Back to Journals » International Journal of General Medicine » Volume 15
Characteristics and Predictors of Long-Time Survivors in Non-Metastatic Gastric Signet Ring Cell Carcinoma: A Large Population-Based Study
Authors Weng Q, Li Z, Xie Y, Guo J , Zhang Y, Ye G
Received 14 December 2021
Accepted for publication 7 March 2022
Published 19 March 2022 Volume 2022:15 Pages 3133—3142
DOI https://doi.org/10.2147/IJGM.S350448
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Qiuyan Weng,1 Zhe Li,2,3 Yaoyao Xie,4 Junming Guo,2– 4 Yong Zhang,5 Guoliang Ye2,3
1Department of Neurology, The Affiliated Hospital of Medical School, Ningbo University, Ningbo, Zhejiang, People’s Republic of China; 2Department of Gastroenterology, The Affiliated Hospital of Medical School, Ningbo University, Ningbo, Zhejiang, People’s Republic of China; 3Institute of Digestive Diseases, Ningbo University, Ningbo, Zhejiang, People’s Republic of China; 4Department of Biochemistry and Molecular Biology and Zhejiang Key Laboratory of Pathophysiology, Ningbo University School of Medicine, Ningbo, Zhejiang, People’s Republic of China; 5Department of Trauma Orthopedics, Ningbo No. 6 Hospital, Ningbo, Zhejiang, People’s Republic of China
Correspondence: Guoliang Ye, Department of Gastroenterology, The Affiliated Hospital of Medical School, Ningbo University, Ningbo, 315020, People’s Republic of China, Tel +86-574-87035171, Fax +86-574-87380487, Email [email protected] Yong Zhang, Department of Trauma Orthopedics, Ningbo No. 6 Hospital, Ningbo, 315000, Zhejiang, People’s Republic of China, Email [email protected]
Objective: Gastric signet ring cell carcinoma (SRCC) is a distinct entity with a relatively poor prognosis. This study analyzed the clinicopathological characteristics of long-time survivors (LTSs) and identified independent predictors of long-term survival (LTS) in non-metastatic gastric SRCC.
Methods: Data from 3906 patients with non-metastatic gastric SRCC were retrieved from Surveillance, Epidemiology and End Results according to the inclusion and exclusion criteria. Patients were randomly divided into training and validation cohorts. Predictors of LTS in the training cohort were identified by multivariate logistic regression. A nomogram-based predictive model for LTS was constructed in non-metastatic gastric SRCC.
Results: There were 800 patients who survived for > 5 years and were defined as TLSs. Young age, other race (not black or white population), female gender, married status, small tumor size, low tumor infiltration, and negative lymph node involvement were independent predictors of LTS in non-metastatic gastric SRCC. These seven variables were incorporated into a nomogram model for predicting LTS. The calibration curve showed good consistency between observed and predicted probability of LTS, and the receiver operating characteristic curve showed acceptable discriminative capacity in the training and validation cohorts.
Conclusion: This study provides an overview of the features of patients with non-metastatic gastric SRCC. Age, race, sex, marital status, tumor size, tumor infiltration, and lymph node involvement were identified as independent predictors of LTS.
Keywords: gastric cancer, signet ring cell carcinoma, long-time survivor, nomogram
Introduction
Gastric cancer is a lethal malignancy that accounts for approximately 8.27% of new cancer cases arising from the digestive system.1 Histopathologically, most gastric tumors are adenocarcinomas.2 Signet ring cell carcinoma (SRCC) is a relatively rare histological type with an extremely poor prognosis due to rapid tumor growth and diffuse infiltration into surrounding tissues;3 it accounts for 16.8% of all gastric cancer cases.4 SRCC is characterized by predominant intracytoplasmic mucin production in tumor cells (>50% size of cell) with the unique appearance of a signet ring.5,6 A population-based analysis indicated that patients with gastric SRCC have significantly worse 5-year and 10-year survival rates than those with gastric adenocarcinoma (5-year survival: 19.2% vs 25.8%; 10-year survival: 16.0% vs 22.1%).4 The baseline demographics and clinical characteristics of gastric SRCC are also distinct from those of common gastric adenocarcinoma, including the mean age of onset, gender ratio, obesity prevalence, tumor grade, and advanced tumor stage.4,7,8
Despite the overall dismal prognosis of gastric SRCC, the long-term survival (LTS) of patients with gastric SRCC has improved in recent decades owing to endoscopic treatment, surgery, and adjuvant and neoadjuvant treatments for early-stage patients, as well as systemic chemotherapy, hyperthermic intraperitoneal chemotherapy, and immunotherapy for metastatic patients.9 However, there are limited data on the optimal management of non-metastatic gastric SRCC possibly because of the rarity of this specific tumor type. Investigating the specific characteristics of patients with gastric SRCC who survive for a long time is thus critical.
In this study, long-term survivors (LTSs) were defined as patients with a cancer-specific survival (CSS) of >5 years.10,11 Early death (ED) was defined as cancer-specific death within 5 years from the diagnosis of non-metastatic gastric SRCC. Eligible patients were identified from the Surveillance, Epidemiology and End Results (SEER) database, and the clinicopathological characteristics of non-LTSs and LTSs were compared. Independent predictors of LTS were identified and used to construct and validate a nomogram model to predict LTS in non-metastatic gastric SRCC.
Materials and Methods
Data Source and Patient Selection
Patients with non-metastatic gastric SRCC were collected from the SEER database between 2004 and 2015 using SEER*State (version 8.3.6). The SEER database includes population-based data from 18 registration centers, which covers approximately 30% of the US population.12 The SEER database includes data on demographics, cancer incidence, tumor-related clinicopathological features, and cancer treatment, thereby providing accessible information for clinical studies of malignant tumors, especially for rare tumor entities.13 Ethical approval was obtained from the Ethics Committee of the Affiliated Hospital of the Medical School of Ningbo University. Written informed consent was not required in this SEER-based retrospective study.
First, 12,846 patients with gastric SRCC were identified from the SEER database between 2004 and 2015 according to the International Classification of Diseases in Oncology (ICD-O-3) (ICD-O-3: 8490). Patients were included if they met the following criteria: (1) patient age ≥18 years; (2) pathologically confirmed gastric SRCC; (3) single primary tumor; (4) active follow-up data; (5) survival time ≥1 month, which might be, otherwise, caused by perioperative complications; (6) clear cause of death; (7) non-metastatic patients with known TNM stage. As a result, 3906 eligible patients were enrolled in the study (Figure 1). Patients were divided into three groups according to age as follows: ≤50 years, 51–70 years, and >70 years.14
 |
Figure 1 Flow chart of patient selection. Abbreviations: SRCC, signet ring cell carcinoma; CSS, cancer-specific survival; LTSs, long-time survivors. |
LTSs were defined as patients with cancer-specific survival (CSS) >5 years. Patients with CSS <5 years were defined as non-LTSs.
For each patient, demographics, tumor features, treatment regimens, and patient survival data were retrieved from the SEER database.
Establishment and Validation of the Nomogram
Patients were randomly divided into the training group (N = 2759) and validation group (N = 1147) at a ratio of 7:3 by setting seed in R software. Independent predictors of LTS were identified by univariate and multivariate logistic regression analyses. Seven independent risk factors (age, race, sex, marital status, tumor size, T stage, and N stage) were used to construct a nomogram model to predict LTS.
The discrimination of the nomogram-based LTS prediction was assessed using a calibration plot in both cohorts. The C-index was also calculated. The predictive accuracy of the nomogram was evaluated using a receiver operating characteristic (ROC) curve and the area under the curve (AUC).
Statistical Analysis
The significance of differences in clinicopathological characteristics between LTSs and non-LTSs was evaluated using the chi square test. Outcomes of interest included overall survival (OS: the time interval from initial diagnosis to all-cause death) and CSS (the duration from initial diagnosis to death caused by gastric cancer). Survival curves were plotted using the Kaplan-Meier method, and survival differences were evaluated using the Log rank test. Potential predictors of LTS were identified by univariate logistic regression analysis, and variables with a P value <0.1 in the univariate analysis were included in the multivariate analysis. Results are expressed as odds ratio (OR) and 95% confidence interval (CI). SPSS statistics version 26.0 software (SPSS Inc, Chicago, United States) and R version 3.6.1 software (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses. A two-sided P value <0.05 indicated statistical significance.
Results
Baseline Characteristics of Patients
In total, 3906 patients with non-metastatic gastric SRCC were included according to the inclusion and exclusion criteria. The baseline characteristics of the patients are shown in Table 1. Specifically, 21.79% of patients were ≤50 years old (N = 851), 46.16% were 51–70 years old (N = 1803), and 32.05% were >70 years old (N = 1252). Most of the patients were white (N = 2642, 67.64%). There were no significant differences between the number of men (N = 2059, 52.71%) and women (N = 1847, 47.29%). Regarding marital status, 58.50% of the patients were married (N = 2285) and 37.15% were unmarried (N = 1451). Most patients showed poorly differentiated tumors (N = 3204, 82.03%). Patients were divided into different groups according to tumor size (Table 1). Regarding T stage, 30.18% (N = 1179), 42.01% (N = 1641), 23.02% (N = 899), and 4.79% (N = 187) of the patients had T1, T2, T3, and T4 tumors, respectively. Over half of the patients had no lymph node involvement (N = 2013, 51.54%), 32.51% had N1 stage (N = 1270), and 15.95% had N2 stage (N = 623). Most patients underwent surgery (N = 2896, 74.14%), 36.82% received radiation (N = 1438), and 57.76% received chemotherapy (N = 2256).
 |
Table 1 Clinicopathological Characteristics of Included Patients |
Differences in Clinicopathological Characteristics Between LTSs and Non-LTSs
The clinicopathological features of LTSs and non-TLSs were compared. Most variables were significantly different between the two groups (Table 2). There was a higher number of young patients (≤50 years) in the LTS group than in the non-LTS group. Regarding race, other race (except black and white) was more common among LTSs. The proportion of women (50.88%) and married patients (66.62%) was higher among LTSs.
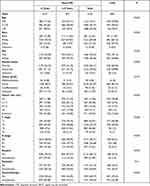 |
Table 2 Differences of Clinicopathological Characteristics Between Non-LTSs and LTSs in Non-metastatic Gastric SRCC |
Predictors of LTS in Non-Metastatic Gastric SRCC
Patients were randomly assigned to training and validation cohorts at a ratio of 7:3. Predictors of LTS were identified using univariate logistic regression analysis. Age, race, sex, marital status, tumor size, T stage, and N stage were associated with LTS (Table 3). These variables were incorporated into the multivariate logistic regression analysis, which showed that younger age, other race (not black or white population), female gender (OR = 1.21, P = 0.028), married status (OR = 1.59, P < 0.001), small tumor size, less advanced T stage, and less advanced N stage were significant independent predictors of LTS (Table 3).
 |
Table 3 Logistic Regression Analysis to Identify Predictors of Long-Time Survival in Non-Metastatic Gastric SRCC in the Training Cohort |
Establishment and Validation of a Nomogram
According to the results of the multivariate logistic regression analysis, age, race, sex, marital status, tumor size, T stage, and N stage were incorporated into a nomogram to predict the probability of LTS in a specific individual. As shown in Figure 2, T stage was the most important predictor of LTS with a maximal score of 100. Other variables had various effects on the probability of LTS.
The nomogram showed good accuracy in predicting LTS in the training cohort, with a C-index of 0.745 (Figure 3A). The calibration plot showed good agreement between predicted and observed LTS (Figure 3A). Similarly, the C-index was 0.720 for the nomogram-based LTS prediction in the validation cohort (Figure 3B). The calibration curve showed good consistency between observed and predicted LTS probability. Finally, a ROC curve was generated to evaluate the predictive power of the nomogram-based prediction model for LTS. The AUC values were 0.745 and 0.720 in the training and validation cohorts, respectively (Figure 4).
 |
Figure 3 Calibration curve of nomogram-predicted and actual probability of long-time survival in the training cohort (A, N = 2759) and the validation cohort (B, N = 1147). |
Age Was an Independent Predictor of LTS in Non-Metastatic Gastric SRCC
Multivariate logistic regression analysis identified age as an independent predictor of LTS. To further examine the association between age and the probability of LTS, we analyzed patient survival stratified by age at diagnosis. As shown in Supplementary Figure 1, patients ≤50 years of age had the best survival, whereas those >70 years had the poorest prognosis.
Discussion
Gastric SRCC is a rare histological type of gastric cancer and is associated with poor survival compared with adenocarcinoma. The incidence of gastric SRCC is increasing, resulting in a considerable health burden to patients.15 Therefore, assessing the clinicopathological features of gastric SRCC is important. In addition, investigating the characteristics of LTSs may provide information essential for improving the clinical management of these patients. However, because of the rarity of gastric SRCC and the small sample size, demographic or survival analyses in a single institution are difficult. Here, we used the SEER database to examine the characteristics of LTSs among patients with non-metastatic gastric SRCC and identified predictors of LTS.
To the best of our knowledge, this is the first large population-based study investigating the characteristics of LTSs in non-metastatic gastric SRCC. Consistent with previous studies,16,17 the incidence of gastric SRCC was higher among women, who accounted for 47.29% of patients in this study. Poorly differentiated or undifferentiated tumors accounted for the majority of tumors (85.18%), which was consistent with previous reports.18 Comparison of the clinicopathological features of LTSs and non-LTSs showed that LTSs included a higher proportion of young patients (≤50 years, 29.88%), other ethnicity (27.75%), female patients (50.88%), married patients (66.62%), those with a relatively smaller tumor size, and less advanced T and N stages (Table 2).
We further examined the independent predictors of LTS. Multivariate logistic regression analysis revealed that younger age, other race (not black or white population), female gender, married status, small tumor size, and less advanced T and N stages significantly predicted LTS. Age was a strong independent predictor of LTS. As shown in Table 3, patients ≤50 years and patients 51–70 years had a two-fold higher probability of LTS than those >70 years of age. The relatively better LTS in the young population was further confirmed by the survival curve (Supplementary Figure 1). Age at diagnosis is an independent predictor in several malignancies, such as colorectal cancer19 and hepatocellular carcinoma.20 Saito et al analyzed 1473 gastric cancer patients who underwent curative gastrectomy and showed that age was an independent prognostic factor, although lymph node dissection was relatively limited and the proportion of patients receiving chemotherapy was lower among elderly patients.21 Consistently, a Japanese team demonstrated that more extensive lymph node dissection and fewer peri-operative complications might explain the better survival in young patients with gastric cancer.22 In addition, married patients were more likely to have LTS than unmarried patients. Spousal support is important for the active treatment and surveillance of patients after a cancer diagnosis,23,24 whereas unmarried patients are more likely to experience undertreatment and lack of social support.25
In addition to the demographic and tumor-specific factors analyzed in this study, several studies have demonstrated that molecular biomarkers are associated with patient prognosis in gastric cancer. Chen et al reported that the GALNT14-rs9679162 genotype was an effective predictor of prognosis in gastric SRCC.26 These authors combined clinicopathological parameters with the GALNT14-rs9679162 genotype to develop a scoring system for stratifying advanced gastric SRCC patients into three distinguishable prognostic subgroups. However, most diagnostic and prognostic biomarkers of gastric SRCC are observed in the laboratory. For instance, hsa-miR-665 and hsa-miR-95 are downregulated in gastric SRCC and may be related to tumor invasion, metastasis, and chemoresistance.27
The present study had several limitations. Firstly, selection bias is unavoidable due to the retrospective nature of the study. Although multivariate logistic regression analysis revealed that age was an independent predictor of LTS in non-metastatic gastric SRCC, the relatively poorer survival of elderly patients might also be associated with inadequate lymph node dissection and less intensive treatment. However, these data cannot be retrieved from the SEER database. Secondly, although the performance of the model was acceptable in both the training and validation cohorts, external validation is warranted to confirm the reliability and generalizability of the nomogram model. A prospective multicenter study with a large sample size is necessary to confirm the present findings. Finally, gastric SRCC with distant metastasis was excluded from the analysis because patients with metastatic disease are mainly treated with palliative systemic therapy and are likely to show a distinct prognostic pattern.28 Thus, another study is necessary to examine this particular subgroup of patients.
In summary, we investigated the clinicopathological characteristics of non-metastatic gastric SRCC and identified predictors of LTS using the SEER database. Young age, other race (not black or white population), female gender, married status, small tumor size, lower tumor infiltration, and negative lymph node involvement were independent predictors of LTS in non-metastatic gastric SRCC. These seven parameters were used to construct a nomogram-based prediction model of LTS, which showed acceptable performance in both training and validation cohorts.
Acknowledgments
The authors are grateful for the efforts of the SEER program in creating the SEER database. This study was supported by the Science and Technology Research on Public Welfare Project of Ningbo, Zhejiang Province (No. 2021S178), Ningbo Nature Fund Project (No.2021J253), Zhejiang Province Public Welfare Technology Application Research Project of China (No. LGF22H160039).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Dicken BJ, Bigam DL, Cass C, et al. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241(1):27–39. doi:10.1097/01.sla.0000149300.28588.23
3. Pernot S, Voron T, Perkins G, et al. Signet-ring cell carcinoma of the stomach: impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21(40):11428–11438. doi:10.3748/wjg.v21.i40.11428
4. Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers (Basel). 2020;12(6):1544.
5. Bu Z, Zheng Z, Li Z, et al. Clinicopathological and prognostic differences between mucinous gastric carcinoma and signet-ring cell carcinoma. Chin J Cancer Res. 2013;25(1):32–38. doi:10.3978/j.issn.1000-9604.2013.01.05
6. Washington MK, Goldberg RM, Chang GJ, et al. Diagnosis of digestive system tumours. Int J Cancer. 2021;148(5):1040–1050. doi:10.1002/ijc.33210
7. Bamboat ZM, Tang LH, Vinuela E, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21(5):1678–1685. doi:10.1245/s10434-013-3466-8
8. Chon HJ, Hyung WJ, Kim C, et al. Differential prognostic implications of gastric signet ring cell carcinoma: stage adjusted analysis from a single high-volume center in Asia. Ann Surg. 2017;265(5):946–953. doi:10.1097/SLA.0000000000001793
9. Li Y, Zhu Z, Ma F, et al. Gastric signet ring cell carcinoma: current management and future challenges. Cancer Manag Res. 2020;12:7973–7981. doi:10.2147/CMAR.S268032
10. Zheng L, Wu C, Xi P, et al. The survival and the long-term trends of patients with gastric cancer in Shanghai, China. BMC Cancer. 2014;14:300. doi:10.1186/1471-2407-14-300
11. Katai H, Ishikawa T, Akazawa K, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer. 2018;21(1):144–154. doi:10.1007/s10120-017-0716-7
12. Hu H, Wang Z, Zhang M, et al. Clinicopathological characteristics and prognosis in endometrial cancer with bone metastasis: a SEER-based study of 584 women. Front Oncol. 2021;11:694718. doi:10.3389/fonc.2021.694718
13. Chen L, Tang K, Wang S, et al. Predictors of lymph node metastasis in Siewert type II T1 adenocarcinoma of the esophagogastric junction: a population-based study. Cancer Control. 2021;28:10732748211026668. doi:10.1177/10732748211026668
14. Lee JG, Kim SA, Eun CS, et al. Impact of age on stage-specific mortality in patients with gastric cancer: a long-term prospective cohort study. PLoS One. 2019;14(8):e0220660. doi:10.1371/journal.pone.0220660
15. Henson DE, Dittus C, Younes M, et al. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128(7):765–770. doi:10.5858/2004-128-765-DTITIA
16. Taghavi S, Jayarajan SN, Davey A, et al. Prognostic significance of signet ring gastric cancer. J Clin Oncol. 2012;30(28):3493–3498. doi:10.1200/JCO.2012.42.6635
17. Shi T, Song X, Liu Q, et al. Survival benefit of palliative gastrectomy followed by chemotherapy in stage IV gastric signet ring cell carcinoma patients: a large population-based study. Cancer Med. 2019;8(13):6010–6020. doi:10.1002/cam4.2521
18. Kwon KJ, Shim KN, Song EM, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014;17(1):43–53. doi:10.1007/s10120-013-0234-1
19. McKay A, Donaleshen J, Helewa RM, et al. Does young age influence the prognosis of colorectal cancer: a population-based analysis. World J Surg Oncol. 2014;12:370. doi:10.1186/1477-7819-12-370
20. Cucchetti A, Sposito C, Pinna AD, et al. Effect of age on survival in patients undergoing resection of hepatocellular carcinoma. Br J Surg. 2016;103(2):e93–9. doi:10.1002/bjs.10056
21. Saito H, Osaki T, Murakami D, et al. Effect of age on prognosis in patients with gastric cancer. ANZ J Surg. 2006;76(6):458–461. doi:10.1111/j.1445-2197.2006.03756.x
22. Fujiwara Y, Fukuda S, Tsujie M, et al. Effects of age on survival and morbidity in gastric cancer patients undergoing gastrectomy. World J Gastrointest Oncol. 2017;9(6):257–262. doi:10.4251/wjgo.v9.i6.257
23. Qiu M, Yang D, Xu R. Impact of marital status on survival of gastric adenocarcinoma patients: results from the Surveillance Epidemiology and End Results (SEER) Database. Sci Rep. 2016;6:21098. doi:10.1038/srep21098
24. Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–3876. doi:10.1200/JCO.2013.49.6489
25. Zhang J, Gan L, Wu Z, et al. The influence of marital status on the stage at diagnosis, treatment, and survival of adult patients with gastric cancer: a population-based study. Oncotarget. 2017;8(14):22385–22405. doi:10.18632/oncotarget.7399
26. Chen TH, Lin WR, Lee C, et al. Prognostic stratification of advanced gastric signet ring cell carcinoma by clinicopathological factors and GALNT14 genotype. J Cancer. 2018;9(19):3540–3547. doi:10.7150/jca.26293
27. Chen J, Sun D, Chu H, et al. Screening of differential microRNA expression in gastric signet ring cell carcinoma and gastric adenocarcinoma and target gene prediction. Oncol Rep. 2015;33(6):2963–2971. doi:10.3892/or.2015.3935
28. Liang X, Zhu J, Li Y, et al. Treatment strategies for metastatic gastric cancer: chemotherapy, palliative surgery or radiotherapy? Future Oncol. 2020;16(5):91–102. doi:10.2217/fon-2019-0495
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


