Back to Journals » Cancer Management and Research » Volume 11
Characteristic And Novel Therapeutic Strategies Of Nasopharyngeal Carcinoma With Synchronous Metastasis
Authors Liao W, Tian M, Chen N
Received 19 June 2019
Accepted for publication 5 September 2019
Published 16 September 2019 Volume 2019:11 Pages 8431—8442
DOI https://doi.org/10.2147/CMAR.S219994
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Rudolph Navari
Wenjun Liao, Maolang Tian, Nianyong Chen
Department of Radiation Oncology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
Correspondence: Nianyong Chen
Department of Radiation Oncology, West China Hospital, Sichuan University, Chengdu 610041, People’s Republic of China
Tel +86 28 8542 3278
Fax +86 28 8542 2952
Email [email protected]
Abstract: Nasopharyngeal carcinoma (NPC) is rare in Western countries, but its incidence in China and Southeast Asia is notably high. NPC shows a high rate of distant metastasis including metachronous metastasis (mmNPC, metastasis after definitive chemo-radiotherapy) and synchronous metastasis (smNPC, metastasis at initial diagnosis). 4–10% of patients would be diagnosed as smNPC annually, and the survival outcomes of these patients are quite poor. As with few clinical trials exclusively focusing on this population, treatment on smNPC is not unified and many problems remain unsolved. To date, systematic chemotherapy (CT) still remains a fundamental treatment in smNPC. Although no randomized trial has been conducted to compare different CT regimens in smNPC, gemcitabine and taxanes in combination with platinum seem optimal in first-line setting. In second-line CT, there is no consensus: mono-chemotherapy with drugs such as gemcitabine, taxanes or capecitabine could be taken into consideration. Immunotherapy based on checkpoint inhibitors shows promising efficacy both in first-line and in the following lines of therapy. In addition to CT, local therapy in smNPC is also very important. Locoregional radiotherapy (RT) for primary tumor in combination with CT could strikingly increase OS with acceptable toxicities. And local treatment, such as surgery and RT, for metastatic lesions could bring extra survival benefit in patients with solitary or limited metastases. Overall, the present study provides an overview of the literature on the various studies of smNPC.
Keywords: nasopharyngeal carcinoma, radiotherapy, chemotherapy, immunotherapy, metachronous
Plain Language Summary
Distant metastasis (DM) is the major causes of death in NPC. At initial diagnosis, 4–10% of patients with NPC are found to have synchronous metastasis (smNPC). Although the smNPC occurs with low incidence, the prognosis of these patients remains dismal. In the past, some studies reported smNPC from different perspectives, however, there are few studies to summarize and analyze these results.
In this review, we firstly examined the metastatic and prognostic characteristics of smNPC, and then we discussed the potential mechanism that might be involved in the early DM of smNPC. Next, we summarized clinical data concerning the efficacy of different treatment approaches on smNPC from two levels (systematic therapy including chemotherapy, targeted therapy and immunotherapy, and local therapy including locoregional radiotherapy for primary tumor and local treatment for metastatic lesions). Based on these, we put forward a treatment mode in the management of this disease. Finally, we provided future directions and strategies in the treatment of smNPC.
Introduction
Nasopharyngeal carcinoma (NPC) is a rare malignancy in the worldwide. However, a distinct geographical variation is evident, with a peak annual incidence approaching 30/100,000 persons in Southern China.1 Over the past years, survival outcomes of patients with locally advanced NPC (LA-NPC) have dramatically improved because of the improvement of radiotherapy (RT) technology and broader application of chemotherapy (CT).2–4
Due to its inherent characteristics, distant metastasis (DM) is more commonly observed in NPC than other head and neck squamous cell carcinoma (HNSCC).5 It is reported that 4–10% of patients with NPC present with synchronous metastasis (smNPC, metastasis at initial diagnosis).6 And 20–30% of patients with LA-NPC would display metachronous metastasis (mmNPC, metastasis after radical chemo-radiotherapy), usually within 3 years.7 With the occurrence of metastasis, the survival outcomes of patients are significantly poor, with a median overall survival (OS) of 12–15 months under treatment with palliative CT.8
While multiple studies have focused on mmNPC, there are few reports on characteristics and management of smNPC. Modalities incorporating systematic CT as well as RT to the primary nasopharynx lesion and regional lymph nodes are recommended by current National Comprehensive Cancer Network (NCCN) guidelines. However, these guidelines only provide a rough direction, and numerous treatment issues remain unaddressed. Herein, we initially examined the clinical characteristics, and discussed possible metastatic mechanism involved in smNPC. Then, we conducted stratified analysis and summary of current clinical studies on smNPC, in order to provide a comprehensive management for this unique cancer. Finally, future directions on this disease are proposed.
The Characteristics Of smNPC
Metastatic Characteristics Of smNPC
Unlike other HNSCC, DM on initial diagnosis is more frequent in NPC (9.1% vs 3%).9 Multivariate analysis showed that advanced T stage, positive N stage, N3 status, and pretreatment EBV DNA levels were all significant risk factors for DM in smNPC.9,10 The most frequently involved sites for metastases are bones (64–67%), liver (32–34%), lungs (15–22%), and distant lymph nodes (12–15%).11,12 In bone metastasis, the frequently common locations are thoracic vertebrae, lumbar vertebrae, sternum, ribs, ilium, and femur.13 Additionally, single organ involved is more common than multiple organs involved (70% vs 30%) in smNPC.12 While more than 70% of patients with smNPC have multiple metastatic lesions for all the involved organs or locations.12,14
Prognostic Characteristics Of smNPC
Patients with smNPC are a heterogeneous group who display a wide range of survival outcomes, which is known to vary relative to different metastatic status. Patients with lung metastasis or limited metastatic lesions are generally thought to have favorable outcomes than those with liver metastasis or multiple metastatic lesions.11,15 Thus, some researchers have thought that current M stage have not truly reflected prognostic characteristics, and further subdivision of M stage should be put forward.15 Wu et al, classified smNPC into three categories: M1a (single lesion located into an isolated organ but not the liver), M1b (single lesion in the liver or multiple lesions in organs except the liver), and M1c (multiple lesions in the liver). Different categories showed significantly diverse prognosis, with median OS for M1a, M1b, and M1c were 46, 25, and 18 months, respectively.16 In the study of Chen et al,12 patients were also divided into three groups: M1a (oligo lesions without liver invaded), M1b (widespread metastases without liver involved), and M1c (liver metastasis without regarding on the number of metastatic lesions). Likely, patients with M1c displayed the worst 3-year OS. In both studies, patients with solitary metastatic lesions except the liver involved had favorable outcomes compared to those with multiple distant lesions. However, once the occurrence of hepatic metastasis with either single or multiple lesions, patient’s outcome decreased markedly.
In addition, even among patients with bone-only metastasis, the survival outcomes differ. Xia et al, reviewed 312 patients with bone-only metastasis, and found that worse OS was associated with metastatic spinal lesions and with >3 metastatic sites.17 In 2019, Mai et al, also examined 226 patients with bone-only metastasis in smNPC. Based on the number of metastatic lesions and EBV DNA status after palliative CT, patients were divided into low-, middle-, and high-risk groups. The 3-year OS of patients in the low-risk was 80%, in the middle 60% and high 40%, separately.18 Table 1 details survival outcomes following prognostic stratification of the metastatic stage.
 |
Table 1 Survival Outcomes Following Prognostic Stratification Of The Metastatic Stage |
Possible Genetic Aberrations In smNPC
At the time of diagnosis, only a minority of patients with NPC present with DM. However, the exact mechanisms that promote or inhibit early metastasis remain unclear. Among patients of colorectal cancer with or without DM at first diagnosis, the fundamental difference between the groups was the aberration of immune-related genes, rather than the tumor-related genes.19 Moreover, the density of immune cells in specimens of patients with or without DM were significant difference: a protective immune signature was more observed in patients without DM. Consequently, the variation in immune status was proved to contribute to early metastasis. NPC is an EB virus-associated malignancy characterized by infiltration of numerous lymphocytes in tissue specimens, which greatly influence the prognosis of patients with NPC.20 In EB virus positive NPC, as high as 28.8% of patients have MHC Class I gene aberrations, and this group of patients consequently was found to have a worse OS than those with MHC Class I wild type.21 These findings indicated that the aberration of MHC genes might have a key role in the process of early DM in NPC, however, which requires further study.
In recent years, some researchers have examined the genomic landscape of NPC specimens using next-generation sequence (NGS). Certain novel metastatic related genes or signal pathways have been identified.21–23 However, these findings have not fully elucidated the mechanism of early DM in smNPC due to a well-matched cohort study including metastatic or non-metastatic NPC at initial diagnosis is not conducted. Future studies should address this issue.
The Landscape Of Systematic Therapy In smNPC
Chemotherapy
First-line CT
Systematic CT is the cornerstone for smNPC. For decades, the most widely used regimens in smNPC are platinum-based doublets.24 It was reported that the complete response (CR) was around 5%, partial response (PR) 50%, stable disease (SD) 32%, and progressive disease (PD) 10% for metastatic sites, respectively.11,25 In spite of numerous of clinical trials on NPC over the past two decades, no randomized studies have compared different CT protocols until 2016: a multicenter, Phase III randomized clinical trial which compared the efficacy of gemcitabine plus cisplatin (GP) and 5-fluorouracil plus cisplatin (PF) in recurrent/metastatic NPC. The results demonstrated that GP was superior to PF, with median progression-free survival (PFS) and OS were 7.0 vs 5.6 months, and 29.1 vs 20.9 months, respectively.26 Although this trial was not specially focus on smNPC population, it should be noticed that 45 (25%) patients in the GP group and 59 (33%) in the PF group were diagnosed as smNPC, providing an evidence that GP might be superior to PF in smNPC.
In addition to 5-fluorouracil or gemcitabine in combination with platinum, taxanes (paclitaxel and docetaxel) combined with platinum have also been frequently employed. One study including 39 patients with smNPC suggested that patients who received a chemotherapeutic protocol including docetaxel achieved a better survival than those without it.27 However, there are few studies that have directly addressed the question concerning whether taxanes-based doublet or triplet combinations, such as TP and TPF (taxanes, platinum, and 5-fluorouracil), are over to GP regimen in smNPC. Future clinical trials are warranted to address this issue.
Actually, it is very difficult to determine which protocol is the best for smNPC, because many studies have focused on mmNPC rather than smNPC, or put smNPC and mmNPC together as metastatic NPC to analyze without distinction. Sometimes, we have to refer to the conclusions that come from the mmNPC, although this might be inappropriate. A retrospective study compared the efficacy of commonly used first-line CT on recurrent/metastatic NPC, which showed that the overall response rate (ORR) of PF, TP, GP, TPF, and BPF (bleomycin, cisplatin and 5-fluorouracil) regimens were 60.2%, 61.7%, 71.1%,74.0%, and 69.1%, respectively.28 Although higher response rates were observed in GP and TPF, it did not translate into long-term survival advantage, with 3-year OS was 23% for GP and TPF, 24% for PF, and TP, 17% for BPF, respectively. However, triplet combination regimens, such as TPF, had more grade 3/4 toxicities compared with cisplatin-based doublets. Based on these, cisplatin-based triplets are not recommended for metastatic NPC at first-line setting.
Cycles Of CT
Although CT is verified as an independent prognostic factor for patients with smNPC, the optimal cycles of CT remain unclear. Some evidences suggested that there was no significant difference between patients who received at least six cycles of CT and those who received fewer than six cycles,29 raising the issue of acquired chemo-resistance in patients with the increased cycles of CT. However, less-intense CT can sometimes be detrimental. Particularly, patients with fewer than four cycles of CT were associated with worse prognosis.11,25 On the basis of these, 4–6 cycles of CT might be considered in smNPC.
Maintenance Chemotherapy
Durable response is rarely encountered in metastatic disease. Hence, maintenance therapy might be considered in smNPC. In a retrospective study, 28 patients with metastatic NPC underwent CT followed by 5-fluorouracil maintenance therapy. The results showed that 35% of patients remained progression-free beyond 1 year, suggesting that a subset of patients might benefit from exposure to a maintenance schedule.30 Even though the evidence was not sufficient, maintenance therapy should be explored for patients with smNPC, particularly with those oral drugs such as capecitabine.
Second-Line CT
In patients with smNPC who develop PD after first-line CT, treatment is challenging. There are few studies to evaluate the efficacy of CT as second-line in smNPC. However, according to the results of second-line CT in mmNPC, mono-chemotherapy such as methotrexate, docetaxel, capecitabine, and gemcitabine could be taken into consideration. The ORR and median PFS of methotrexate, docetaxel, capecitabine, and gemcitabine were 25% and 4.6 months,31 37% and 5.3 months,32 37% and 5 months,33 48% and 5.1months,34 respectively. The grade 3/4 hematological toxicity was around 10%. Double agents, however, did not obviously bring survival benefit. It was showed that the ORR was 37.7% and median PFS was 5.2 months for gemcitabine plus vinorelbine as second-line in the treatment of mmNPC.35 However, 20% of patients had grade 3/4 hematological toxicity.
Targeted Therapy
Epidermal growth factor receptor (EGFR) is highly expressed in NPC and inhibiting EGFR signal pathway has been taken into consideration for a long time.36,37 Unfortunately, there are no clinical trials or retrospective studies on exclusively evaluating the efficacy of EGFR inhibitors in smNPC. However, in view of the data from mmNPC, we could find that the activity of EGFR inhibitors, such as gefitinib and erlotinib, were disappointing as second or third-line therapy in the treatment of metastatic NPC, with no OR.38,39 In addition, a Phase II study tested cetuximab, an anti-EGFR monoclonal antibody, in association with carboplatin in 59 recurrent or metastatic patients: again, the response was low (ORR 11.7%) and the grade 3/4 toxicities were high (51.7%).40
Vascular endothelial growth factor (VEGF) is also important in NPC and it interacted with its receptor VEGF-R to promote tumor survival.41 However, the efficacy of agents targeting VEGF signal pathway to treat metastatic NPC was discouraging, with weaker response and more hemorrhagic events than that found for CT.42,43
PD-1/PD-L1 Inhibitors
Except for traditional therapy as mentioned above in smNPC, immunotherapy targeting immune checkpoints, as a new kind of treatment, has begun to be studied in NPC.
The keynote-028 trial including 27 metastatic NPC who were heavily failure on prior therapy (70.4% patients had received three or more therapies), showed that pembrolizumab (PD-L1 inhibitor) had desirable antitumor activity, with the ORR was 25.9%.44 Similar results also obtained in another multicenter study (NCI-9742) including 44 metastatic NPC who treated with Nivolumab (PD-1 inhibitor), with the ORR was 20.5% and 1-year PFS 19.3%.45 Anti-PD-1/PD-L1 as single therapy had promising activity in metastatic NPC, combined therapy could further improve efficacy.
In 2018, a single arm, Phase I trial evaluated the combination of camrelizumab (PD-1 inhibitor) plus GP on the treatment-naïve metastatic NPC. The results showed that 91% (20/22) of patients had an ORR with a median follow time of 10.2 months, and the toxicity profile was manageable.46 More importantly, the median PFS of patients had already exceeded 12 months in this study, 5 months more than that reported in GP alone.26 In this trial, there were 5 (23%) cases were diagnosed as smNPC as well. It is suggested that combined PD-1 inhibitors with systematic CT could also increase the outcomes of smNPC. Based on these, we are conducting a clinical trial to evaluate the efficacy and safety of a PD-1 inhibitor plus GP in the treatment of smNPC (ChiCTR1900022683).
In conclusion, for patients with smNPC, the first-line systematic therapy is platinum-based doublets, particularly GP and TP. In the second-line treatment of smNPC, the choice depends on the previous treatment. And the interval to recurrence and drug toxicities should also be considered. In patients pretreated with platinum and progression within 6 months, it might be preferable to consider mono-chemotherapy, with relatively desirable antitumor activity and comparable toxicities. On the contrary, in patients pretreated without platinum or patients with the interval to progression beyond 6 months, the re-introduction of platinum-based CT could be considered. In addition, immune checkpoint inhibitors should be strongly encouraged, particularly in combination with CT. As to molecular-targeted therapy, it should not be recommended owing to the results are not convinced.
The Perspective Of Locoregional RT In smNPC
The Value Of Locoregional RT
Although some clinical trials are being conducted to test the efficacy of locoregional RT for primary tumor in smNPC (NCT02111460), increasing evidences have proved the potential value of it. A SEER analysis performed by Lu et al, demonstrated a 50% reduced risk of mortality in patients who received RT for their primary tumor, compared to CT alone.47 In 2017, Karam et al, compared survival outcomes of 718 patients who received CT, with or without locoregional RT through the National Cancer Data Base (NCDB).48 Their results also confirmed that CT in combination with locoregional RT was associated with improved 5-year OS (28% vs 10%). Further, long-term survival >10 years was only observed in the combined cohort. Table 2 presents the studies including locoregional RT in the treatment of patients with smNPC.
 |
Table 2 Studies Including Locoregional RT In The Treatment Of Patients With smNPC |
In order to control for the likelihood that more patients with good performance status were allocated to the RT cohort, sequential landmarks were done for both studies mentioned above. These results suggested that differences in survival were unlikely to be driven by selection bias, and further underscored the importance of RT.48 Additionally, combined CT with RT did not increase the incidence of adverse events, with reported grade 2 to 3 mucositis was 42% and skin reactions 25%.11,25
The survival benefit associated with locoregional RT might result from the reduction of tumor load. On the one hand, the reduction of primary tumor volume could decrease the likelihood of death from severe complications, such as intractable bleeding.47 On the other hand, localized control reduced the odds of disseminated disease through cancer cell “self seeding”.49 High tumor burden was associated with elevated circulating tumor cell (CTC) levels.50 CTCs originate from the primary or metastatic tumors and enter the systemic circulation, potentially inciting subsequent metastases.51 For patients with NPC, the number of CTCs was negatively associated with prognosis.52
The Sequence And Interval Of CT And Locoregional RT In smNPC
The sequence and interval of CT and RT have great effect on prognosis of patients with LA-NPC. It is proved that delayed RT (interval > 30 days between induction CT and RT) is associated with higher risk of DM and death in patients with LA-NPC.53 However, the timing and combination of CT in relation to RT in smNPC are still unclear.54
Karam et al, found that there was no difference in OS between concurrent chemo-radiotherapy or CT followed by RT (sequential RT) in smNPC.48 Whether RT initiation was within 10 days, or even within 120 days of CT initiation, the OS curves were similar. Likewise, Lin et al, investigated the sequence and time of RT initiation, and obtained similar results.55 Therefore, it is suggested that the sequence and interval between CT and locoregional RT have no significant effect on the prognosis of patients with smNPC. Nevertheless, some researchers believe that it is more reasonable that patients received CT alone followed by a presumed interval evaluation of tumor response, and subsequently radical locoregional RT. It is reported that locoregional RT could statistically improve survival in patients with CR or PR compared to that with SD after CT.11 This sequential-based mode could allow us to check the patient’s response and consider both treatment benefits. Locoregional RT might be withheld in patients who are not sensitive to CT.56
The Effect Of New Radiation Techniques On Patients With smNPC
In the previous studies, the majority of patients with smNPC underwent locoregional RT with two-dimensional conventional radiotherapy (2D-CRT). Currently, intensity-modulated radiation therapy (IMRT) is the predominant radiation technique employed in NPC. The clinical benefit of IMRT with respect to local disease control and adverse toxicities is obvious in LA-NPC.57 Some studies have also indicated that compared with 2D-CRT, IMRT provided patients with smNPC with more survival advantage.24 On the one hand, IMRT could increase locoregional control, which might translate into survival benefit in patients with smNPC. On the other hand, relatively low incidence of grade 3/4 acute toxicities was witnessed in IMRT cohort, which might enhance patient’s compliance and treatment intensity.24 Except for conventional IMRT technique, more advanced technologies such as volumetric-modulated arc therapy (VMAT) and Helical TomoTherapy (HT) have been used in NPC in recent years. Compared with conventional IMRT and VMAT, HT appears to have better dosimetric distribution.58,59 Long-term results also showed that HT resulted in excellent disease control (5-year PFS 84.6%) in NPC with mild acute and late toxicity.60
In addition, charged particles, such as proton beam and carbon ion beam have begun to be used in NPC. Owing to the virtue of Bragg peak, particles therapy can better spare normal tissues than photon therapy while maintaining effective delivery of does to the target.61 However, few studies have reported the clinical outcomes of the use of intensity-modulated proton therapy (IMPT) for patients with NPC, and carbon therapy is mainly used as salvage treatment for locally recurrent NPC.61,62
As to patients with smNPC, we should balance RT-related toxicities and disease control when locoregional RT, as a supplement treatment, is administrated. In order to maximize benefits of locoregional RT in smNPC, these advanced radiation techniques characterized by physical or biological superiority provide a direction to explore, particularly in patients with bulky tumor.
Doses And Fractions Of Locoregional RT
To date, the optimal doses for primary tumor in smNPC remain controversial. Mounting evidences have suggested that increased doses of RT are associated with improved OS. One study examined four groups stratified by RT doses: <30, 30–49.9, 50–69.9, and ≥70Gy. A survival advantage was observed when RT dose was ≥50Gy, and when the RT dose was ≥70Gy, there was an additional survival benefit.48 Similar results also indicated that patients’ outcomes were improved when ≥65Gy to the primary tumor, compared to those who received a lower doses.63 Thus, based on existing data, definitive high RT doses for primary tumor are preferable.
IMRT with conventional fraction (1.8-2Gy/F and 5 times a week) is most widely used in NPC, which necessitates an average treatment duration of 6–7 weeks. However, patients with smNPC have relatively short PFS (the median PFS is about 6 months),54 prolonged course of RT, sometimes, might be inappropriate. Hence, hypofractionated RT (hypo-RT) becomes available.
Hypo-RT involves a very accurate delivery of a high dose in a few number of fractions (typically 5 or fewer fractions) to a target with narrow margins.64 In some cases of recurrent head and neck cancer, hypo-RT could be highly effective and features an acceptable toxicity profile.65 In the early 1990s, Ang et al, reported desirable safety and efficacy of using higher doses per fraction (>5Gy) in patients with head and neck cancer.66 Hypofractionation (18Gy/3F) has also been used as a boost treatment for localized recurrent NPC, with an ORR of 90% and only 19% of patients exhibiting severe late toxicities.67
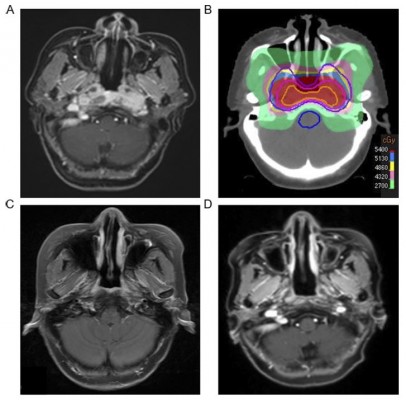
Although no data have been reported about the safety and efficacy of hypo-RT for primary tumor in patients with smNPC, it holds as a new RT technique in the treatment of smNPC. Firstly, compared to conventional fractionated RT, hypo-RT can deliver similar biological equivalent dose (BED) within a shorter period of time. Secondly, hypo-RT does not increase obvious acute toxicities, particularly in combination with systematic CT, and it can be administrated with flexible. On the contrary, long duration of RT might cause the probability of acute RT-related complications, thus affecting the implementation of systematic CT. Thirdly, hypo-RT could reduce the cost and stress of hospital stay for patients with smNPC, because less time is needed. Finally, the main pattern of treatment failure in smNPC is still metastases including the appearance of new lesions and progression of primary metastatic sites, suggesting that the control of metastatic sites is more important than control of primary tumor. In our hospital, two patients with smNPC were received hypo-RT (54-48Gy/9-8F/21d qod) for primary tumor after systematic CT. We did not observe serious acute toxicities (grade ≥ 3) during hypo-RT. And one patient has been followed for more than one year, we did not find any signs of local recurrence and serious late toxicities (Figure 1). Of course, this is just a preliminary attempt, more clinical data are needed.
Local Treatment For Metastatic Sites
The metastatic locations and numbers determine the way of local therapy for metastatic lesions. Generally, aggressive local therapy for patients with solitary or oligometastatic lesions could bring survival benefit. The most frequently used local therapy including surgery, RT, transcatheter hepatic artery chemoembolization (TACE), and radiofrequency ablation (RFA). However, studies on local treatment for DM in smNPC remain scare, most focus on mmNPC.
One study published by Ma et al, which included 105 patients with solitary lung metastasis from NPC who underwent operation or operation plus CT, RT or radio-chemotherapy and CT alone, showed that surgery for metastatic lesions obviously increased local control rate (LCR) and prolonged survival, with the LCR were 96.4% vs 88% vs 53.8%, and median OS 82.4 vs 49.6 vs 29 months, respectively.68 Likewise, Pan et al, showed that patients with limited lung metastases who received RFA had longer metastatic OS than those without it (77.1 vs 32.4months).69
Huang et al, compared the outcome of patients with synchronous or metachronous liver metastases from NPC who underwent partial hepatectomy or TACE. Seventy-six percent (22/30) patients in this study presented with < 3 metastatic lesions and 63%(19/30) patients had the largest metastasis size < 5cm. The results showed that the OS of patients who received partial hepatectomy was significantly superior to those who received TACE, with 1-, 3-, and 5-year OS were 85.7%, 64.2%, and 40.2% vs 53.3%, 26.6%, and 20.0%, respectively.70 In addition, Pan et al, showed that RFA in the treatment of patients with 1–3 hepatic metastases from NPC prolonged survival compared to those who underwent CT alone (median OS 48.1 vs 25.9 months).71
As to bone metastasis, local RT is the main treatment. In 2019, Sun et al, reviewed 226 bone-only metastasis NPC at initial diagnosis, and of these, 68 patients experienced Local RT. The results, unexpectedly, showed that local RT based on systematic CT did not confer survival benefit.18 However, in the study of Shen et al, they demonstrated that patients with solitary bone metastasis who underwent local RT combined CT had longer survival than those who received CT or local RT alone (5-year OS 57.3% vs 11.2%). However, for patients with multiple bone metastasis, this impact was not significant.17 These findings suggested that the value of local RT for bone metastasis is warranted to further study: to validate which proportion of patients would benefit most.
Although most patients underwent 30–60 RT doses with conventional fractions in the previous studies, there is no consensus on the optimal doses and fractions of RT for bone metastasis. The regimens most widely employed are 30Gy/10F, 20Gy/5F, and 8Gy/F.72,73 According to an updated systematic review, the single fraction and multiple fraction RT had similar outcomes and toxicities, with the ORR and CR were 61% vs 62%, and 23% vs 24%, respectively. While re-treatment was significantly more frequent in single fraction arm than multiple fraction arm (20% vs 8%).74 Therefore, some author recommended that multiple fraction RT might be better for bone metastasis from NPC, considering the rate of re-treatment.75
Notably, another problem remains unanswered that the optimal time for managing the metastatic lesions in smNPC. Mai et al, found that the sequence of local treatment for metastasis with systematic CT did not affect OS of patients with mmNPC.76 The same conclusion, however, might not be totally generalized to smNPC. Besides, it remains unclear that whether local therapy is still needed when metastatic lesions reach CR after CT.
Conclusion
Due to the lower proportion of smNPC in all newly diagnosed NPC, it is difficult to conduct large scale, prospective, randomized clinical trials. Almost all clinical data on smNPC in this review come from retrospective studies, thus biases are inevitable existed. Nevertheless, we summarized available evidences and provided a treatment mode for smNPC (Figure 2). In short, 4–6 cycles of GP or TP are preferred for smNPC in first-line setting. In the second-line setting, mono-chemotherapy is recommended and doublets CT with caution. On the basis of systematic CT, RT with high radiation doses (66-70Gy) for primary tumor and regional lymph nodes could further improve OS. In addition, for patients with oligometastatic lesions, local therapy for metastatic sites such as RT or surgery should be recommended. As with multiple metastases, local treatment was only considered to relieve symptoms.
The future directions for smNPC may be thematically summed as follows. Firstly, immunotherapy based on checkpoint inhibitors is a promising strategy. Its efficacy was confirmed both as a first-line and second-line therapy for patients with recurrent or metastatic NPC.45,46 More Phase II and III trials are warranted to assess the efficacy of immunotherapy combined with CT as first-line therapy in the treatment of smNPC. Due to short PFS of patients with smNPC, we should additionally explore the value of immunotherapy as maintenance therapy.
Secondly, we should further explore how to optimize the combination of locoregional RT and systematic CT such as the indications and timing of RT, and which patients would benefit most from locoregional RT.56 Besides, with the arrival of precise RT era, utilizing the short-course, hypo-RT for primary tumor of smNPC might be adopted as a new approach to explore, especially in combination with immunotherapy. There are evidences that besides killing cancer cells, hypo-RT could change immune microenvironment, thus increasing the efficacy of immunotherapy.77
Thirdly, on the basis of different prognostic characteristics of smNPC, we might consider setting up a new metastatic stage system to facilitate individualized, humanized management. For instance, it was showed that systematic CT plus locoregional RT could benefit patients in low and middle risk groups according to the subdivision of metastatic status, whereas it was not found in the high-risk group.18
Finally, smNPC might represent a particular subtype. Fundamental questions regarding molecular mechanisms that promote or inhibit early metastasis remain unclear. Future studies should consider to use bioinformatics or conduct cohort study to determine difference between patients with or without metastasis at initial diagnosis, thus unveiling the metastatic mechanisms.
Overall, well-designed prospective clinical trials are still warranted to determine the best treatment paradigm for patients with smNPC.
Ethics Approval And Informed Consent
Patient’s clinical treatment information in our hospital was approved to be used by the institutional ethics, and all patients were provided written informed consent.
Author Contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30(2):114–119.
2. Sun Y, Li W-F, Chen N-Y, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–1520. doi:10.1016/S1470-2045(16)30410-7
3. Liu GY, Lv X, Wu YS, et al. Effect of induction chemotherapy with cisplatin, fluorouracil, with or without taxane on locoregionally advanced nasopharyngeal carcinoma: a retrospective, propensity score-matched analysis. Cancer Commun (Lond). 2018;38(1):21. doi:10.1186/s40880-018-0283-2
4. Liao J-F, Zhang Q, Du X-J, et al. Concurrent chemoradiotherapy with weekly docetaxel versus cisplatin in the treatment of locoregionally advanced nasopharyngeal carcinoma: a propensity score-matched analysis. Cancer Commun. 2019;39:1. doi:10.1186/s40880-019-0380-x
5. The 150 most important questions in cancer research and clinical oncology series: questions 6–14: edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):33. doi:10.1186/s40880-017-0200-0
6. Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23(2):261–270. doi:10.1016/0360-3016(92)90740-9
7. Li AC, Xiao WW, Shen GZ, et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of IMRT: long-term results and benefits of chemotherapy. Oncotarget. 2015;6(27):24511–24521. doi:10.18632/oncotarget.4312
8. Loong HH, Ma BB, Chan AT. Update on the management and therapeutic monitoring of advanced nasopharyngeal cancer. Hematol Oncol Clin North Am. 2008;22(6):1267–1278. doi:10.1016/j.hoc.2008.08.012
9. Liu JC, Bhayani M, Kuchta K, Galloway T, Fundakowski C. Patterns of distant metastasis in head and neck cancer at presentation: implications for initial evaluation. Oral Oncol. 2019;88:131–136. doi:10.1016/j.oraloncology.2018.11.023
10. Tang LQ, Chen QY, Fan W, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2013;31(23):2861–2869. doi:10.1200/JCO.2012.46.0816
11. Woloschak GE, Zeng L, Tian Y-M, et al. Retrospective analysis of 234 nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis: therapeutic approaches and prognostic factors. PLoS One. 2014;9(9):e108070.
12. Zou X, You R, Liu H, et al. Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur J Cancer. 2017;77:117–126. doi:10.1016/j.ejca.2017.02.029
13. Zhao CL, Qian GQ, Chen XY, Chen C. Retrograde analysis of clinical characteristics of bone metastasis in 1,031 cases of preliminarily diagnosed nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2014;15(8):3785–3788. doi:10.7314/apjcp.2014.15.8.3785
14. Jiang R, You R, Pei XQ, et al. Development of a ten-signature classifier using a support vector machine integrated approach to subdivide the M1 stage into M1a and M1b stages of nasopharyngeal carcinoma with synchronous metastases to better predict patients’ survival. Oncotarget. 2016;7(3):3645–3657. doi:10.18632/oncotarget.6436
15. Pan -C-C, Lu JIN, Yu J-R, et al. Challenges in the modification of the M1 stage of the TNM staging system for nasopharyngeal carcinoma: A study of 1027 cases and review of the literature. Exp Ther Med. 2012;4(2):334–338. doi:10.3892/etm.2012.584
16. Shen L-J, Wang S-Y, Xie G-F, et al. Subdivision of M category for nasopharyngeal carcinoma with synchronous metastasis: time to expand the M categorization system. Chin J Cancer. 2015;34:3. doi:10.1186/s40880-015-0031-9
17. Shen L, Dong J, Li S, et al. M1 stage subdivision and treatment outcome of patients with bone-only metastasis of nasopharyngeal carcinoma. Oncologist. 2015;20(3):291–298. doi:10.1634/theoncologist.2014-0206
18. Sun XS, Liang YJ, Liu SL, et al. Subdivision of nasopharyngeal carcinoma patients with bone-only metastasis at diagnosis for prediction of survival and treatment guidance. Cancer Res Treat. 2019. doi:10.4143/crt.2018.652
19. Mlecnik B, Bindea G, Kirilovsky A, et al. The tumor microenvironment and immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2016;8(327):327ra326. doi:10.1126/scitranslmed.aaf0746
20. Ono T, Azuma K, Kawahara A, et al. Prognostic stratification of patients with nasopharyngeal carcinoma based on tumor immune microenvironment. Head Neck. 2018;40(9):2007–2019. doi:10.1002/hed.25189
21. Li YY, Chung GTY, Lui VWY, et al. Exome and genome sequencing of nasopharynx cancer identifies NF-κB pathway activating mutations. Nat Commun. 2017;8:14121.
22. Lin DC, Meng X, Hazawa M, et al. The genomic landscape of nasopharyngeal carcinoma. Nat Genet. 2014;46(8):866–871. doi:10.1038/ng.3006
23. Zheng H, Dai W, Cheung AKL, et al. Whole-exome sequencing identifies multiple loss-of-function mutations of NF-κB pathway regulators in nasopharyngeal carcinoma. Proc Natl Acad Sci. 2016;113(40):11283–11288. doi:10.1073/pnas.1607606113
24. Hu S-x, He X-h, Dong M, et al. Systemic chemotherapy followed by locoregional definitive intensity-modulated radiation therapy yields prolonged survival in nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis. Med Oncol. 2015;32(9). doi:10.1007/s12032-015-0663-2
25. Tian YH, Zou WH, Xiao WW, et al. Oligometastases in AJCC stage IVc nasopharyngeal carcinoma: A subset with better overall survival. Head Neck. 2016;38(8):1152–1157. doi:10.1002/hed.24345
26. Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388(10054):1883–1892. doi:10.1016/S0140-6736(16)31388-5
27. Shuang H, Feng J, Caineng C, et al. The value of radical radiotherapy in the primary tumor of newly diagnosed oligo-metastatic nasopharyngeal carcinoma patients. Clin Transl Oncol. 2018;21(2):213–219. doi:10.1007/s12094-018-1911-7
28. Jin Y, Cai X-Y, Shi Y-X, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;138(10):1717–1725. doi:10.1007/s00432-012-1219-x
29. Chen M-Y, Jiang R, Guo L, et al. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer. 2013;32(11):604–613. doi:10.5732/cjc.013.10148
30. Leong SS, Wee J, Rajan S, et al. Triplet combination of gemcitabine, paclitaxel, and carboplatin followed by maintenance 5-fluorouracil and folinic acid in patients with metastatic nasopharyngeal carcinoma. Cancer. 2008;113(6):1332–1337. doi:10.1002/cncr.23687
31. Dugan M, Choy D, Ngai A, et al. Multicenter phase II trial of mitoxantrone in patients with advanced nasopharyngeal carcinoma in Southeast Asia: an Asian-Oceanian Clinical Oncology Association Group study. J Clin Oncol. 1993;11(1):70–76. doi:10.1200/JCO.1993.11.1.70
32. Ngeow J, Lim WT, Leong SS, et al. Docetaxel is effective in heavily pretreated patients with disseminated nasopharyngeal carcinoma. Ann Oncol. 2010;22(3):718–722. doi:10.1093/annonc/mdq425
33. Chua D, Wei WI, Sham JST, Au GKH. Capecitabine Monotherapy for Recurrent and Metastatic Nasopharyngeal Cancer. Jpn J Clin Oncol. 2008;38(4):244–249. doi:10.1093/jjco/hyn022
34. Foo KF, Tan EH, Leong SS, et al. Gemcitabine in metastatic nasopharyngeal carcinoma of the undifferentiated type. Ann Oncol. 2002;13(1):150–156. doi:10.1093/annonc/mdf002
35. Chen C, Wang F-h, Wang Z-q, et al. Salvage gemcitabine–vinorelbine chemotherapy in patients with metastatic nasopharyngeal carcinoma pretreated with platinum-based chemotherapy. Oral Oncol. 2012;48(11):1146–1151. doi:10.1016/j.oraloncology.2012.05.021
36. Ma BB, Poon TC, To KF, et al. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma–a prospective study. Head Neck. 2003;25(10):864–872. doi:10.1002/hed.10307
37. Beeram M, Patnaik A, Rowinsky EK. Raf: a strategic target for therapeutic development against cancer. J Clin Oncol. 2005;23(27):6771–6790. doi:10.1200/JCO.2005.08.036
38. Chua DT, Wei WI, Wong MP, Sham JS, Nicholls J, Au GK. Phase II study of gefitinib for the treatment of recurrent and metastatic nasopharyngeal carcinoma. Head Neck. 2008;30(7):863–867. doi:10.1002/hed.20792
39. You B, Le Tourneau C, Chen EX, et al. A Phase II trial of erlotinib as maintenance treatment after gemcitabine plus platinum-based chemotherapy in patients with recurrent and/or metastatic nasopharyngeal carcinoma. Am J Clin Oncol. 2012;35(3):255–260. doi:10.1097/COC.0b013e31820dbdcc
40. Chan AT, Hsu MM, Goh BC, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol. 2005;23(15):3568–3576. doi:10.1200/JCO.2005.02.147
41. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi:10.1038/nature04483
42. Chan SL, Ma BB. Novel systemic therapeutic for nasopharyngeal carcinoma. Expert Opin Ther Targets. 2012;16 Suppl 1:S63–S68. doi:10.1517/14728222.2011.635646
43. Almobarak AA, Jebreel AB, Abu-Zaid A. Molecular targeted therapy in the management of recurrent and metastatic nasopharyngeal carcinoma: a comprehensive literature review. Cureus. 2019;11(3):e4210. doi:10.7759/cureus.3882
44. Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 2017;35(36):4050–4056. doi:10.1200/JCO.2017.73.3675
45. Ma BBY, Lim WT, Goh BC, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the mayo clinic Phase 2 consortium (NCI-9742). J Clin Oncol. 2018;36(14):1412–1418. doi:10.1200/JCO.2017.77.0388
46. Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19(10):1338–1350. doi:10.1016/S1470-2045(18)30495-9
47. Hu J, Kong L, Gao J, Hu W, Guan X, Lu JJ. Use of radiation therapy in metastatic nasopharyngeal cancer improves survival: a SEER analysis. Sci Rep. 2017;7:1.
48. Rusthoven CG, Lanning RM, Jones BL, et al. Metastatic nasopharyngeal carcinoma: patterns of care and survival for patients receiving chemotherapy with and without local radiotherapy. Radiother Oncol. 2017;124(1):139–146. doi:10.1016/j.radonc.2017.03.019
49. Comen E, Norton L, Massague J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8(6):369–377. doi:10.1038/nrclinonc.2011.64
50. Kaifi JT, Kunkel M, Dicker DT, et al. Circulating tumor cell levels are elevated in colorectal cancer patients with high tumor burden in the liver. Cancer Biol Ther. 2015;16(5):690–698. doi:10.1080/15384047.2015.1026508
51. Kim M-Y, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. doi:10.1016/j.cell.2009.11.025
52. Chen Z, Xu L, Xu X, Yuan C. The clinical value of detecting circulating tumour cells in the peripheral blood of nasopharyngeal carcinoma patients. Oncol Lett. 2018;5:6283–6290.
53. Peng L, Liu JQ, Xu C, et al. The prolonged interval between induction chemotherapy and radiotherapy is associated with poor prognosis in patients with nasopharyngeal carcinoma. Radiat Oncol. 2019;14(1):9. doi:10.1186/s13014-019-1213-4
54. Yin Z, Zhang X, Wang Y, Wang P, Yuan Z. The combination of systemic therapy and locoregional radiotherapy prolongs survival in newly diagnosed metastatic nasopharyngeal carcinoma patients. Onco Targets Ther. 2017;10:5677–5683. doi:10.2147/OTT.S150035
55. Verma V, Allen PK, Simone CB
56. Sun XS, Liu LT, Liu SL, et al. Identifying optimal candidates for local treatment of the primary tumor among patients with de novo metastatic nasopharyngeal carcinoma: a retrospective cohort study based on Epstein-Barr virus DNA level and tumor response to palliative chemotherapy. BMC Cancer. 2019;19(1):92. doi:10.1186/s12885-019-5281-5
57. Hsieh JC-H, Du T, Xiao J, Qiu Z, Wu K. The effectiveness of intensity-modulated radiation therapy versus 2D-RT for the treatment of nasopharyngeal carcinoma: a systematic review and meta-analysis. PLoS One. 2019;14(7):e0219611.
58. Lee FK-h, Yip CW-y, Cheung FC-h, Leung AK-c, Chau RM-c, Ngan RK-c. Dosimetric difference amongst 3 techniques: tomoTherapy, sliding-window intensity-modulated radiotherapy (IMRT), and RapidArc radiotherapy in the treatment of late-stage nasopharyngeal carcinoma (NPC). Med Dosim. 2014;39(1):44–49. doi:10.1016/j.meddos.2013.09.004
59. Lee T-F, Fang F-M, Chao P-J, Su TJ, Wang LK, Leung SW. Dosimetric comparisons of helical tomotherapy and step-and-shoot intensity-modulated radiotherapy in nasopharyngeal carcinoma. Radiother Oncol. 2008;89(1):89–96. doi:10.1016/j.radonc.2008.05.010
60. Leung SW, Lee TF. Treatment of nasopharyngeal carcinoma by tomotherapy: five-year experience. Radiat Oncol. 2013;8:107. doi:10.1186/1748-717X-8-107
61. Holliday EB, Frank SJ. Proton therapy for nasopharyngeal carcinoma. Chin Clin Oncol. 2016;5(2):25. doi:10.21037/cco.2016.03.05
62. Hu J, Bao C, Gao J, et al. Salvage treatment using carbon ion radiation in patients with locoregionally recurrent nasopharyngeal carcinoma: initial results. Cancer. 2018;124(11):2427–2437. doi:10.1002/cncr.31318
63. Lin S, Tham IWK, Pan J, Han L, Chen Q, Lu JJ. Combined high-dose radiation therapy and systemic chemotherapy improves survival in patients with newly diagnosed metastatic nasopharyngeal cancer. Am J Clin Oncol. 2012;35(5):474–479. doi:10.1097/COC.0b013e31821a9452
64. Lo SS, Fakiris AJ, Teh BS, et al. Stereotactic body radiation therapy for oligometastases. Expert Rev Anticancer Ther. 2009;9(5):621–635. doi:10.1586/era.09.15
65. Baliga S, Kabarriti R, Ohri N, et al. Stereotactic body radiotherapy for recurrent head and neck cancer: a critical review. Head Neck. 2017;39(3):595–601. doi:10.1002/hed.24633
66. Ang KK, Byers RM, Peters LJ, et al. Regional radiotherapy as adjuvant treatment for head and neck malignant melanoma. Preliminary results. Arch Otolaryngol Head Neck Surg. 1990;116(2):169–172. doi:10.1001/archotol.1990.01870020045012
67. Wu SX, Chua DT, Deng ML, et al. Outcome of fractionated stereotactic radiotherapy for 90 patients with locally persistent and recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2007;69(3):761–769. doi:10.1016/j.ijrobp.2007.03.037
68. Ma J, Wen ZS, Lin P, Wang X, Xie FY. The results and prognosis of different treatment modalities for solitary metastatic lung tumor from nasopharyngeal carcinoma: a retrospective study of 105 cases. Chin J Cancer. 2010;29(9):787–795.
69. Pan C-c, Wu P-h, Yu J-r, et al. Comparative survival analysis in patients with pulmonary metastases from nasopharyngeal carcinoma treated with radiofrequency ablation. Eur J Radiol. 2012;81(4):e473–e477. doi:10.1016/j.ejrad.2011.05.037
70. Huang J, Li Q, Zheng Y, et al. Partial hepatectomy for liver metastases from nasopharyngeal carcinoma: a comparative study and review of the literature. BMC Cancer. 2014;14:818. doi:10.1186/1471-2407-14-818
71. Pan C, Wu P, Yu J, et al. CT-guided radiofrequency ablation prolonged metastatic survival in patients with liver metastases from nasopharyngeal carcinoma. Int J Hyperthermia. 2011;27(6):549–554. doi:10.3109/02656736.2011.593019
72. Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient. Cancer. 2013;119(4):888–896. doi:10.1002/cncr.27616
73. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–1436. doi:10.1200/JCO.2006.09.5281
74. Rich SE, Chow R, Raman S, et al. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol. 2018;126(3):547–557. doi:10.1016/j.radonc.2018.01.003
75. Lu T, Guo Q, Cui X, et al. Prognostic evaluation of nasopharyngeal carcinoma with bone-only metastasis after therapy. Yonsei Med J. 2016;57(4):840–845. doi:10.3349/ymj.2016.57.4.840
76. Liang YJ, Sun XS, Yang ZC, et al. Effect of local treatment for metastasis and its sequence with chemotherapy on prognosis of post-treatment metastatic nasopharyngeal carcinoma patients. Oral Oncol. 2019;92:40–45. doi:10.1016/j.oraloncology.2019.03.015
77. Kobiela J, Spychalski P, Marvaso G, et al. Ablative stereotactic radiotherapy for oligometastatic colorectal cancer: systematic review. Crit Rev Oncol Hematol. 2018;129:91–101. doi:10.1016/j.critrevonc.2018.06.005
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.