (EOS count <300 G/l) were mainly female (67.2%) of middle age (42.8% had 40 to 60 years of age). They were frequently overweight (35%) or obese (29%). Most patients (58.2%) were never smokers while 11.2% were reported as current smokers. Approximately two-thirds (69.2%) had a history of personal atopy and 54.9% had at least one ENT (ear, nose, and throat) disease. 41.1% of EOS- patients had chronic rhinitis and 13.6% had polyposis. A large proportion of EOS- patients (78.6%) presented at least one non-ENT comorbidity, mainly anxiety (45.5%), gastroesophageal reflux disease (GERD, 45.2%), or high blood pressure (30.2%). Few statistically significant differences were observed between the EOS- and EOS+ groups: as compared with EOS+ patients, EOS- patients were more frequently female (p = 0.03); they less frequently had at least one ENT disease (p < 0.01), especially chronic sinusitis (p < 0.001), or polyposis (p < 0.001). They were more frequently affected by at least one other comorbidity (p < 0.001), such as GERD (p < 0.001) or high blood pressure (p = 0.02) (Table 1).
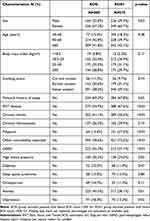 |
Table 1 Comparison of Patients’ Profile According to Blood Eosinophilic Count: EOS- versus EOS+ |
Overall, 68.9% of EOS- patients reported sensitization to allergens and 50.4% had total serum IgE level >150 IU/mL. No statistically significant difference was observed between the EOS- and EOS+ groups regarding sensitization to allergens (p = 0.35) whereas the proportion of patients with total serum IgE level (>150 IU/mL) was higher in the EOS+ than EOS- group (67.2% vs 50.4%, p < 0.01) (Table 2).
 |
Table 2 Comparison of Asthma and Asthma Management According to Blood Eosinophilic Count: EOS- Vs EOS+ |
Virtually all EOS- patients (93.6%) received long-acting β2-agonists (LABAs) combined with ICS, and 53.6% took antileukotrienes. Inhaled long-acting muscarinic antagonists (LAMAs) or biotherapy (omalizumab) were taken by approximately one-third of EOS- patients. No difference was observed between the EOS- and EOS+ groups in percentages of patients treated by each therapy except for LAMAs which were more frequently taken by EOS- patients (p < 0.01) and long-term oral corticosteroids (OCS) which were less frequently taken by EOS- patients (p < 0.01) (Table 2).
For EOS- patients, mean asthma duration was 30 years (SD, 18.3). In the last 12 months, 78.9% of them had presented more than one exacerbation, 74.4% an exacerbation requiring ICS dose increase or rescue corticosteroid therapy, and 28.4% an exacerbation requiring hospitalization. The median number of exacerbations per subject was 4.5. With 47.5% of the patients with an ACT score <15 and 28.9% with an ACT score between 15 and 19, EOS- asthma appeared to be poorly controlled. Asthma duration was significantly longer (p < 0.01), and asthma less well controlled in EOS- than EOS+ patients (p < 0.01) (Table 2). Similar results were observed when EOS- asthma was defined with an EOS threshold set at 150 G/l (Figure 2).
Allergic Vs Non-Allergic Severe Non-Eosinophilic Asthma
Among the 500 EOS- patients (EOS count <300 G/l), 293 (58.6%) had an ALL+ profile and 132 (26.4%) an ALL- profile based on allergic sensitization tests; the allergic profile of the asthma of 75 patients (15%) was not assessed. Few significant differences were observed between the ALL+ and ALL- patients. As compared to ALL- patients, ALL+ patients were significantly younger (67.5% vs 42.4% were <60 years of age, p < 0.01) and underweight (37.2% vs 30.3% were <25 kg/m², p = 0.03); they more frequently reported a personal history of atopy (91.3% vs 26.6%, p < 0.01) and of chronic rhinitis (46.0% vs 34.6%, p < 0.001) (Table 3). Asthma duration was longer in ALL+ than ALL- patients (32.4 vs 18.2 years, p < 0.0001), and total serum IgE levels were more frequently high in ALL+ patients (57.9% vs 35.6%, p < 0.001). Regarding asthma treatments, ALL+ patients more frequently received antileukotrienes (58.4% vs 47.0%, p = 0.03) or omalizumab (39.2% vs 22.7%, p < 0.001), and less frequently received OCS at study inclusion (13.3% vs 21.2%, p = 0.04) than ALL- patients. No difference was observed in asthma control (Table 4 and Figure 3).
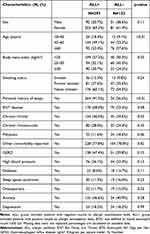 |
Table 3 Comparison of EOS- Patients’ Profile According to Allergic Profile: ALL+ Vs ALL |
 |
Table 4 Comparison of EOS- Patients According to the Allergic Profile of the Patients: ALL+ Vs ALL |
IgE- Vs IgE+ Severe Non-Eosinophilic Asthma
Among EOS- patients (EOS count <300 G/l), 188 (37.6%) had low total serum IgE level (IgE-) and 191 (38.2%) had total high serum IgE level (IgE+); no IgE data was available for 121 patients (24.2%). Few differences were observed between the IgE+ and IgE- patients. IgE+ patients more frequently reported a personal history of atopy (75.9% vs 63.0%, p < 0.01) and sensitization to allergens (78.6% vs 59.6%, p < 0.001) than IgE- patients. No difference was observed between the IgE- and IgE+ patients in asthma therapy, except for omalizumab intake which was more frequent in IgE+ patients (48.7% vs 31.4%, p < 0.001). No significant difference between IgE- and IgE+ patients was observed in asthma control, although there was a tendency towards a better control in IgE+ patients: ACT score indicated good asthma control, poor asthma control, and uncontrolled asthma for 26.7%, 30.0%, and 43.3% of IgE+ patients vs 19.0%, 26.4%, and 54.6% of IgE- patients, respectively (Supplementary Table 3).
Drug Therapy in Severe EOS- and EOS+ Asthma
EOS- patients (EOS count <300 G/l) were less frequently treated with OCS than EOS+ patients: 16% vs 23.7%. Among EOS- patients, patients treated with OCS were more frequently former smokers and less frequently active smokers (41.3% vs 28.6% and 2.5% vs 12.9%, p < 0.01), more frequently affected by osteoporosis (37.8% vs 10.4%, p < 0.01), and less frequently sensitized to allergens (58.2% vs 70.9%, p = 0.04). Regarding asthma therapy (excluding OCS), no difference was observed between EOS- patients treated and non-treated with OCS, except for LAMA and theophylline which were more frequently taken by EOS- patients (48.8% vs 35.2%, p = 0.02 and 15.0% vs 7.9%, p = 0.04). Asthma control was poorer in EOS- patients treated than non-treated with OCS: higher proportion of frequent exacerbators (91.4% vs 76.4%, p < 0.01), higher median number of exacerbations in the prior 12 months (7 vs 4, p < 0.01), higher percentage of patients with at least one moderate exacerbation (83.8% vs 72.6%, p = 0.04), higher proportion of patients with at least one exacerbation requiring hospitalization (43.8% vs 25.5%, p < 0.01), and lower ACT scores. ACT score was <15 indicating uncontrolled asthma in 65.8% of patients who received OCS vs 44.0% of patients who did not (p < 0.01) (Supplementary Table 4).
Discussion
The present secondary analysis of the FASE CPHG database which was dedicated to severe asthma patients offered the possibility to improve knowledge about EOS- asthma, such as the characteristics of patients with EOS- asthma and level of control and management of their asthma.
This analysis showed that EOS- asthma (<300 G/l of blood eosinophil) was approximately as frequent as EOS+ asthma (47% vs 53%). This result was in line with the literature until recently.9,16 A recent article published by Heaney et al concluded that the occurrence of a severe EOS- asthma was rare; 83.8% of the patients of their study were identified as most likely to have severe EOS+ asthma. In their study, EOS+ asthma was defined using a multicomponent “eosinophil gradient algorithm”. This composite criterion considered blood EOS count, OCS use, elevated fractional exhaled nitric oxide, nasal polyps, and adult-onset asthma.17
In the literature, EOS- asthma had been associated with environmental and/or host factors, such as smoking, pollution, work-related agents, infections, and obesity.9 In our analysis, the rates of obese patients, current or former smokers, or atopic patients were similar between patients with severe EOS- and EOS+ asthma. GERD, hypertension, anxiety, or depression were more frequently reported in EOS- patients, and ENT diseases including chronic rhinitis or polyposis as well as high level of IgE (>150 IU/mL) were more frequently reported in EOS+ patients.
Among the significant differences between patients with severe EOS- and EOS+ asthma, the most important was probably the poor asthma control in the EOS- population as assessed by the ACT. The poor control of asthma in EOS- patients was observed regardless of the cutoff value chosen to define eosinophilic asthma. As the accurate definition of EOS- asthma is not well established in the literature and guidelines, the description of patients with EOS- asthma, including their comparison with patients with EOS+ asthma was performed with two hypotheses: blood eosinophil count <300 G/l (primary hypothesis) or <150 G/l (secondary hypothesis). The recommended threshold to define eosinophilic asthma based on blood eosinophil counts was derived from pharmacological studies of biologics targeting eosinophils, and ranges between 150 G/l and 300 or 400 G/l. Values below the threshold are used to define non-eosinophilic asthma.8 It should be noted that eosinophilic counts were performed in blood samples and not sputum as sputum processing is rarely performed in clinical practice, and blood eosinophil count is commonly used as a surrogate marker to identify eosinophilic asthma.
Previous studies have reported the poor response to standard asthma therapies in EOS- asthma, especially the poor response to ICS. The present analysis partially confirmed this result as in comparison to EOS+ patients, EOS- patients tended to have less-controlled asthma (ACT score <18, 48% vs 40%), although largely treated with ICS combined with LABAs, and more frequently treated with LAMAs. Patients with severe EOS- asthma were as likely to have moderate or severe exacerbations as patients with severe EOS+ asthma. This result was surprising. Data from placebo arms of late phase trials for biologics in severe asthma tended to indicate that increased eosinophilic blood count was associated with increased exacerbation risk.18 Because long-term OCS intake could have influenced eosinophilia, an additional analysis was performed in EOS- patients to compare patients with and without OCS treatment. In our study, EOS- patients with OCS treatment were rare (16%) and less numerous than EOS+ patients taking OCS (24%). As expected, OCS were mainly used in patients with poor asthma control and frequent exacerbation occurrences. No other pertinent patients’ characteristics were markedly associated with OCS use except osteoporosis.
Anxiety and depression were more frequently reported for EOS- than EOS+ patients. It is well known that anxiety and depression are common in patients with severe asthma and often associated with uncontrolled asthma or lower ACT scores.19–21 However, to date, very few data were available on severe EOS- asthma and its impact on psychic disorders. The low disease control as perceived by the patients and the lack of current or new effective treatments could possibly contribute to the bad mood of patients with severe EOS- asthma.
More than half of patients with severe EOS- asthma (59%) had an allergic profile (ALL+) confirming that allergic asthma could be combined or not with high blood eosinophils. ALL+ patients had allergy markers (atopy history, chronic rhinitis, younger age) and a higher risk of obesity. However, the allergic profile was not associated with impaired asthma control or increased number of exacerbations. It should be noted that about 15% of EOS- patients had an unknown allergic profile, indicating that, at the time of the study, efforts were required to increase health care professionals’ awareness of the usefulness of allergic testing.
Additionally, total IgE level remained unknown for about 25% of patients, which complied with recommendations stating that IgE should only be measured when omalizumab is scheduled to be used. When known, about 50% of EOS- patients had high IgE levels (>150 IU/l). Patients’ profiles and asthma characteristics were similar and close to those reported in ALL+ patients. High IgE levels did not influence asthma control or increase the risk for exacerbations.
The present study had some limitations. Its main limitation was its cross-sectional design, which did not ensure causal associations and did not allow follow-up description. Constitution of cohorts with longitudinal follow-up would help to confirm these results and assess their persistence. Collected data were declarative. EOS+ asthma was based on a cutoff value of either 150 or 300 G/l for patients treated and untreated with OCS. As treatment with OCS is known to suppress blood eosinophilia, a solution could have been to define EOS+ asthma on a composite variable (ie, 150 G/l and 300 G/l in patients with and without OCS treatment, respectively). As long-term OCS treatment was prescribed in 15% of patients with eosinophilic level <150 G/l, this would lead to exclude from the EOS- group 44 patients. However, this study had also some strengths. The FASE CPHG database constitutes a large, exhaustive, and interesting tool to improve knowledge and provide a complete and recent overview of severe asthma patients in France. Additionally, the database comprises prevalent patients offering the opportunity to assess patients with asthma while being under treatment and followed by physicians.
Conclusion
This secondary analysis of the data issued from the FASE CPHG study which was dedicated to severe asthma offered the possibility to improve knowledge about patients with severe non-eosinophilic asthma. Using a large sample of patients, it provided information on their characteristics and on asthma management and burden in real life. Patients with severe EOS- asthma showed specific profiles; they reported more frequently GERD, hypertension, anxiety, and depression. Whereas ENT diseases, including chronic rhinitis or polyposis, were less frequent in EOS- patients, the rates of obese, current/former smokers, and atopic patients were similar between EOS- and EOS+ patients, which was also true for high level of IgE. In addition to combined ICS and LABA treatment, patients with severe EOS- asthma frequently received LAMAs. Despite this reinforced inhaled treatment, patients with EOS- asthma had a poorer asthma control and were as likely to have moderate or severe exacerbations as patients with EOS+ asthma. Anxiety and depression were more frequently reported in EOS- than EOS+ patients.
Furthermore, the use of OCS concerns a low proportion of patients with severe EOS- asthma, but it is correlated with osteoporosis, poorer control of asthma, and more frequent exacerbations. With regard to the prevalence of comorbidities, the burden associated with severe EOS- asthma was heavy due to more comorbidities. Allergic asthma is as frequent in EOS+ as in EOS- patients and among EOS- patients, and the presence of allergy does not worsen the clinical features.
Abbreviations
ACT, Asthma Control Test; ALL, allergic; ATS, American Thoracic Society; CCTIRS, Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé; CI, confidence interval; CPHG, Collège des Pneumologues des Hôpitaux Généraux; CXCR2, chemokine receptor 2; EOS, eosinophil or eosinophilic; ENT, ear, nose, and throat; ERS, European Respiratory Society; G, giga; GERD, gastroesophageal reflux disease; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroids; IgE, immunoglobulin E; IQR, interquartile range; IU, international unit; LABA, long-acting β2 agonist; LAMA: long-acting muscarinic antagonist; MAPK, mitogen-activated protein kinase; N, number; OCS, long-term oral corticosteroids; PDE, phosphodiesterase; PI, phosphoinositide; PPARγ, peroxisome proliferator-activated receptor gamma; SD, standard deviation; TSLP, thymic stromal lymphopoietin; WSAS, West Sweden Asthma Study.
Data Sharing Statement
The database is available upon request to Kappa Santé, a contract research organization.
Acknowledgments
The authors would like to thank the Collège des Pneumologues des Hôpitaux Généraux (CPHG, France) which initiated the FASE-CPHG study, Kappa Santé company (Paris, France) for the management of collected data, and Abelia Science (Saint-Georges-sur-Baulche, France) for providing medical writing support.
The authors also thank the FASE-CPHG participants and investigators for making this study possible: Dr Parrat (CH Papeete); Dr Nocent (CH Bayonne); Dr Mangiapan (CHI Créteil); Dr Prud’homme and Dr Courdeau-Labourie and Dr Demaegdt (CH Tarbes); Dr Oster and Dr Moreau and Dr Allibe (CH Colmar); Dr Portel and Dr Roy (CH Libourne); Dr Appere De Vecchi (CH Argenteuil); Dr Maurer (CH Montfermeil); Dr Lepoulain Doubliez (CH Charleville Mezières); Dr Iamandi (CH Mulhouse); Dr Gourcerol (CH Pau); Dr Didi, Dr Decroisette and Dr Bertocchi (CH Pringy); Dr Barbare and Dr Moncelly (CH Meaux); Dr Tannous (CH Forbach); Dr Kelkel (CH Chambéry); Dr Rolland (CH Cannes); Dr Jouveshomme (Hôpital St-Joseph, Paris); Dr Bernier (CH Dinan); Dr Hauss, Dr Ould, Dr Vincent, Dr Van Mossevelde and Dr Gallego (CH Elbeuf); Dr Merzoug (CH Fougères); Dr Haddad (CH Lourdes); Dr Guerrero, Dr Jarjour, Dr Haouachi and Dr Goutorbe (CH Béziers); Dr Morel, Dr Lemaire and Dr Russier (CH Orléans); Dr Roge (CH Morlaix); Dr Dumont (CH Chauny); Dr Cavestri and Dr Just (CH Roubaix); Dr Colin (CH Versailles); Dr Goupil, Dr Mansour and Dr Paris (CH Le Mans); Dr Philippe and Dr Boitiaux (CH Cergy-Pontoise); Dr Simon (CH Compiègne); Dr Marcq, Dr Bizieux, Dr Guibert and Dr Caby (CH La Roche sur Yon); Dr Lecuyer (CH Saint-Quentin); Dr Merlusca (CH Sedan); Dr Blanc (CH Aix en Provence); Dr Langelot (CH Les Sables d’Olonne); Dr Tagu (CH Bar le Duc); Dr Leveiller, Dr Duriel-Niel and Dr Coëtmeur (CH Saint-Brieuc); Dr Ilie (CH Dunkerque); Dr Raspopa (CH Toulon); Dr Lerousseau and Dr Rotomondo (CH Antibes); Dr Gramada (CH Sarreguemines); Dr Cornu and Dr Petit (CH Verdun); Dr Kraemer (CH Fréjus); Dr Guy (CH Vannes); Dr Gentil, Dr Luciani and Dr Lucena (CH Bourgoin Jallieu); Dr Michaux (CH Macon); Dr Maitre (CH Vesoul); Dr De Faverges (CH Nevers); Dr Fouret (CH Villeneuve Saint-Georges); Dr Goarant (CH Saint-Malo); Dr Bara and Dr Hamoudi (CH Angoulême); Dr Maetz (CH Douai); Dr Yousef and Dr Lungoci (CH Le Puy en Velay); Dr Al Freijat (CH Belfort); Dr Assemi (CH Saverne); Dr Beysens (CH Saintes); Dr Clarissou (CH Beaumont sur Oise); Dr Karimo (CH Saint-Omer); Dr Saadi (CH Barbezieux Saint Hilaire); Dr Barre and Dr Farny (CH Cahors); Dr Romand (CH Contamine sur Arve); Dr Leleu (CH Abbeville); Dr Bernard (CH Quimper); Dr Marangoni (CH Saint-Dié); Dr Fevrier (CH Saumur); Dr Paillot (CH Metz); Dr Bonnefoy (CH Jonzac); Dr Manoila (CH Evreux); Dr Abraham (CH Dax); Dr Lacroix (CH Périgueux); Dr Virally (CH Aulnay sous Bois).
Funding
The FASE-CPHG study was supported by contributions made through the CPHG from ALK, AstraZeneca, Boehringer Ingelheim, GSK, and Le Nouveau Souffle. The funding bodies had no role on the conception of this manuscript, they did not participate in any way in the design of the FASE-CPHG study. AstraZeneca supported the secondary analysis of the data presented in the present article.
Disclosure
CFV, AC and GT are employees of AstraZeneca. NT and DS are employees of Kappa Santé. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Asthma (GINA). Pocket guide for asthma management and prevention. A pocket guide for health professionals; 2021. Available from: https://ginasthma.org/pocket-guide-for-asthma-management-and-prevention.
2. Rönnebjerg L, Axelsson M, Kankaanranta H, et al. Severe asthma in a general population study: prevalence and clinical characteristics. J Asthma Allergy. 2021;14:1105–1115. doi:10.2147/JAA.S327659
3. Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1):1900588. doi:10.1183/13993003.00588-2019
4. Global Initiative for Asthma (GINA). Diagnosis and management of difficult-to-treat and severe asthma. Difficult-to-treat & severe asthma in adolescent and adult patients. A GINA Pocket Guide for health professionals. V3.0; 2021. Available from: https://ginasthma.org/severeasthma.
5. Bourdin A, Fabry-Vendrand C, Ostinelli J, et al. The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract. 2019;7(5):1477–1487. doi:10.1016/j.jaip.2018.12.029
6. Gruffydd-Jones K. Unmet needs in asthma. Ther Clin Risk Manag. 2019;15:409–421. doi:10.2147/TCRM.S160327
7. Brusselle GG, Koppelman GH. Biologic Therapies for Severe Asthma. N Engl J Med. 2022;386(2):157–171. doi:10.1056/NEJMra2032506
8. Carr TF, Zeki AA, Kraft M. Eosinophilic and noneosinophilic asthma. Am J Respir Crit Care Med. 2018;197(1):22–37. doi:10.1164/rccm.201611-2232PP
9. Esteban-Gorgojo I, Antolín-Amérigo D, Domínguez-Ortega J, Quirce S. Non-eosinophilic asthma: current perspectives. J Asthma Allergy. 2018;11:267–281. doi:10.2147/JAA.S153097
10. Haute Autorité de Santé (HAS). Asthmes sévères: quels traitements proposer et dans quels cas? Communiqué de presse [Severe asthma: which treatments to offer and in which cases?]; 2018. French. French: https://www.has-sante.fr/jcms/c_2823952/fr/asthmes-severes-quels-traitements-proposer-et-dans-quels-cas.
11. Thomson NC. Novel approaches to the management of noneosinophilic asthma. Ther Adv Respir Dis. 2016;10(3):
12. Abe Y, Suga Y, Fukushima K, et al. Advances and challenges of antibody therapeutics for severe bronchial asthma. Int J Mol Sci. 2021;23(1):83. doi:10.3390/ijms23010083
13. Portel L, Parrat E, Nocent-Ejnaini C, et al. FASE-CPHG study: a panoramic snapshot of difficult-to-treat, severe asthma in French nonacademic hospitals. ERJ Open Res. 2019;5(4):00069–2019. doi:10.1183/23120541.00069-2019
14. Coëtmeur D, Parrat E, Nocent-Ejnaini C, et al. Activité physique et asthme sévère: résultats de l’étude FASE-CPHG [Physical activity in severe asthma: results of the FASE-CPHG Study]. Rev Mal Respir. 2020;37(4):320–327. French. doi:10.1016/j.rmr.2019.12.004
15. Raherison-Semjen C, Parrat E, Nocent-Eijnani C, et al. CPHG (College of French Non-academic Hospitals). FASE-CPHG Study: identification of asthma phenotypes in the French Severe Asthma Study using cluster analysis. Respir Res. 2021;22(1):136. doi:10.1186/s12931-021-01723-x
16. Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57(7):643–648. doi:10.1136/thorax.57.7.643
17. Heaney LG, Perez de Llano L, Al-Ahmad M, et al. Eosinophilic and noneosinophilic asthma: an expert consensus framework to characterize phenotypes in a global real-life severe asthma cohort. Chest. 2021;160(3):814–830. doi:10.1016/j.chest.2021.04.013
18. Casale TB, Chipps BE, Rosén K, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018;73(2):490–497. doi:10.1111/all.13302
19. Yonas MA, Marsland AL, Emeremni CA, Moore CG, Holguin F, Wenzel S. Depressive symptomatology, quality of life and disease control among individuals with well-characterized severe asthma. J Asthma. 2013;50(8):884–890. doi:10.3109/02770903.2013.810750
20. Porsbjerg C, Menzies-Gow A. Co-morbidities in severe asthma: clinical impact and management. Respirology. 2017;22(4):651–661. doi:10.1111/resp.13026
21. Ciprandi G, Schiavetti I, Rindone E, Ricciardolo FLM. The impact of anxiety and depression on outpatients with asthma. Ann Allergy Asthma Immunol. 2015;115(5):408–414. doi:10.1016/j.anai.2015.08.007
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.