Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Characteristics of 12-Month Readmission for Hospitalized Patients with COPD: A Propensity Score Matched Analysis of Prospective Multicenter Study
Authors Xu T, Sun W, Zhao H, Wang X, Yuan Q, Zhang X, Mao S, Zhang X, Zhao M, Sheng Z, Zhang M , Huang M, Ji N
Received 1 June 2022
Accepted for publication 9 September 2022
Published 20 September 2022 Volume 2022:17 Pages 2329—2341
DOI https://doi.org/10.2147/COPD.S376909
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Tingting Xu,1,* Wei Sun,2,* Hongqing Zhao,3,* Xinmin Wang,4 Qi Yuan,1 Xijie Zhang,1 Shan Mao,5 Xiuwei Zhang,6 Mingming Zhao,7 Zebo Sheng,8 Mingshun Zhang,9 Mao Huang,1 Ningfei Ji1
1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Xishan People’s Hospital of Wuxi City, Wuxi, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, Wuxi Second People’s Hospital, Wuxi, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, Shuyang Hospital of Traditional Chinese Medicine, Suqian, People’s Republic of China; 5Department of Respiratory and Critical Care Medicine, Nanjing First Hospital, Nanjing, People’s Republic of China; 6Department of Respiratory and Critical Care Medicine, Nanjing Jiangning Hospital, Nanjing, People’s Republic of China; 7Department of Respiratory and Critical Care Medicine, Nanjing Gaochun People’s Hospital, Nanjing, People’s Republic of China; 8Department of Respiratory and Critical Care Medicine, Changshu First People’s Hospital, Suzhou, People’s Republic of China; 9Department of Immunology, Nanjing Medical University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mao Huang; Ningfei Ji, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Nanjing Medical University, No. 300, Guangzhou Road, Nanjing, 210029, People’s Republic of China, Tel/Fax +86-13813886116 ; +86-18951080699, Email [email protected]; [email protected]
Purpose: Hospitalization for acute exacerbations of chronic obstructive pulmonary disease (AECOPD) is considered as severe exacerbations. Readmission for severe exacerbations is a crucial event for COPD patients. However, factors associated with readmission for severe exacerbations are incomplete. The study aimed to investigate different characteristics between the severe and non-severe exacerbation groups.
Patients and Methods: Patients hospitalized for severe AECOPD were included in multi-centers, and their exacerbations in next 12 months after discharge were recorded. According to exacerbations, patients were separated into the severe-exacerbation group and the non-severe exacerbation group. Propensity-score matching (PSM) and multivariable analyses were performed to compare the baseline characteristics of two groups. The Hosmer-Lemeshow test and receiver operating characteristic curve were applied to evaluate how well the model could identify clusters.
Results: The cohort included 550 patients with severe AECOPD across 27 study centers in China, and 465 patients were finally analyzed. A total of 41.5% of patients underwent readmission for AECOPD within 1 year. There were no significant differences in baseline characteristics between groups after PSM. Severe exacerbations in the 12 months were related to some factors, eg, the duration of COPD (13 vs 8 years, P< 0.001), the COPD Assessment Test (CAT) score (20 vs 17, P< 0.001), the blood eosinophil percentage (1.5 vs 2.0, P< 0.05), and their inhaler therapies. Patients readmitted with AECOPD had a longer time of diagnosis (≥ 9 years), more symptoms (CAT ≥ 10), and lower blood eosinophils (Eos < 2%). A clinical model was derived to help identify patients at risk of readmission with severe exacerbations.
Conclusion: These analyses confirmed the relevance of COPD at admission with future severe exacerbations. A lower blood eosinophils percentage appears to be related to readmission when combined with clinical history. Further studies are needed to evaluate whether this study can predict the risk of exacerbations.
Keywords: chronic obstructive pulmonary disease, acute exacerbation, eosinophils, prognosis
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease underpinned by diverse clinical phenotypes and biological endotypes,1,2 with episodes of exacerbations leading to hospitalization and mortality.3–5 There were 212.3 million cases of COPD globally with an estimated 3.3 million deaths relevant to COPD in 2019.6 Acute exacerbation of COPD (AECOPD), defined as worsening of respiratory symptoms that results in additional therapy, is classified into mild, moderate and severe exacerbation. Patients are severe when the patient visits an emergency room or is hospitalized for the event.7,8
For patients underlying hospitalization for AECOPD, readmission to hospital is a vital problem. Hence, it is important to identify risk factors for severe exacerbation of COPD-related readmission in advance. Previous studies have identified various factors in predicting COPD outcomes. For example, Zemans et al reported that a combination of soluble Receptor for Advanced Glycation Endproducts and C-Reactive Protein may be predictive of total exacerbation frequency in the ECLIPSE study.9 García-Sanz et al demonstrated that age, exacerbation severity and comorbidities influenced 5-year mortality in a retrospective study.10 Furthermore, studies mostly focused on short-term readmission such as 30 days or 90 days after discharge.11,12 Several studies have explored rates and risk factors related to readmission within 12 months, most were retrospective studies. The rates of readmission range from 25%-87%, and risk factors vary a lot among studies.13 Notably, in our country, limited studies have been conducted to investigate factors relevant to 12-month readmission in a prospective way.
Severe exacerbations rely on self-reported symptoms and lack reliable biomarkers to distinguish future risk of exacerbations. Blood eosinophils tests are feasible in clinical practice. Furthermore, blood eosinophils are recommended as a biomarker to guide inhaled corticosteroids (ICS) use according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 20208. However, there is controversy regarding the predictive value of blood eosinophil levels for severe exacerbation related readmission.14–20 Whether eosinophil levels and other clinical characteristics at admission for AECOPD have an effect on readmission in the next year remains unclear and warrants investigation in large, longitudinal cohorts. Variation in the prevalence, and controversy in relationship between eosinophils and severe exacerbations demonstrate that risk factors cannot be generalized, which implies the interventions should be tailored to local healthcare system. Thus, the main objective of our study was to investigate the similarities and differences of patients with or without severe exacerbations of COPD in the following 12 months after discharge from the hospital.
Materials and Methods
Study Design
Our study is a multi-center, prospective, observational cohort study conducted in China. The study complied with the Declaration of Helsinki and Good Clinical Practice and was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University, China (2019-SR-129). The outbreak of coronavirus disease 2019 (COVID-19) influenced the number of enrolled patients, so we have to reapply to the hospital federal committee to prolong the study. All participants provided written informed consent. Respiratory physicians across 27 study centers enrolled patients with the main diagnosis of hospitalization for AECOPD from October 2019 to October 2020 and followed them in the next 12 months after discharge, due to the pandemic of COVID-19, sporadic cases make it difficult for all enrolled patients to attend the visit to the hospital, which pushes researchers to contact part of them online or by telephone.
Study Patients
Patients were diagnosed with COPD by symptoms, spirometry and the standard definition of the Global Initiative for Chronic Obstructive Lung Disease.8 In detail, the recruitment criteria included a post-bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio <0.7, hospitalization for AECOPD and blood tests within 24 hours after admission. Blood tests included complete blood cell count which was collected from peripheral venous blood. Upon candidate reassessment, exclusion criteria were lack of pulmonary function test within five years, mislabeled asthmatics, COPD without exacerbation and no access to 12-month follow-up. Patients with proceeding cancers and those taking systemic corticosteroids 48 hours before admission were also excluded.
Study Variables
At baseline, some characteristics (eg, the patients’ self-reported symptoms, smoking history, residence, time since COPD diagnosis, COPD Assessment Test (CAT) score, spirometry within 5 years, comorbid conditions, inhaler therapy and corticosteroid use within 48 hours before admission) were documented at study entry with the use of research collaborations platform of YiduCloud (https://research.yiducloud.com.cn/). Complete blood cell counts taken before the use of systemic corticosteroids were also documented and uploaded as pictures onto the platform. In line with previous studies, high eosinophil counts were defined as blood eosinophils ≥2% of the total white blood cell count, which has shown high sensitivity in identifying sputum eosinophilia.15,21 Exacerbations in the next year after hospitalization were recorded by hospital database, telephone and internet due to the unexpected pandemic.22
Study Outcomes
The occurrence of severe exacerbations was a critical outcome. Physicians were instructed to make their decision on common clinical practice without specific criteria of an exacerbation. The primary outcome was 12-month readmission for severe exacerbation of COPD after discharge. A severe exacerbation was defined as hospitalization for COPD.7 To investigate the factors related to 12-month readmission after discharge, we divided patients into two groups: the severe exacerbation group and the non-severe exacerbation. The severe exacerbation group indicates patients who are hospitalized for AECOPD in the next 12 months, while patients without readmission for AECOPD are defined as the non-severe exacerbation group.
Study Analysis
Demographic data were summarized using basic descriptive statistics. For continuous variables, data were presented as the means ± standard deviations (SD) if they met normal distribution, or medians (25–75% interquartile range) if not. For categorical variables, data were presented as numbers (percentages). Continuous variables were analyzed using Student’s t-test or nonparametric tests. Categorical variables were tested by the Chi-square test, Fisher’s exact test or Mann–Whitney U-test, as appropriate. To help reduce the effects of confounding on nonrandomized design, propensity-score matching was applied. Considering the difficulty in conducting pulmonary function tests during acute exacerbations, the covariates (eg, age, sex, smoking status, body mass index, residence and FEV1% pre) were included in the PSM. Patients were 1:1 PS-matched by the near neighbor methodology with a maximum caliper of 0.02 of the deviation of the logit of the PS.
A binary logistic regression model was used to estimate factors associated with the probability of 12-month readmission for an individual. Variables that were explored in the univariate analysis were considered in the multivariate model, while a conservative significance threshold of 0.01 was applied to assess data for entry into or deletion from the model. Furthermore, the Hosmer-Lemeshow test was conducted to evaluate the goodness of fit of the binary logistic regression models. Receiver operating characteristic (ROC) curves were generated to assess the predictive ability of the models to correctly identify the onset of 12-month readmission. All statistical analyses were conducted using the IBM-SPSS version 26.0. Variables with a two-sided P<0.05 were accepted as statistically significant.
Results
Recruitment of Patients
The procedure of sample selection is shown in Figure 1. A total of 550 patients were assessed for eligibility from October 2019 to October 2020 in 27 study centers in China. Therein, 85 patients were excluded because they did not completely fulfill the enrollment criteria. Thus, 465 patients were included in the analysis.
 |
Figure 1 Flowchart of cohort selection. Abbreviation: COPD, chronic obstructive pulmonary disease. Note: Severe exacerbation indicates patients experienced readmission for exacerbations. |
Since the unexpected COVID-19 pandemic, the number of recruited patients was sharply decreased from 90 to 5 patients per month (see Figure 2). Concerning the study centers, most of the hospitals attributed much to the study. Specifically, half of the centers enrolled more than 15 patients, and the most active center included 69 patients (Figure 3).
 |
Figure 2 The number of recruited patients over time. Abbreviation: COVID-19, coronavirus disease 2019. |
 |
Figure 3 Number of patients from 27 study centers in China. |
The demographic and baseline characteristics of the full cohort are presented in Table 1. Briefly, the enrolled patients were mostly men (71.8%) with smoking history. The primary symptoms were dyspnea, cough and expectoration. The mean (SD) duration of COPD diagnosis was 9.27 (9.36) years, the mean (SD) FEV1/FVC was 53.15% (12.16%), and the median (IQR) eosinophil percentage was 1.71 (3.07). Among those COPD patients, 41.5% (n=193) required hospitalization (range 1–8) with severe exacerbations in the following 12 months. In terms of COPD medications, most patients were prescribed ICS plus long-acting β2 agonist (LABA) combination or ICS plus LABA and long-acting muscarinic antagonist (LAMA) combination (24.1% and 29.5%, respectively).
 |
Table 1 Demographic and Clinical Data of Cohort Patients |
Characteristics of Patients with or Without Severe Exacerbations Before and After Propensity-Score Matching
Demographic According to the current GOLD strategy document, patients were categorized into severe exacerbation (termed group A) and non-severe exacerbation (termed group B) groups. The severe exacerbation group comprised 193 patients (41.5%) who experienced hospitalization for AECOPD and the non-severe exacerbation group consisted of 272 patients (58.5%) who were not hospitalized for COPD in the following 12 months. Baseline characteristics were compared between the two groups.
As shown in Table 2, patients with severe exacerbations in the next 12 months were older, which showed a greater proportion of the rural population, a lower ratio of current smokers, a longer duration of disease, a decreased eosinophil percentage, and fewer individuals with high blood eosinophils. Moreover, they complied with more respiratory symptoms, more severe airflow limitation, and more complex comorbidities. In addition, they were more likely to keep inhaling medicine regularly. The proportion of patients in the GOLD grade III was the highest in both the group A and the group B. In addition, the percentage of GOLD grade IV was significantly higher in group A than that in group B.
 |
Table 2 Characteristics of Patients with or Without Severe Exacerbations, Before and After 1:1 Propensity Score Matching |
Given differences in the baseline characteristics between groups A and B, PSM was performed to reduce the effects of confounding. The following variables (eg, age, sex, residence, BMI, and smoking status) were included in the PSM model. Spirometry is crucial in the management of COPD; however, it is not routinely performed in AECOPD due to worsening symptoms and failure to cooperate effectively. Furthermore, it is necessary to explore factors regardless of pulmonary function test. Thus, FEV1 of predicted was also included in the model. Of the entire COPD patient cohort, 172 of 193 group A cases could be matched (1:1) to group B using the nearest neighbor method with a maximum caliper width equal to 0.02 of the PS. After matching, the two groups were well-balanced in baseline characteristics and showed a similar distribution of characteristics to the unmatched analysis. Notably, FVC% predicted and cor pulmonale were not included in the model but they were balanced after propensity-score adjustment, indicating that we were able to balance key unmeasured factors as far as feasible.
Factors Independently Associated with Severe Exacerbations
Before matching, four factors were related to severe exacerbations in multivariable logistic regression analysis: years of COPD greater than or equal to 9 years, presence of dyspnea, CAT ≥10 and eosinophils <2%, as shown in Table 3. Nevertheless, no significant difference was observed in the comorbidity of cor pulmonale between two groups. After propensity matching, the prevalence of cor pulmonale was similar between the two groups, and only three factors remained independently associated with severe exacerbation: years of COPD ≥9 years (odds ratio (OR), 2.16; 95% CI, 1.38 to 3.39; P<0.001), CAT ≥10 (OR, 3.40; 95% CI, 1.36 to 8.51; P<0.001), and eosinophils <2% (OR, 1.68; 95% CI, 1.07 to 2.64; P<0.05).
 |
Table 3 Multivariate Regression Analysis of Associations with Severe Exacerbation (Group A) |
Clinical Rules to Predict Future Severe Exacerbations
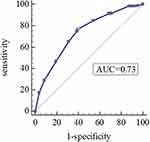
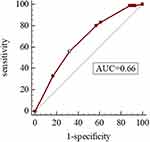
As shown in Table 4, the Hosmer-Lemeshow test was used to evaluate the goodness of fit in the model, and the H-L test values were 0.728 and 0.837, respectively, which suggested that the model was well-fitted. Moreover, we derived ROC curves from the results of the multivariate logistic regression analysis. The AUCs of 0.727 (95% CI 0.681–0.773, P<0.05) and 0.662 (95% CI 0.605–0.720, P<0.05) demonstrated a fair accuracy of the model to predict the severe exacerbation (Figures 4 and 5).
 |
Table 4 The Goodness of Fit and Accuracy of the Model |
Discussion
In this multicenter observational study, we found that patients admitted to the hospital with a COPD exacerbation were mostly male, and 41.5% of them would be readmitted within 1 year. For patients who were readmitted to the hospital with severe exacerbations in the following 12 months, they had a significantly longer duration of COPD (OR, 2.16; 95% CI, 1.38 to 3.39; P<0.001), higher CAT score (OR, 3.40; 95% CI, 1.36 to 8.51; P<0.001), and lower eosinophils (OR, 1.68; 95% CI, 1.07 to 2.64; P<0.05) than those without severe exacerbations. There was a trend for a higher eosinophil percentage in patients without severe exacerbations, with a ≥2% blood eosinophils before and after PSM. To date, the relationship between the risk of severe exacerbation and blood eosinophil count remains controversial.14,15,17–20 Our study may compensate for some information for those at risk of the most severe exacerbations (ie, those requiring hospitalization). However, given the observational design, the small sample size, and the loss of annual follow-up in 59 patients, the study should not be taken to extrapolate either low or high eosinophils in severe exacerbations. From a clinical perspective, it is challenging for doctors to identify patients at risk of exacerbations when insufficient follow-up is yet available to provide. Based on a real-world clinical setting, our study confirmed that long duration, multi-symptoms and low eosinophil percentage were associated with a higher incidence of severe exacerbation in the next 12 months, which may contribute to evaluating the severity of disease by history taking and blood eosinophils in clinical practice.
Among all study centers, tertiary referral centers enrolled more severe patients than rural hospitals, which may be due to the hierarchical medical system in our country. The system requires doctors to transfer severe patients from rural hospitals to referral hospitals, and transfer mild or moderate patients conversely. The severe exacerbation phenotype is defined as patients with COPD experiencing hospitalization or emergency room visits because of worsening symptoms, which leads to poorer outcomes.23 Consistent with previous research, COPD patients were mainly male.24 Non-pharmacological treatments for COPD are a crucial part of treatment plan, including vaccinations and pulmonary rehabilitation. Our study revealed patients were more likely to take pharmacotherapies than non-pharmacological treatments, and very few patients had access to Flu, pneumonia vaccine, pulmonary rehabilitation or other palliative care. Unlike our results, Mir et al reported that palliative care was similar with pulmonary rehabilitation and possibly involved in reducing COPD-related readmission rates.25 Therefore, before widespread adoption, further studies and streamlined implementation of procedures are needed in our country to advocate non-pharmacological treatments.
Our results are partially concordant with some studies that found such differences between the two groups. For example, the EXACO study recorded the number of moderate and severe COPD exacerbations for 4 years and found that severe exacerbations were related to clinical variables including a COPD diagnosis of more than 7 years, daily sputum production and a modified Medical Research Council dyspnea score of 3–4.26 However, the timepoints essential to COPD development might differ between individuals. Our mean time since COPD diagnosis was 9 years, which was employed as a cutoff value in our multivariate analysis. Moreover, the mean years of COPD diagnosis in the severe exacerbation group were nearly twice than non-severe exacerbation group. Pathological alterations of the airway structure and resistance to ICS might account for the phenomenon. At the early stage of COPD, inhaled exposures induce oxidant injury and reprogramming of airway epithelium to defend against damage.27,28 At the advanced stage of COPD, accumulation of the epithelium reprogramming causes changes of airway microenvironment, immune responses and remodeling.29 The structure alterations might result in increased respiratory symptoms and accelerated decline of lung function, leading to the onset and progression of COPD.30 Our findings emphasize the importance of early interventions to prevent from severe exacerbations of COPD, which has been verified by the Tie-COPD study.31 Moreover, the CAT score involving cough, sputum production and dyspnea was calculated for a comprehensive assessment of symptoms, which has been widely used to evaluate the severity and prognosis of exacerbations. Previous studies analyzed participants with COPD of SPIROMICS cohort and IMPACT cohort, and revealed that higher CAT score was associated with increased rates of AECOPD.32,33 Pulido Herrero et al had similar findings that the CAT score increased during exacerbation and decreased 2 months later.34 These studies might indicate that symptom burden is related to the exacerbation status, which implies that reduction of symptoms probably contributes to preventing exacerbations.35 An ICS in combination with a LABA or a LAMA is the mainstay therapy for managing severe COPD.36 The link that we observed between severe exacerbation and inhale treatment has been partially noted previously.37 In the ECLIPSE study, an independent association between worse quality of life and future risk of exacerbation was observed, and the proportion of medications for COPD raised as the severity increased.38 In our study, patients in the severe exacerbation group had a higher tendency to use regular medications and more combinations of drugs, with the ratio of ICS/LABA/LAMA being nearly 1.5 times higher than that in the non-severe exacerbation group even after PSM (34.9% and 24.4%, P<0.05). According to Palmiotti et al, patients with severe exacerbations were critically ill and primarily at GOLD IV stage, who showed better adherence to the GOLD guidelines using ICS/LABA/LAMA.39
The predicted value of eosinophils for the incidence and prognosis of future severe exacerbation was diverse, with either no relationship or a positive relationship reported.18,40,41 Our findings are concordant with prior studies demonstrating a high prevalence (46%) of elevated eosinophil counts, defined as ≥2% of the total leukocyte count.14 Many studies such as SUNSET, WISDOM and SPIROMICS have reported exacerbations correlating with blood eosinophils.15,16,42,43 In our dataset, the proportion of high eosinophils in patients without severe exacerbation was approximately 1.7 times greater than that in the severe exacerbation group, and low eosinophil percentage was associated with increased 12-month COPD-related readmission before and after adjustment. Unlike our study, the SUNSET study reported that patients with higher blood eosinophils had a higher risk of future exacerbations, but their enrolled patients were stable COPD with a history of tobacco use and the endpoint was moderate or severe exacerbations.16 On the other hand, the SPIROMICS cohort study, recruited for a range of COPD severity for smokers and a control group of non-smokers, reported that elevated blood eosinophils only in combination with elevated sputum eosinophils were associated with COPD exacerbations.32 Consistent with our study, MacDonald et al found that the low eosinophil group was associated with increased mortality at 12 months, providing the clinical utility of eosinophils as biomarkers during acute exacerbations of COPD (AECOPD).18 The DECAF score identified eosinopenia as one of five key predictors of inpatient mortality in severe exacerbation of COPD.44 In addition, some studies comparing low and high eosinophil clusters have shown that high eosinophil was correlated with shorter length of hospital stay, lower risk of subsequent exacerbation, and better response to systemic corticosteroid treatment.19,45,46 The biology underlying these discrepancies is uncertain, but there could be several reasons. First, the criteria of enrolled patients were different among studies, such as stable COPD, moderate and severe exacerbation of COPD or non-smokers without COPD. Such a recruitment strategy does not permit us to conclude the predictive value of blood eosinophils in all COPD groups. Second, the primary endpoints were diverse including mortality in hospital, 28-day readmission, first exacerbation time and 12-month readmission; however, the mechanisms lack molecular and biological research. Biologically, the high eosinophil group was related to fewer neutrophil percentages, a lower risk of bacterial infection at exacerbation, and a better response to systemic corticosteroid treatment, which may account for our results.47–50
The study has some strengths and limitations. A main strength of this work is the use of multiple centers in patients with severe exacerbations of COPD and the online access to follow-up data during the period of the COVID-19 pandemic, which may be representative of a real-world practice.51 Another important strength is that we divided patients based on severe exacerbation in the following 12 months, which recorded comprehensive symptoms by CAT score and blood eosinophils at baseline to explore the relationship between eosinophil percentage and incidence of severe exacerbation within 1 year. Additionally, considering that pulmonary function is not recommended during severe acute exacerbation, we wanted to explore the characteristics between the two groups regardless of the severity of airflow limitation. Hence, we adjusted for potential bias, including age, sex, residence, body mass index and predicted FEV1% by PSM and multiple regression. Despite this extensive adjustment, it is still possible that some amount of unmeasured confounding remains. Additional limitations of our study include the lack of follow-up of 59 patients, potential inaccuracies by telephone or internet, and failure to timely measure blood eosinophils and lung function after 1 year due to the COVID-19 pandemic, which somewhat restricts the generalizability of this study.52 Large prospective studies are needed to further explore the impact of eosinophils on the clinical outcomes of AECOPD.
Conclusion
In summary, for patients with or without 12-month readmission for AECOPD, their baseline characteristics differed greatly in past history, symptoms, eosinophils and prescriptions. Patients with duration ≥9 years, CAT ≥10 and eosinophil <2% tend to be associated with increased 12-month readmission for AECOPD. These results are clinically relevant and highlight the consideration of specific COPD phenotypes.
Abbreviations
AECOPD, acute exacerbations of chronic obstructive pulmonary disease; PSM, propensity-score matching; ROC, receiver operating characteristic; CAT, COPD Assessment Test; Eos, eosinophil; GOLD, Global Initiative for Chronic Obstructive Lung Disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PS, propensity score; SD, standard deviation; IQR, interquartile range; COVID-19, coronavirus disease 2019; ICS, inhaled corticosteroid; LABA, long-acting β2 agonist; LAMA, long-acting muscarinic antagonist; AUC, area under the curve.
Data Sharing Statement
The datasets used or analyzed in the study are not publicly available because the patients are still in follow-up for further data but are available from Ningfei Ji upon reasonable request.
Ethics Approval and Informed Consent
The study complied with the Declaration of Helsinki and Good Clinical Practice. Besides, the study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University, China, with ethical approval reference number IRB-2019-SR-129. Before the actual data collection, written informed consent was obtained from the study participants. Our study is a multi-center, prospective, observational cohort study conducted in China.
Acknowledgments
The authors would like to thank staff members from 27 centers and all data collectors for their cooperation throughout the study, for example, Shuguang Han, Wei Gu, Bing Wan, Yanping Hang and Hui Qiu. In addition, we would like to thank Wei He and Min Wang for quality control. Special thanks to all study participants who agreed to participate in the study.
Author Contributions
All authors made a significant contribution to the multi-center work, whether the contribution was in the conception, study design, execution, acquisition of data, analysis and interpretation of data, took part in drafting, revising or critically reviewing the article, and approved the final version of the manuscript before submission to an agreed upon journal. All authors agree to be accountable for all aspects of the work.
Funding
This study was funded by Nanjing Medical University, the Jiangsu Provincial Key Research and Development Program (Grant Number BE2020616), and Bethune Charitable Foundation (BJ-RW2020008J). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cho MH, Hobbs BD, Silverman EK. Genetics of chronic obstructive pulmonary disease: understanding the pathobiology and heterogeneity of a complex disorder. Lancet Respir Med. 2022;10(5):485–496. doi:10.1016/s2213-2600(21)00510-5
2. Barnes PJ. Inflammatory endotypes in COPD. Allergy. 2019;74(7):1249–1256. doi:10.1111/all.13760
3. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/s0140-6736(19)30427-1
4. Kim V, Aaron SD. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur Respir J. 2018;52(5):1801261. doi:10.1183/13993003.01261-2018
5. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi:10.1016/s0140-6736(17)31222-9
6. Safiri S, Carson-Chahhoud K, Noori M, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. BMJ. 2022;378:e069679. doi:10.1136/bmj-2021-069679
7. Celli BR, Fabbri LM, Aaron SD, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med. 2021;204(11):1251–1258. doi:10.1164/rccm.202108-1819PP
8. Global Initiative for Chronic Obstructive Lung Disease. 2020 Report: global strategy for prevention, diagnosis and management of COPD; 2020.
9. Zemans RL, Jacobson S, Keene J, et al. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir Res. 2017;18(1):117. doi:10.1186/s12931-017-0597-7
10. García-Sanz MT, Cánive-Gómez JC, Senín-Rial L, et al. One-year and long-term mortality in patients hospitalized for chronic obstructive pulmonary disease. J Thorac Dis. 2017;9(3):636–645. doi:10.21037/jtd.2017.03.34
11. Alqahtani JS, Aldabayan YS, Aldhahir AM, Al Rajeh AM, Mandal S, Hurst JR. Predictors of 30- and 90-day COPD exacerbation readmission: a prospective cohort study. Int J Chron Obstruct Pulmon Dis. 2021;16:2769–2781. doi:10.2147/copd.S328030
12. Alqahtani JS, Njoku CM, Bereznicki B, et al. Risk factors for all-cause hospital readmission following exacerbation of COPD: a systematic review and meta-analysis. Eur Respir Rev. 2020;29:156. doi:10.1183/16000617.0166-2019
13. Njoku CM, Alqahtani JS, Wimmer BC, et al. Risk factors and associated outcomes of hospital readmission in COPD: a systematic review. Respir Med. 2020;173:105988. doi:10.1016/j.rmed.2020.105988
14. Kim VL, Coombs NA, Staples KJ, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J. 2017;50(4):1700853. doi:10.1183/13993003.00853-2017
15. Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi:10.1016/s2213-2600(17)30432-0
16. Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329–339. doi:10.1164/rccm.201803-0405OC
17. David B, Bafadhel M, Koenderman L, De Soyza A. Eosinophilic inflammation in COPD: from an inflammatory marker to a treatable trait. Thorax. 2021;76(2):188–195. doi:10.1136/thoraxjnl-2020-215167
18. MacDonald MI, Osadnik CR, Bulfin L, et al. Low and high blood eosinophil counts as biomarkers in hospitalized acute exacerbations of COPD. Chest. 2019;156(1):92–100. doi:10.1016/j.chest.2019.02.406
19. Jabarkhil A, Moberg M, Janner J, et al. Elevated blood eosinophils in acute COPD exacerbations: better short- and long-term prognosis. Eur Clin Respir J. 2020;7(1):1757274. doi:10.1080/20018525.2020.1757274
20. Vogelmeier CF, Kostikas K, Fang J, et al. Evaluation of exacerbations and blood eosinophils in UK and US COPD populations. Respir Res. 2019;20(1):178. doi:10.1186/s12931-019-1130-y
21. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi:10.1164/rccm.201104-0597OC
22. Dagens A, Sigfrid L, Cai E, et al. Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review. BMJ. 2020;369:m1936. doi:10.1136/bmj.m1936
23. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464–471. doi:10.1164/rccm.201710-2029OC
24. Koreny M, Arbillaga-Etxarri A, de Basea MB, et al. Urban environment and physical activity and capacity in patients with chronic obstructive pulmonary disease. Environ Res. 2022;214:113956. doi:10.1016/j.envres.2022.113956
25. Mir WAY, Siddiqui AH, Paul V, et al. Palliative care and Chronic Obstructive Pulmonary Disease (COPD) readmissions: a narrative review. Cureus. 2021;13(8):e16987. doi:10.7759/cureus.16987
26. Le Rouzic O, Roche N, Cortot AB, et al. Defining the ”Frequent Exacerbator” phenotype in COPD: a hypothesis-free approach. Chest. 2018;153(5):1106–1115. doi:10.1016/j.chest.2017.10.009
27. Hoffmann RF, Jonker MR, Brandenburg SM, et al. Mitochondrial dysfunction increases pro-inflammatory cytokine production and impairs repair and corticosteroid responsiveness in lung epithelium. Sci Rep. 2019;9(1):15047. doi:10.1038/s41598-019-51517-x
28. O’Beirne SL, Shenoy SA, Salit J, et al. Ambient pollution-related reprogramming of the human small airway epithelial transcriptome. Am J Respir Crit Care Med. 2018;198(11):1413–1422. doi:10.1164/rccm.201712-2526OC
29. Brandsma CA, de Vries M, Costa R, Woldhuis RR, Königshoff M, Timens W. Lung ageing and COPD: is there a role for ageing in abnormal tissue repair? Eur Respir Rev. 2017;26(146):170073. doi:10.1183/16000617.0073-2017
30. Karakioulaki M, Papakonstantinou E, Stolz D. Extracellular matrix remodelling in COPD. Eur Respir Rev. 2020;29(158):190124. doi:10.1183/16000617.0124-2019
31. Zhou Y, Zhong NS, Li X, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377(10):923–935. doi:10.1056/NEJMoa1700228
32. Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi:10.1016/s2213-2600(17)30207-2
33. Thomashow B, Stiegler M, Criner GJ, et al. Higher COPD assessment test score associated with greater exacerbations risk: a post hoc analysis of the IMPACT trial. Chronic Obstr Pulm Dis. 2022;9(1):68–79. doi:10.15326/jcopdf.2021.0259
34. Pulido Herrero E, García Gutiérrez S, Antón Ladislao A, et al. Chronic obstructive pulmonary disease assessment test: usefulness for monitoring recovery and predicting poor course of disease after exacerbations. Emergencias. 2019;31(1):21–26.
35. Cookson W, Moffatt M, Rapeport G, Quint J, Pandemic A. Lesson for global lung diseases: exacerbations are preventable. Am J Respir Crit Care Med. 2022;205(11):1271–1280. doi:10.1164/rccm.202110-2389CI
36. Wang MT, Lai JH, Huang YL, et al. Comparative effectiveness and safety of different types of inhaled long-acting β(2)-agonist plus inhaled long-acting muscarinic antagonist vs inhaled long-acting β(2)-agonist plus inhaled corticosteroid fixed-dose combinations in COPD A propensity score-inverse probability of treatment weighting cohort study. Chest. 2021;160(4):1255–1270. doi:10.1016/j.chest.2021.05.025
37. Chen L, Chen S. Prediction of readmission in patients with acute exacerbation of chronic obstructive pulmonary disease within one year after treatment and discharge. BMC Pulm Med. 2021;21(1):320. doi:10.1186/s12890-021-01692-3
38. Celli B, Locantore N, Yates JC, et al. Markers of disease activity in COPD: an 8-year mortality study in the ECLIPSE cohort. Eur Respir J. 2021;57(3). doi:10.1183/13993003.01339-2020
39. Palmiotti GA, Lacedonia D, Liotino V, et al. Adherence to GOLD guidelines in real-life COPD management in the Puglia region of Italy. Int J Chron Obstruct Pulmon Dis. 2018;13:2455–2462. doi:10.2147/copd.S157779
40. Vestbo J, Fabbri L, Papi A, et al. Inhaled corticosteroid containing combinations and mortality in COPD. Eur Respir J. 2018;52(6):1801230. doi:10.1183/13993003.01230-2018
41. Martínez-Gestoso S, García-Sanz MT, Calvo-álvarez U, et al. Variability of blood eosinophil count and prognosis of COPD exacerbations. Ann Med. 2021;53(1):1152–1158. doi:10.1080/07853890.2021.1949489
42. Ferguson GT, Shaikh A, Tetzlaff K, Mueller A, Magnussen H, Watz H. Effect of inhaled corticosteroid withdrawal on chronic obstructive pulmonary disease exacerbations in patients taking triple therapy at baseline. Int J Chron Obstruct Pulmon Dis. 2020;15:2879–2888. doi:10.2147/copd.S237408
43. Dalin DA, Løkke A, Kristiansen P, et al. A systematic review of blood eosinophils and continued treatment with inhaled corticosteroids in patients with COPD. Respir Med. 2022;198:106880. doi:10.1016/j.rmed.2022.106880
44. Echevarria C, Steer J, Bourke SC. Comparison of early warning scores in patients with COPD exacerbation: DECAF and NEWS score. Thorax. 2019;74(10):941–946. doi:10.1136/thoraxjnl-2019-213470
45. Greulich T, Tüffers J, Mager S, et al. High eosinophil blood counts are associated with a shorter length of hospital stay in exacerbated COPD patients - a retrospective analysis. Respir Res. 2020;21(1):106. doi:10.1186/s12931-020-01365-5
46. Ko FWS, Chan KP, Ngai J, et al. Blood eosinophil count as a predictor of hospital length of stay in COPD exacerbations. Respirology. 2020;25(3):259–266. doi:10.1111/resp.13660
47. Choi J, Oh JY, Lee YS, et al. The association between blood eosinophil percent and bacterial infection in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:953–959. doi:10.2147/copd.S197361
48. Kolsum U, Donaldson GC, Singh R, et al. Blood and sputum eosinophils in COPD; relationship with bacterial load. Respir Res. 2017;18(1):88. doi:10.1186/s12931-017-0570-5
49. Sivapalan P, Lapperre TS, Janner J, et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): a multicentre, randomised, controlled, open-label, non-inferiority trial. Lancet Respir Med. 2019;7(8):699–709. doi:10.1016/s2213-2600(19)30176-6
50. Lonergan M, Dicker AJ, Crichton ML, et al. Blood neutrophil counts are associated with exacerbation frequency and mortality in COPD. Respir Res. 2020;21(1):166. doi:10.1186/s12931-020-01436-7
51. Halpin DMG, Criner GJ, Papi A, et al.; Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24–36. doi:10.1164/rccm.202009-3533SO
52. Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi:10.1016/s0140-6736(22)00470-6
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.