Back to Journals » Journal of Pain Research » Volume 15
Characteristics of Persons Seeking Care for Moderate to Severe Pain Due to Chronic Low Back Pain and Osteoarthritis: A Cross-Sectional Study
Authors Pagé MG , Tousignant-Laflamme Y , Dorais M, Beaudry H, Fernet M
Received 3 February 2022
Accepted for publication 30 March 2022
Published 19 April 2022 Volume 2022:15 Pages 1125—1139
DOI https://doi.org/10.2147/JPR.S360314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Michael A Ueberall
M Gabrielle Pagé,1,2 Yannick Tousignant-Laflamme,3 Marc Dorais,4 Hélène Beaudry,5 Mireille Fernet6
1Research Center, Centre Hospitalier de l’Université de Montréal, Montreal, QC, Canada; 2Department of Anesthesiology and Pain Medicine, Université de Montréal, Montreal, QC, Canada; 3School of Rehabilitation, Université de Sherbrooke, Sherbrooke, QC, Canada; 4StatSciences Inc., Notre-Dame-de-l’Ile-Perrot, QC, Canada; 5Quebec Pain Research Network, Sherbrooke, QC, Canada; 6Medical Affairs Division, Pfizer Canada, Montreal, QC, Canada
Correspondence: M Gabrielle Pagé, Research Center, Centre hospitalier de l’Université de Montréal, S01-122, St-Antoine Tower, 850 St-Denis, Montreal, QC, H2X 0A9, Canada, Tel +1 514-890-8000, ext. 31601, Email [email protected]
Purpose: To assess the associations between pain severity or physical (pQoL) and mental (mQoL) health-related quality of life and disability status or health-care utilization among persons living with moderate/severe pain due to chronic low back pain (CLBP) or osteoarthritis (OA), who received treatments in Quebec’s tertiary care pain centers.
Materials and Methods: This retrospective study was carried out using the Quebec Pain Registry (Canada) from 2008 to 2014 and contains data on persons referred to tertiary pain management clinics. Participants were selected if they were diagnosed with CLBP (N = 2663) or OA (N = 139) of more than 3 months duration and of pain intensity ≥ 5 on the Numeric Rating Scale (0– 10) and completed baseline questionnaires.
Results: Less than 5% of persons were hospitalized in the 6 months before their first visit at the pain clinic, and 11.9% and 18.9% of persons with OA and CLBP, respectively, had a pain-related emergency room (ER) visit. Less than 1/5 and more than 1/4 of persons with OA and CLBP were receiving disability benefits, respectively. Persons with CLBP who had visited the ER, those on disability and those receiving disability benefits, reported higher levels of pain severity, interference, and lower levels of mQoL (and pQoL for those on disability or receiving benefits) compared to those who did not consult the ER, those not on disability or not receiving disability benefits, respectively (all p < 0.05). For OA, disability status was the only variable associated with pain interference and QoL (all p < 0.05).
Conclusion: Pain severity, pain interference and mQoL were associated with health-care utilization and disability status in persons with CLBP. These results were globally not found among persons with OA, which might be due to smaller sample size or unique characteristics of this population.
Keywords: osteoarthritis, Quebec Pain Registry, healthcare utilization, back pain, quality of life
Introduction
Low back pain (LBP) is a common health problem estimated to be experienced by 80% of persons at least once in their lifetime, with a point prevalence of 7.5%1 and increasing with age, and with one-in-four persons experiencing a recurrent episode within one year.2,3 Broad and varying definitions of what constitutes the diagnosis of LBP4 make prevalence difficult to calculate, however. Acute bouts of LBP can last upwards of 6 weeks, however 5–10% of persons with LBP transition into chronicity.5 The economic burden of LBP is far reaching to include not only direct medical costs, but indirect costs such as those associated with work absenteeism/presenteeism. Accelerated population aging, contributed by the baby boomer generation, is expected to continue through to the year 2031. Persons 65 years of age and older will constitute roughly 24% of Canada’s total population by 2063, making the potential human and economic burden of LBP exceptionally high.6 LBP is the most common form of chronic pain and the leading cause of global years-lived-with-disability.7
Similarly to CLBP, osteoarthritis (OA) is also a chronic pain condition that is prevalent (age-standardized point prevalence of 3754 per 100,0008), is associated with high rates of limitations and disability (118.8 global age-standardized years lived with disability in 20178), is localized to a few body areas, and includes typically ineffective endogenous pain control and central sensitization mechanisms.9 Osteoarthritis (OA) is a chronic joint disease characterized by the progressive degeneration of the articular cartilage which covers and protects the terminal ends of bones within a joint.10 Females and the elderly are the highest-risk population for developing OA, however there are many other “person- or joint level” risk factors (eg, obesity and joint trauma) that are linked to OA development.7,10,11 OA has life-long debilitating effects on a person’s daily activities of living, and therefore, their overall quality of life (QoL).7 Although OA can occur in any joint, the knees, hips, hands, facet joints of the spine, and the feet are most often affected, with many persons reporting OA in multiple joints.7,10 Due to the diversity of diagnostic definitions, prevalence and incident rates are difficult to establish in OA. However, the Public Health Agency of Canada reports that an estimated 14% of the Canadian adult population (≥20 years of age)12 are living with OA. Persons 65 years of age and older are the fastest growing cohort of the population and therefore, the number of Canadians living with OA is projected to increase to almost 6 million by 2031.13 Furthermore, the current economic burden of OA is substantial and will continue to rise dramatically with estimates reaching $7.6 billion CAD annually in total OA-related costs.12
To this date, no studies have examined the associations between moderate to severe pain in persons living with OA or chronic low back pain (CLBP) and health-care utilization or disability status, in the province of Québec, Canada. This study aims at describing persons with OA and CLBP attending tertiary care pain management clinics in the province of Quebec to gain a better understanding of the impact of this disease on the persons and society. Current literature typically examines prevalence of CLBP or OA within emergency room settings or hospitalization,14,15 rather than rates of ER visits from samples of persons with chronic pain who are accessing specialized pain care. While this information is useful to understand gaps in adequate access to pain management, it does not help understand patterns of health-care utilization among those already referred to specialized pain services. Such knowledge would help understand gaps in health-care services and identify modifiable risk factors.
In addition, studies have aimed to understand factors associated with disability levels among various chronic pain populations. Systematic reviews of disability among persons with CLBP often show the importance of psychosocial factors in predicting disability, often more so than pain characteristics.16–18 However, given that the experience of pain is multidimensional and often co-occurs with other comorbid conditions, one might wonder whether more global measures of health such as quality of life in chronic pain cohorts might also be an important indicator of disability. The literature is scarce on this topic, but a recent study conducted on persons with musculoskeletal pain found that physical health-related quality of life was associated with disability pension.19
The purpose of this cross-sectional study is to assess the associations between pain severity and physical (pQoL) and mental (mQoL) health-related quality of life and disability status and health-care utilization among persons with moderate/severe pain due to CLBP or OA receiving treatment in tertiary care pain centers in Quebec (Canada).
OA and CLBP were chosen as the focus of this study because they represent different types of chronic pain (degenerative joint conditions and a musculoskeletal condition, respectively) that share similar burden and typically include ineffective endogenous pain control and central sensitization mechanisms.
Materials and Methods
This study selected persons from the Quebec Pain Registry (QPR) with moderate to severe pain due to OA or CLBP at first visit and who consented that their QPR data be used for research purposes. Ethical review for the present study was conducted independently by the Research Ethics Board (REB) at the Centre hospitalier de l’Université de Montréal (CHUM no. 20.286). The data collection process and manuscript comply with the Declaration of Helsinki.
Québec Pain Registry
This descriptive retrospective study was carried out with existing structured data contained in the QPR, a large research database of persons living with various chronic pain syndromes who were referred to one of five tertiary care pain management clinics in the province of Quebec (Canada).20 Unlike many registries, the QPR provides rich information regarding clinical/medical data and outcome measures, including specific standardized diagnostic codes assigned by treating physician and treatment and medical history collected through a telephone interview with a nurse.20,21
Setting and Inclusion/Exclusion Criteria for the QPR
This study was carried out using the data collected in the QPR.20 The QPR research database includes data on 9418 persons who were referred to the participating pain clinics between November 2008 and November 2014 (inclusively). Persons seen in an ambulatory setting, aged ≥18 years and able to understand and read French or English were enrolled in the QPR registry. Persons who were unable to complete questionnaires due to severe physical or cognitive inability were excluded. Persons were enrolled in the QPR prior to their first visit at the pain clinic; they completed a self-reported questionnaire composed of well-validated scales and measurement tools (Patient Questionnaire: see below for details). They were also interviewed by a research nurse who collected clinical data using a structured interview protocol (Nurse Questionnaire: see below for details). Eligible persons were informed that their data along with those of other persons who gave their permission could be used for research purposes. If they agreed, they were invited to sign the REB-approved consent form of the QPR.
Participants
Persons from the QPR database who met all the following criteria were eligible for inclusion in the present study:
Diagnostic codes established by pain physician indicative of OA or LBP at baseline were based on the consensual QPR grid of pain diagnoses elaborated by four experienced pain physicians with background in anesthesiology or neurosurgery.20 For CLBP, this included lumbar pain with or without radicular pain, radicular pain only, diffuse pain in the lumbar region, lumbar pain due to postherpetic neuralgia, lumbar pain of uncertain origin, other type of lumbar pain, sacral pain or coccygeal pain and the absence of a diagnosis of OA. For OA, this included osteoarthritis of the knee, hip or more than one location, in the absence of a secondary diagnosis of CLBP.
Persons who reported that pain was present for at least 3 months prior to baseline.
Persons scored their average pain intensity from 5 to 10 within the 7 days prior to baseline on the Numeric Rating Scale (NRS) to capture moderate, severe and very severe pain levels.22
Persons were excluded if they also lived with rheumatoid arthritis or cancer-related pain, and if they were present in both CLBP and OA cohorts simultaneously.
Data Sources and Measurement
Two questionnaires, one administered by the nurse and one filled by the patient, were used to collect data at patient’s first visit at the pain clinic.20
Patient Questionnaire
Demographic characteristics included age, sex, education, work status (disability status considered both temporary or permanent disability), disability benefits and income source. Disability benefits were determined based on reported source of income. Those reporting receiving benefits Commission de la Santé et Sécurité au travail (Commission for health and security at work), Indemnisation des victimes d’actes criminels (Indemnisation of victims of criminal acts), Société d’assurance-automobile du Québec (Society of auto-insurance of Quebec), disability payment though employer or Canada disability pension were considered as receiving disability.
Pain characteristics included pain severity (mean of pain scores from the NRS (0–10) for average, worst, and current pain within the 7 days prior to baseline)23,24 and pain interference (total score on the 7 interference items of the Brief Pain Inventory24). Psychological distress was assessed using the Beck Depression Inventory25,26 and Pain Catastrophizing Scale.27 Alcohol and drug consumption were measured using items documenting frequency of use (never, < once per month, once per month, 2–3 times per month, once a week, 2–3 times per week, 4–6 times per week, every day), as well as the CAGE-AID28 (for persons enrolled prior to May 2012) and the Opioid Risk Tool29 (as part of the nurse administered questionnaire for persons enrolled after May 2012). Physical (pQoL) and Mental (mQoL) health-related QoL were measured using norm-based subscale scores of the Short Form-12 Health Survey v2.30
Nurse-Administered Questionnaire
Pain characteristics included pain duration, pain frequency, circumstances surrounding pain onset, and neuropathic components (Questionnaire Douleur Neuropathique 4 (DN4)31). Health-care utilization was measured as a function of number of pain-related emergency visits or hospitalizations in the past 6 months, past and current pharmacological pain treatments, type and severity of side effects of current pharmacological pain treatments, and type of current and past physical and psychological pain treatments, including procedures (such as blocks), psychological techniques, self-management strategies, physical therapies and complementary alternative therapies. Type and number of health-care providers consulted since pain onset was also collected as well as mobility support required inside/outside of home. Finally, current and past medical history was also gathered using a standardized list. Health comorbidities were also gathered using a standardized list of common chronic conditions (this list was modified in May 2012 and as such some conditions are only evaluated on a subgroup of patients).
Statistical Methods
Descriptive analyses (number and percentage for categorical variables; mean with standard deviation (SD) for continuous variables) were produced in each cohort to depict characteristics of CLBP and OA cohorts.
The associations between pain-related emergency department visit (yes (at least 1 visit) vs no (0 visit)) or pain-related hospitalization (yes/no) and (1) pain severity, (2) pain interference and (3) pQoL and mQoL scores were assessed using Wilcoxon Mann–Whitney test.
The associations between disability status and receiving/applying for disability benefits (yes/no) and (1) pain severity, (2) pain interference, and (3) pQoL and mQoL scores also were assessed using Wilcoxon Mann–Whitney test.
All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA). A p-value less than 5% was considered statistically significant.
Results
Descriptive Statistics
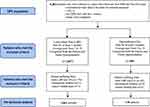
Out of the 8482 participants enrolled in the Quebec Pain Registry, 2663 persons with CLBP and 139 persons with OA were included in the analyses (see Figure 1). A majority of participants were female (CLBP = 55.4%; OA = 59.0%) and approximately 50% had completed college or university. Forty percent of persons with CLBP and 27.6% of persons with OA were working. Less than 1/5 and more than 1/4 of persons with OA and CLBP were receiving disability benefits, respectively.
In addition to demographic characteristics presented in Table 1, variables collected at the persons’ first visit at the pain clinic (baseline assessment) are presented for each person cohort separately, ie, persons with CLBP and OA of moderate to severe pain intensity in Table 2.
 |
Table 1 Demographic Characteristics of the CLBP and OA Patients Enrolled in the Quebec Pain Registry |
 |  |  |  |
Table 2 Clinical Characteristics, Quality of Life, and Health-care Utilization – CLBP and OA Cohorts |
Persons with CLBP were mostly diagnosed with lumbar pain with radicular pain (47.1%) or lumbar pain without radicular pain (33.6%) and approximately two-thirds (63.4%) had symptoms compatible with neuropathic pain.
The average pain (NRS, 0 = no pain, 10 = worst possible pain score) at baseline was 7.3±1.4 and 7.2±1.4 out of 10 for CLBP and OA, respectively. The pain interference score on daily activities was 61.5±19.5 and 64.6±18.8 out of 70 for CLBP and OA, respectively. Participants had pain on average for 27.6±2.4 and 27.9±0.5 days per month for CLBP and OA, respectively.
Persons living with CLBP had consulted on average with 5.8±3.3 different types of health-care professionals for pain since onset, whereas for persons with OA, this number was slightly lower at 5.0±3.1. Hospitalizations were rare for both CLBP and OA (<5%) and less than one-fifth of participants reported an emergency room visit in the past 6 months (OA = 11.9%; CLBP = 18.9%). Physical therapies, psychological techniques, and self-management strategies had been used by 92.6%, 17.6% and 56.1% of persons with CLBP, respectively. For persons with OA, physical therapies were used by 89.2% of persons, while 50.4% were using self-management strategies and 15.8% were using psychological techniques. The most commonly used medications for pain relief were acetaminophen, NSAIDs and opioids for both groups. Anticonvulsants were also frequently used by CLBP patient.
Participants reported on average 3.6±2.3 and 4.4±2.9 comorbidities for CLBP and OA, respectively. Most frequently reported comorbidities for persons with CLBP were depressive disorders (40.4%), hypertension (38.5%), chronic headache/migraine (35.6%), hypercholesterolemia (33.5%), and anxiety disorders (32.9%). Most frequently reported comorbidities for persons with OA were hypertension (49.6%), depressive disorders (45.3%), hypercholesterolemia (44.6%), chronic snoring (36.7%), anxiety disorders (31.7%), and chronic headache/migraine (31.0%). Levels of depressive symptoms were moderate to severe for more than 40% of persons in both cohorts. Levels of pain catastrophizing were in the 4th quartile in 59.1% and 62.6% of persons with CLBP and OA, respectively. Norm-based scores on pQoL were very low, namely 26.7±7.6 and 25.6±7.7 for persons with CLBP and OA, respectively. Most participants (54.6% for CLBP; 61.2% for OA) were drinking alcohol once a month or less.
Association Analyses for CLBP and OA Cohorts
The second objective was to determine the associations between pain intensity scores, pain interference scores, and QoL scores with health-care utilization and disability, from variables collected before the persons’ first visit at the pain clinic. These associations among persons living with moderate-to-severe pain due to CLBP or OA are presented in Table 3.
 |
Table 3 Analysis of Association Between Pain Scores or Quality of Life and Health-care Utilization or Disability Status |
Pain Severity, pain interference and quality of life among persons with CLBP. In the CLBP cohort, those reporting having had emergency visits, being hospitalized in the past 6 months, being on disability or receiving disability benefits were reporting higher levels of pain severity and pain interference compared to those not reporting those events (all p < 0.05). These results might have been influenced in part by the fact that approximately half of persons with CLBP experienced radicular pain, suggesting a neuropathic component to their painful experience. Neuropathic pain has been associated with increased pain severity, health-care utilization and poorer treatment response compared to non-neuropathic pain.32–34
There were no significant associations between pQoL and health-care utilization in the CLBP subgroup, but both hospitalizations and emergency room visits were associated with lower mQoL life (p < 0.05). Disability status and receiving disability benefits were both associated with lower pQoL and mQoL (all p < 0.01).
Pain Severity, pain interference and quality of life among persons with OA. Results did not identify significant associations between health-care utilization and pain severity, pain interference, pQoL or mQoL among persons with OA. Disability status or benefits were not significantly associated with pain severity. The pain interference was significantly associated with disability status (p = 0.0036), but not with disability benefits, such that those with a disability status reported higher levels of pain interference compared to those not on disability. Finally, disability status was the only significant variable associated with pQol and mQoL. More specifically, those on disability reported lower levels of pQoL and mQoL compared to those not on disability (p = 0.0064 and 0.0401, respectively).
Discussion
Participants were in majority females, between 1/3 and half of participants were on temporary or permanent disability and approximately one-quarter were receiving disability benefits. This is comparable to other pain registries35 and also to data from the whole chronic pain sample of the QPR that showed 59.1% of females and 37.2% of participants reported being on temporary or permanent disability.20
Health-care utilization as defined by pain-related emergency visits or hospitalizations was relatively infrequent in the 6 months prior to initiating tertiary care treatments. This finding is somewhat surprising considering that those persons had not initiated tertiary care treatments yet and were on the waitlist. It is possible that their pain condition, despite being moderate to severe, was stable enough and did not require punctual emergency care or hospitalization. When looking at the literature on prevalence of chronic pain in the ER, results suggest that approximately 4.4% of persons have low back pain in the ER14 and these rates seem to increase with time.15 In addition to the possible stability of their chronic pain condition, the chronicity of persons’ pain condition, with an average pain duration of approximately 7 years might influence health-care utilization in that they might have developed strategies to cope with acute bounds of pain and are looking for longer-term, specialized pain care. It is also possible that these persons were followed in primary or secondary care until their first appointment at the tertiary care clinic, thus decreasing the need to access treatment through the ER or hospitalization.
Most participants were not on disability or receiving disability benefits. This could in part be explained by the large proportion of persons in an older age range (mean age CLBP = 55.7; mean age OA = 61.5) and retired (CLBP: 43.3%; OA: 57.0%). Indeed, persons above the age of 65 years cannot receive disability benefits, as this is changed to retirement pensions. This might be more a reflection of sociodemographic characteristics of the study sample rather than a reflection of pain conditions and their degree of interference.
Levels of psychological distress in the form of depressive symptoms and pain catastrophizing were high in this study sample. This is consistent with the literature that suggests higher levels of depressive symptoms in persons with higher degrees of pain severity.36 Even though the strength of evidence is low quality, pain catastrophizing is often found in studies to be associated with pain severity and disability.37
Persons who had visited the ER or were hospitalized and those on disability or receiving disability benefits reported higher levels of pain severity, pain interference and lower levels of mQoL compared to those who did not consult the ER, were not hospitalized, were not on disability or not receiving disability benefits, respectively. These results are consistent with a European study that found a positive association between pain severity and health utilities, healthcare resource use (hospitalization and ER visits), and activity impairment.38 It is also consistent with a systematic review of chronic pain and frequent use of ER that found those frequently using ER present with higher levels of disability compared to those who do not frequently use the ER.39 From a clinical standpoint, these results are important in that they suggest that persons, despite having upcoming appointments in specialized pain treatment centers, continue to seek urgent care for their pain, especially when pain is severe and disabling. It is possible that by improving access for pain flares or rapid changes in patients’ pain condition, we could reduce the use of emergency care services for this population. A recent study of pain in the ER showed that while 5% of patients presenting with chronic pain complaints had consulted a pain specialist, 78% of patients were awaiting for a pain consultation.40 From a research standpoint, identifying specific reasons that drove patients to consult the ER for their pain (eg, lack of rapid access to medical consultation, increased distress during a pain flare, lack of comprehension of self-management options) could further optimize patient care.
No significant association was found between health-care utilization and pQoL, unlike for disability status and disability benefits. Health-care utilization was rather significantly associated with mQoL. It is important to consider that QoL is a global health indicator that might be influenced by pain but also a multitude of factors such as comorbidities and socioeconomic characteristics. Indeed, health-related QoL represents an assigned value attributed to a perceived state of health that is affected by disease, injury or treatment.41 The pQoL score is comprised of elements associated with limitations in physical activities, usual role activities, bodily pain and general health perceptions. It is as such possible that health-care utilization with regard to pain management is not related to the overall construct of pQoL but rather some specific dimensions of the pain experience. With regard to the association between mQoL and health-care utilization, previous studies have shown that mental health aspects, such as depressive symptoms, and chronic pain independently predict ER visits,42 and that targeting psychological distress through behavioural health consultations can reduce health-care utilization or ER services among persons with chronic pain.43 In addition, disability status and benefits might be due to pain condition but are not pain-specific characteristics. They influence one’s pain condition, but also more broadly one’s socioeconomic status. It is thus not surprising to see a positive association in this study between disability status and benefits and pQoL.
Among persons living with OA, those on disability reported higher levels of pain interference and lower levels of pQoL and mQoL compared to those not on disability. Health-care utilization in this subpopulation was not associated with pain severity or interference. These results could in part be explained by the small sample size included in this subgroup. It might also be due to the target population, namely those seen in tertiary care clinic. Hospitalizations among persons with OA are typically associated with joint replacements (eg, hip or knee replacements),44 which is not a procedure that patients typically undergo when waiting for an initial appointment at a specialized pain center. In addition, a large study based in the United States showed that some factors, such as opioid use, are a stronger predictor of health-care utilization among persons with OA compared to pain interference.45 It is possible that some factors not accounted for in the present study better predict ER visits and hospitalization in this population.
The lack of association between disability status and benefits and pain severity or interference might be attributable to the low proportion of persons with OA who were on the workforce (only 46.8% of this sample) and older age. Given the small sample size, age, and work status in this subpopulation, it might not have been possible to detect such association.
Limitations
The QPR characterizes only a small proportion of the chronic pain population, that is, those who are referred to tertiary care clinics, such that the results cannot be generalized to other populations of persons treated in primary or secondary care settings. Access to tertiary care clinics in the province of Quebec requires a physician referral; access to these clinics does not incur any direct costs for the patient but limited due to relatively long waiting lists as is the case in other Canadian provinces. As a result, it is unclear how the data obtained in the QPR compare to what would be obtained in other health-care systems (self-referrals or other systems of access to the specialized pain clinics). Furthermore, the analysis is limited to a specific point in time and did not evaluate the evolution of these persons.
In addition, the QPR population is not a homogeneous one (eg, pain diagnoses, comorbidities, psychological distress, socioeconomic status), reflecting real-world persons visiting multidisciplinary pain treatment clinics. Furthermore, the captured data do not cover all the resources of health-care utilization and the associated costs. It is a limited/conservative evaluation of health-care utilization. Costs of pain-related emergency visits and hospitalizations will have to be extrapolated with further analysis.
Another limitation of our findings pertains to the use of self-reported data. However, all medical/clinical data (eg, pharmacological and non-pharmacological pain treatments) were collected by well-trained research nurses while persons’ diagnoses were established by experienced pain physicians. Furthermore, our data source is an observational one in which sampling and confounding biases may occur and thereby compromise the validity of some of our conclusions.
Finally, the relatively small sample size of the population suffering from OA limits our ability to properly describe this population and establish association between pain, QoL and other clinically important outcomes. It would be important to replicate study results in a larger cohort of persons diagnosed with OA.
Results of this study are generalizable to other populations of CLBP and OA who are seeking specialized treatments for pain in a public healthcare system. Study population is comparable to other similar chronic pain registries in other developed countries. However, it is important to consider that persons included in the present analysis reported moderate to severe pain intensity and as such might not represent the experiences of persons seeking care for pain of lesser intensities.
Conclusion
Among persons seeking specialized care for CLBP for moderate-to-severe pain, health-care utilization and disability status are negatively associated with pain severity, pain interference, and QoL. These results are largely coherent with the existing literature. While the study design does not allow for the identification of causal factors, results suggest pain severity and interference and important characteristics that influence disability status and health-care utilization and should be considered when designing and evaluating treatment approaches. Better understanding how pain severity and interference lead to ER consultations when access to MPT is underway would help better care for these patients and prevent deterioration of their condition while waiting for treatment.46
Except for disability status, these results were not found among persons living with from moderate-to-severe OA pain, which might be due to smaller sample size or unique characteristics of this pain condition.
Acknowledgments
MG Pagé is a Junior 1 Research Scholar from the Fonds de recherche du Québec – Santé (FRQS). The QPR was supported by the Quebec Pain Research Network (QPRN) which was itself funded by a governmental grant from the FRQS. The QPRN was also supported by the Quebec Health Ministry, Pfizer Canada Inc, Astra Zeneca Inc. and to a lesser extent by Janssen Inc whose contributions were all channeled through the FRQS through an official financial partnership.
Funding
Study was funded by Pfizer Canada Medical Division.
Disclosure
M Fernet is a medical advisor for/employee of Pfizer Canada ULC Medical Division who sponsored and funded the study. The analyses were conducted independently by M Dorais and only aggregated results were shared with study sponsor. MG Pagé reports grants from Pfizer Canada ULC during the conduct of the study and received personal fees/honoraria from Canopy Growth for work unrelated to the present manuscript. The authors report no other potential conflicts of interest for this work.
References
1. Wu A, March L, Zheng X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med. 2020;8(6):299. doi:10.21037/atm.2020.02.175
2. Goode AP, Carey TS, Jordan JM. Low back pain and lumbar spine osteoarthritis: how are they related? Curr Rheumatol Rep. 2013;15(2):305. doi:10.1007/s11926-012-0305-z
3. Beaudet N, Courteau J, Sarret P, Vanasse A. Prevalence of claims-based recurrent low back pain in a Canadian population: a secondary analysis of an administrative database. BMC Musculoskelet Disord. 2013;14(1):151. doi:10.1186/1471-2474-14-151
4. Massé-Alarie H, Angarita-Fonseca A, Lacasse A, et al. Low back pain definitions: effect on patient inclusion and clinical profiles. Pain Rep. 2022;7(2):e997. doi:10.1097/PR9.0000000000000997
5. Manchikanti L, Singh V, Falco FJ, Benyamin RM, Hirsch JA. Epidemiology of low back pain in adults. Neuromodulation. 2014;17(Suppl 2):3–10. doi:10.1111/ner.12018
6. Statistics Canada. Statistics Canada population projections for Canada (2013 to 2063), provinces and territories (2013 to 2038); 2013. Available from: https://www150.statcan.gc.ca/n1/pub/91-520-x/91-520-x2014001-eng.htm.
7. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196.
8. Safiri S, Kolahi A, Smith E, et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2019;79(6):819–828. doi:10.1136/annrheumdis-2019-216515
9. Staud R. Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia. Curr Rheumatol Rep. 2011;13(6):513–520. doi:10.1007/s11926-011-0206-6
10. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105(1):185–199. doi:10.1093/bmb/lds038
11. Allen KD, Golightly YM. State of the evidence. Curr Opin Rheumatol. 2015;27(3):276–283. doi:10.1097/BOR.0000000000000161
12. Public Health Agency of Canada. Prevalence of chronic diseases among Canadian adults; 2019. Available from: https://www.canada.ca/en/public-health/services/chronic-diseases/prevalence-canadian-adults-infographic-2019.html.
13. Sharif B, Kopec J, Bansback N, et al. Projecting the direct cost burden of osteoarthritis in Canada using a microsimulation model. Osteoarthritis Cartilage. 2015;23(10):1654–1663. doi:10.1016/j.joca.2015.05.029
14. Edwards J, Hayden J, Asbridge M, Gregoire B, Magee K. Prevalence of low back pain in emergency settings: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2017;18(1):143. doi:10.1186/s12891-017-1511-7
15. Ferreira GE, Herbert R, Machado GC, et al. Low back pain presentations to New South Wales emergency departments: trends over time and geographical variation. Emerg Med Australas. 2021;33(5):868–874. doi:10.1111/1742-6723.13745
16. Alhowimel AS, Alotaibi MA, Alenazi AM, et al. Psychosocial predictors of pain and disability outcomes in people with chronic low back pain treated conservatively by guideline-based intervention: a systematic review. J Multidiscip Healthc. 2021;14:3549–3559. doi:10.2147/JMDH.S343494
17. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91(5):712–721. doi:10.2522/ptj.20100280
18. Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine. 2002;27(5):E109–120. doi:10.1097/00007632-200203010-00017
19. Haukenes I, Farbu EH, Riise T, Tell GS. Physical health-related quality of life predicts disability pension due to musculoskeletal disorders: seven years follow-up of the Hordaland Health Study Cohort. BMC Public Health. 2014;14(1):167. doi:10.1186/1471-2458-14-167
20. Choiniere M, Ware MA, Page MG, et al. Development and implementation of a registry of patients attending multidisciplinary pain treatment clinics: the Quebec Pain Registry. Pain Res Manag. 2017;2017:8123812. doi:10.1155/2017/8123812
21. Moisset X, Page MG. Interest of registries in neuropathic pain research. Rev Neurol. 2021;177(7):843–848. doi:10.1016/j.neurol.2021.07.011
22. Jensen MP, Tomé-Pires C, de la Vega R, Galan S, Solé E, Miro J. What determines whether a pain is rated as mild, moderate, or severe? The importance of pain beliefs and pain interference. Clin J Pain. 2017;33(5):414–421. doi:10.1097/AJP.0000000000000429
23. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. In: Handbook of Pain Assessment.
24. Cleeland CS. The Brief Pain Inventory User Guide. The University of Texas MD Anderson Cancer Center; 2009.
25. Beck AT, Steer RA, Garbin GM. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. doi:10.1016/0272-7358(88)90050-5
26. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. doi:10.1001/archpsyc.1961.01710120031004
27. Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524. doi:10.1037/1040-3590.7.4.524
28. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94(3):135–140.
29. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid risk tool. Pain Med. 2005;6(6):432–442. doi:10.1111/j.1526-4637.2005.00072.x
30. Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi:10.1097/00005650-199603000-00003
31. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36. doi:10.1016/j.pain.2004.12.010
32. McDermott AM, Toelle TR, Rowbotham DJ, Schaefer CP, Dukes EM. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain. 2006;10(2):127–135. doi:10.1016/j.ejpain.2005.01.014
33. O’Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27(2):95–112. doi:10.2165/00019053-200927020-00002
34. Harden N, Cohen M. Unmet needs in the management of neuropathic pain. J Pain Symptom Manage. 2003;25(5 Suppl):S12–17. doi:10.1016/S0885-3924(03)00065-4
35. Vaegter HB, Christoffersen LO, Enggaard TP, et al. Socio-demographics, pain characteristics, quality of life and treatment values before and after specialized interdisciplinary pain treatment: results from the Danish Clinical Pain Registry (PainData). J Pain Res. 2021;14:1215–1230. doi:10.2147/JPR.S306504
36. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. doi:10.1001/archinte.163.20.2433
37. Martinez-Calderon J, Jensen MP, Morales-Asencio JM, Luque-Suarez A. Pain catastrophizing and function in individuals with chronic musculoskeletal pain: a systematic review and meta-analysis. Clin J Pain. 2019;35(3):279–293. doi:10.1097/AJP.0000000000000676
38. Witt EA, Kenworthy J, Isherwood G, Dunlop WC. Examining the association between pain severity and quality-of-life, work-productivity loss, and healthcare resource use among European adults diagnosed with pain. J Med Econ. 2016;19(9):858–865. doi:10.1080/13696998.2016.1178127
39. Depelteau A, Racine-Hemmings F, Lagueux E, Hudon C. Chronic pain and frequent use of emergency department: a systematic review. Am J Emerg Med. 2020;38(2):358–363. doi:10.1016/j.ajem.2019.158492
40. Small RN, Shergill Y, Tremblay S, et al. Understanding the impact of chronic pain in the emergency department: prevalence and characteristics of patients visiting the emergency department for chronic pain at an urban academic health sciences centre. Can J Pain. 2019;3(1):106–113. doi:10.1080/24740527.2019.1587290
41. Vetter TR. Health-related quality of life in pain medicine: a review of theory and practice. In: Preedy VR, Watson RR, editors. Handbook of Disease Burdens and Quality of Life Measures. New York, NY: Springer; 2010:3917–3932.
42. Choi NG, Marti CN, Bruce ML, Kunik ME. Relationship between depressive symptom severity and emergency department use among low-income, depressed homebound older adults aged 50 years and older. BMC Psychiatry. 2012;12(1):233. doi:10.1186/1471-244X-12-233
43. Woodhouse J, Peterson M, Campbell C, Gathercoal K. The efficacy of a brief behavioral health intervention for managing high utilization of ED services by chronic pain patients. J Emerg Nurs. 2010;36(5):399–403. doi:10.1016/j.jen.2009.02.008
44. Wright EA, Katz JN, Cisternas MG, Kessler CL, Wagenseller A, Losina E. Impact of knee osteoarthritis on health care resource utilization in a US population-based national sample. Med Care. 2010;48(9):785–791. doi:10.1097/MLR.0b013e3181e419b1
45. Zhao X, Shah D, Gandhi K, et al. The association of pain interference and opioid use with healthcare utilization and costs, and wage loss among adults with osteoarthritis in the United States. J Med Econ. 2019;22(11):1192–1201. doi:10.1080/13696998.2019.1658590
46. Choiniere M, Dion D, Peng P, et al. The Canadian STOP-PAIN project - Part 1: Who are the patients on the waitlists of multidisciplinary pain treatment facilities? Can J Anaesth. 2010;57(6):539–548. doi:10.1007/s12630-010-9305-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.