Back to Journals » International Journal of General Medicine » Volume 14
Changing Patterns in Clinicopathological Characteristics of Breast Cancer and Prevalence of BRCA Mutations: Analysis in a Rural Area of Southern China
Authors Wang Q, Wu H , Lan Y, Zhang J, Wu J, Zhang Y, Li L, Liu D, Zhang J
Received 12 August 2021
Accepted for publication 18 October 2021
Published 29 October 2021 Volume 2021:14 Pages 7371—7380
DOI https://doi.org/10.2147/IJGM.S333858
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Qiuming Wang,1,* Heming Wu,2,* Yongquan Lan,1 Jinhong Zhang,1 Jingna Wu,1 Yunuo Zhang,1 Liang Li,1 Donghua Liu,1 Jinfeng Zhang1
1Department of Medical Oncology, Meizhou People’s Hospital (Huangtang Hospital), Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Precision Medicine and Clinical Translational Research of Hakka Population, Meizhou People’s Hospital (Huangtang Hospital), Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinfeng Zhang
Department of Medical Oncology, Meizhou People’s Hospital (Huangtang Hospital), Meizhou Academy of Medical Sciences, Huangtang Road, Meijiang District, Meizhou, Guangdong, 514031, People’s Republic of China
Tel/Fax +86 753-220-2723
Email [email protected]
Purpose: Although the burden of breast cancer remains especially high in rural China, data on the clinicopathological characteristics and prevalence of the breast cancer susceptibility gene 1/2 (BRCA1/2) mutations in patients with breast cancer remain limited. We investigated the clinicopathological characteristics, changing patterns, and prevalence of BRCA1/2 mutations in patients with breast cancer.
Patients and Methods: The clinicopathological characteristics of 3712 women with pathologically confirmed primary breast cancer treated at Meizhou People’s Hospital between January 2005 and December 2018 were evaluated. The prevalence of BRCA1/2 mutations in 340 patients with breast cancer diagnosed between January 2017 and September 2018 was also evaluated.
Results: The median age at diagnosis was 49± 10.5 (range, 20– 94) years. Positivity for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) was observed in 59.0%, 52.5%, and 24.9% of patients, respectively. Time trend analysis revealed that an increasing trend was observed for age at diagnosis (p = 0.001), proportion of patients without a reproductive history (p < 0.001), postmenopausal patients (p = 0.001), invasive pathological cancer type (p = 0.008), ER-positive rate (p < 0.001), PR-positive rate (p = 0.008), and HER2-positive rate (p < 0.001). Compared with patients without BRCA1/2 mutations, those with BRCA1/2 mutations were more likely to have a family history of breast or ovarian cancer (p < 0.001) and have triple-negative breast cancer (TNBC) (p < 0.001). Family history of breast or ovarian cancer (odds ratio [OR], 103.58; 95% confidence interval [CI], 20.58– 521.45; p < 0.001) and TNBC subtype (OR, 5.97; 95% CI, 1.16– 30.90; p = 0.033) were independent predictors for BRCA1/2 mutation.
Conclusion: The clinicopathological characteristics of patients with breast cancer in this rural area have changed during the past decade. BRCA1/2 testing should be performed in patients with breast cancer with a family history of breast or ovarian cancer and TNBC.
Keywords: BRCA1/2 mutation, breast neoplasm, molecular subtype, stage
Introduction
Breast cancer is the most common malignancy among women in urban areas of China and second most common malignancy among women in rural areas. In recent years, the incidence of breast cancer has increased in China, and the extent of the increase is greater in the countryside.1,2 The disparities of clinical outcomes, such as presentation, treatment strategies, and survival, between patients with breast cancer in the city and countryside have long been reported.3–9 Due to the socioeconomic disparities, patients with breast cancer in rural areas face more difficulties with access to a high level of cancer care and preventive services.3,6 They are more likely to present with a later disease stage, lower reconstruction rates, and lower overall survival rates than their urban counterparts.7–9 As a developing country, the population living in the countryside makes up more than one-third of the Chinese population. The disparities in China may be even more extreme.
Breast cancer is a heterogenetic disease. Each subtype has a distinct molecular mechanism, presents with unique clinical presentation and prognosis, and responds differently to treatments. Understanding the diversity of gene expression can better predict survival and the treatment efficacy in patients with breast cancer.10 Breast cancer susceptibility gene 1/2 (BRCA1/2) mutations are closely related to the occurrence and development of breast cancer and can also predict the treatment effect of platinum-based chemotherapy and poly-adenosine diphosphate-ribose polymerase inhibitors (PARPis).11–13 Patients with breast cancer with BRCA1/2 mutations display distinctive clinical features: a family history of breast or ovarian cancer, younger age at diagnosis, or triple-negative cancer subtype.14 Most large-scale studies on the clinicopathological characteristics of BRCA-associated breast cancer have been on Western patients, while studies on Asian populations have been small and sporadic.
Limited data are available regarding the clinicopathological characteristics of and prevalence of BRCA1/2 mutations in patients with breast cancer in rural China. Therefore, this study aimed to investigate the clinicopathological characteristics of, changing patterns in, and prevalence of BRCA1/2 mutations in patients with breast cancer admitted to Meizhou People’s Hospital, which is located in the mountainous region of eastern Guangdong Province, southern China. The results are expected to provide a reference for the prevention and control of breast cancer.
Materials and Methods
Data Sources
Patient cohorts in this study are presented in Figure 1. Female patients were included if they were newly diagnosed with primary breast cancer at Meizhou People’s Hospital, Meizhou, China, between January 1, 2005 and December 31, 2018. Patients with unknown stage, occult breast cancer, or primary history of other cancer were excluded. Clinicopathological data, including age at diagnosis; menopausal status; reproductive history; stage; pathological type; estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) statuses; and treatment strategies, were collected from patients’ medical records. Finally, 3712 patients with breast cancer were included. We had not assessed BRCA1/2 mutations until 2017. Due to the high costs of the next-generation sequencing test, not all patients could be tested. Only 340 patients had the test performed between 2017 and 2018.
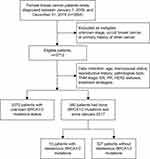 |
Figure 1 Patient cohort selection in this study. |
Molecular Subtypes and Tumor Staging
The ER, PR, and HER2 statuses were assessed by board-certified pathologists in the Department of Pathology at Meizhou People’s Hospital based on immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) findings. ER and PR positivity were defined as ≥ 1% positive tumor cells with nuclear staining. ER and PR negativity was defined as <1% positive tumor cells with nuclear staining. Hormone receptor (HR) positivity was defined as either ER or PR positivity, and HR negativity was defined as both ER and PR negativity. HER2 positivity was defined as an IHC staining score of 3+ or gene amplification detected using FISH. HER2 negativity was defined as an IHC staining score of 0/1+ or a negative FISH result. HER2 IHC (2+) without FISH was categorized as “unknown”. Patients with breast cancer were classified as HR-positive (HR+)/HER2-negative (HER2−), HR+/HER2-positive (HER2+), HR-negative (HR−)/HER2− (triple-negative breast cancer [TNBC]), HR−/HER2+, or “unknown” according to HR and HER2 expression. Staging was categorized based on the American Joint Committee on Cancer (AJCC) tumor–node–metastasis (TNM) staging system during diagnosis.15
BRCA1/2 Mutation Testing
Next-generation sequencing was used to test the peripheral blood samples of 340 patients with breast cancer for BRCA1/2 mutations between January 2017 and September 2018. Two milliliters of peripheral blood samples were collected from each participant. Genomic DNA was extracted from leukocytes using the QIAamp DNA Blood Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. The quality of the DNA was quantified using NanodropTM 2000 spectrophotometer (ThermoFisher Scientific, Waltham, MA), and good quality DNA samples were stored at −80°C until analyzed. A DNA library was constructed using the IonPlus Fragment Library Kit (Life Technologies, Carlsbad, CA), and semiconductor sequencing was performed using an Ion Proton instrument (Life Technologies). High-throughput sequencing and bioinformatics analysis were used to detect the presence of BRCA1 and BRCA2 gene mutations in subjects. In this study, pathogenic mutation, likely pathogenic mutation, mutation with uncertain significance, and benign mutation were named according to the Human Genome Variation Society guidelines using the following reference sequences: BRCA1 (LRG_292t1, NM_007294.3) and BRCA2 (LRG_293t1, NM_000059.3). Deleterious BRCA1/2 mutations were considered pathogenic and likely pathogenic mutations.
Statistical Analyses
A descriptive analysis of clinicopathological data of 3712 female patients with breast cancer was performed. The frequency of each characteristic was calculated for all patients, including those with missing data. Patients were divided into five groups according to their year of diagnosis as follows: 2005–2009, 2010–2012, 2013–2014, 2015–2016, and 2017–2018. Changes in patterns of characteristics between these five periods were analyzed. The time trend of age at diagnosis was evaluated using a one-way analysis of variance. The time trend of other clinicopathological characteristics was analyzed using a linear-by-linear association test. The difference in categorical variables between the BRCA1/2 mutation-positive and -negative groups were compared using chi-squared tests or Fisher’s exact test. Age at diagnosis was compared using a one-way analysis of variance. AJCC stage distribution was compared using the Mann–Whitney U-test. Binary logistic regression was performed to evaluate the relationship between the BRCA1/2 mutation and clinicopathological characteristics. A p-value<0.05 was considered statistically significant. However, a p-value threshold of ≤0.2 was used in the univariate analysis for inclusion of BRCA 1/2 mutation risk factors in the multivariate analysis. Statistical analyses were performed using SPSS (version 25.0; IBM Corp., Armonk, NY, USA).
Ethics Approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Meizhou People’s Hospital (Huangtang Hospital), Meizhou Academy of Medical Sciences. Before BRCA mutation testing, written informed consent was obtained from all participants.
Results
Changing Patterns in Clinicopathological Characteristics
The clinicopathological characteristics of the 3712 patients with breast cancer included in this study are summarized in Table 1. The mean age at diagnosis was 49.9±10.5 (range, 20–94) years. Patients without a reproductive history accounted for 1.1%. The proportion of women who were postmenopausal was 39.5%. The most common pathological type was invasive ductal carcinoma (91.8%), followed by invasive lobular carcinoma (2.1%), mucinous carcinoma (1.6%), ductal carcinoma in situ (DCIS)/lobular carcinoma in situ (LCIS) (1.5%), medullary carcinoma (0.8%), and “others” (2.3%). The most common TNM stage was stage II (48.6%), followed by stage III (27.6%), stage I (17.1%), stage IV (5.2%), and stage 0 (DCIS/LCIS, 1.5%). The proportions of ER+ and PR+ patients were 59.0% and 52.5%, respectively. In total, 923 (24.9%) patients were HER2+. HR+/HER2− breast cancer was the most common molecular subtype (44.1%), followed by HR−/HER2− (15.5%), HR−/HER2+ (13.4%), and HR+/HER2+ (11.5%).
 |
Table 1 Characteristics of Female Patients with Breast Cancer and Changes in Trends Between 2005 and 2018 |
Table 1 also summarizes the time trend of breast cancer during 2005–2018. An increasing trend was observed in age at diagnosis (p=0.001), proportion of patients with no reproductive history (p<0.001), postmenopausal patients (p=0.001), invasive pathological type (p=0.008), ER-positive rate (p<0.001), PR-positive rate (p=0.008), and HER2-positive rate (p<0.001). However, the proportion of AJCC stage (p=0.878) and molecular subtype (p=0.707) did not reach statistical significance (p>0.05). The trends for T stage, N stage, and treatment strategies were also analyzed (Supplementary Table S1). An increasing trend was observed in the proportion of T1 stage cancer, breast-conserving surgery, sentinel lymph node biopsy, neoadjuvant therapy, trastuzumab treatment, adjuvant radiotherapy, and endocrine therapy. The proportion of N stage and chemotherapy did not reach statistical significance (p>0.05).
Prevalence of BRCA1/2 Mutations
Three hundred forty patients with breast cancer were tested for BRCA1/2 mutations. Deleterious BRCA1/2 mutations were found in 3.8% (13/340) of patients. Five patients (5/340, 1.5%) had BRCA1 mutations, and eight (8/340, 2.4%) had BRCA2 mutations. Among those 13 patients, 10 had pathogenic mutations and three had likely pathogenic mutations. Among the 13 BRCA1/2 mutations, the most prevalent mutation type was a frameshift mutation (6/13, 46.2%), followed by nonsense mutation (4/13, 30.8%), missense mutation (2/13, 15.4%), and intron mutation (1/13, 7.7%).
Compared with patients without BRCA1/2 mutation, patients with BRCA1/2 mutation were more likely to have a family history of breast or ovarian cancer (p<0.001) and more likely to have TNBC (p<0.001) (Table 2).According to the univariate logistic regression analysis, family history (p<0.001) and TNBC (p=0.013) were significantly related to BRCA1/2 mutation (Table 3). These factors and HER2 status (p=0.082) were included in the multivariate logistic regression analysis (Table 3). The results showed that family history of breast cancer or ovarian cancer (odds ratio [OR], 103.58; 95% confidence interval [CI], 20.58–521.45; p<0.001) and TNBC subtype (OR, 5.97; 95% CI, 1.16–30.90; p=0.033) were independent predictors for BRCA1/2 mutation (Table 3).
 |
Table 2 Difference in Characteristics Between Patients with Breast Cancer with BRCA1/2 Mutation and without BRCA1/2 Mutation |
 |
Table 3 Multivariate Logistic Regression Analysis of Possible Factors Independently Predicting BRCA1/2 Mutation |
Discussion
In this study, we found that the clinicopathological characteristics of patients with breast cancer in our area had significantly changed during the past decades. The age at diagnosis had increased, and more patients with postmenopausal status were diagnosed. The positive rates of ER, PR, and HER2 had also increased. However, the distribution of TNM stage had not changed, and the proportion of invasive carcinoma was still very high. The deleterious BRCA1/2 mutation rate was 3.8% in our study. A family history of breast or ovarian cancer and TNBC subtype were independently associated with BRCA1/2 mutation in patients with breast cancer.
The number of patients with breast cancer admitted to our hospital has continually increased in recent years. Studies16–19 have shown that many factors can increase the risk of breast cancer, and more Chinese women are at risk in both rural and urban areas.20,21 In this study, the mean age at diagnosis was 49.9±10.5 years, with premenopausal women accounting for 60.5% of the cases. However, we found that age at diagnosis and the proportion of postmenopausal patients had increased over time, in agreement with results of previous studies.1,22 Invasive ductal carcinoma was the most prevalent pathological subtype in this region. Patients who were diagnosed with DCIS/LCIS accounted for <2.0% of the cases. These findings were in agreement with those of another study23 of patients with breast cancer in the neighboring Chaoshan area. However, compared with breast cancer cases in Japan, South Korea, and the urban areas of China, there were more cases of invasive breast cancer and fewer cases of DCIS/LCIS in this region.22,24,25 Furthermore, a greater proportion of patients in this study presented with more advanced breast cancer (stages III and IV, 32.9%), and N0 tumors only accounted for 47.1% of the cases. The prevalence of nodal involvement has remained high in recent years. Although there was a steady increase in the proportion of patients presenting with stage I breast cancer, the proportion of patients presenting with stage III disease remained high, and there was an increase in the proportion of patients presenting with stage IV disease. Moreover, the proportion of patients with carcinoma in situ in this region has not increased in the last 14 years. The women in this rural area do not have access to regular breast cancer screening and have substandard awareness of breast cancer prevention, which may contribute to a more invasive pathological type and advanced presentation.
The ER+ rate (59.0%) was lower in patients with breast cancer in this study than in those from urban areas of China, South Korea, Japan, and the USA.4,14,16,17 This may be related to the greater proportion of premenopausal patients with breast cancer in our study. However, the proportion of patients with ER+ breast cancer in this region has increased in recent years, which is similar to the trend observed in previous studies.4,22,25 Although the exact cause is unclear, studies17,26 suggest that the trend may be related to increased levels of obesity and lower fertility rates. In our study, we found a trend of a decrease in the fertility rate. Meanwhile, a recent study27 also found that there was a steady increase in the mean body mass index and obesity prevalence of women in rural areas in China during 2004–2018. These findings may in some way contribute to the increase in ER-positive rates. We should pay more attention to these risk factors in future studies. The HER2+ rate in this study was 24.9%, which is consistent with that of previous reports in China and other Asian countries22,24,25 but is higher than that reported by studies conducted in the USA.4 These differences in the receptor expression of breast cancer across countries may be attributed to variations in the prevalence of risk factors26 and genetic predisposition.28 In addition, they may be attributed to selection bias because of the study design.
Individuals who harbor germline BRCA1/2 mutations have a predisposition to breast cancer. In this study, the deleterious BRCA1/2 mutation rate was 3.8%. The most prevalent type of the BRCA1/2 mutation in our study was a frameshift mutation, followed by a nonsense mutation, missense mutation, and intron mutation, in line with findings of a previous study on a Chinese population.29 A family history of breast or ovarian cancer and the TNBC subtype were independent predictors for BRCA1/2 mutations. These results are also consistent with those reported in previous studies.11,30,31 However, the large CIs that are present in our study may be related to the limited sample size. Future studies with a larger sample size are needed to better illustrate the predictive value of these risk factors. BRCA1/2 mutations can also predict the treatment effect of platinum-based chemotherapy12 and PARPis, such as olaparib,13 in the treatment of metastatic breast cancer. A recent study32 showed that patients with high-risk early breast cancer and BRCA1/2 mutations could also benefit from 1 year of olaparib treatment after completion of standard adjuvant therapy. However, BRCA1/2 mutation may not be the only indicator of efficacy in olaparib treatment. Studies have shown that homologous recombination deficiency, high tumor-infiltrating lymphocyte counts, or high programmed death-ligand 1 expression are related to olaparib response in addition to BRCA1/2 mutation.33,34 These findings are important for managing TNBC, a heterogeneous subtype with an unfavorable prognosis and no effective treatment. More relevant studies are needed to better identify candidates for PARPis treatment among patients with TNBC.
The single-center retrospective design of our study may have introduced several biases and limitations. First, the number of patients with breast cancer in this area may have been underestimated because patients who had been hospitalized in other hospitals of the region were not included. Second, critical data were missing in the records of patients diagnosed before 2010, such as data on HR and HER2 status. Finally, in the absence of regular follow-ups, survival data and treatment outcomes of these patients could not be presented.
Conclusion
This study showed that the clinicopathological characteristics of patients with breast cancer in this rural area have changed. However, a large number of patients still have locally advanced or metastatic disease and invasive pathology. Family history of breast cancer or ovarian cancer and TNBC subtype were independent predictors for BRCA1/2 mutation. Strategies should be adopted to help people adhere to a healthy lifestyle and to increase public awareness regarding the importance of early breast cancer detection. BRCA1/2 testing should be performed for patients with breast cancer with a family history of breast cancer or ovarian cancer or who have TNBC.
Abbreviations
BRCA1/2, Breast cancer susceptibility gene 1/2; CI, Confidence interval; DCIS, ductal carcinoma in situ; ER, Estrogen receptor; FISH, Fluorescence in situ hybridization; HR, Hormone receptor; LCIS, Lobular carcinoma in situ; OR, Odds ratio; PR, Progesterone receptor; TNBC, Triple-negative breast cancer.
Data Sharing Statement
The datasets used and/or analyzed in the current study will be available from the corresponding author upon reasonable request immediately after article publication and for a period of 5 years.
Ethics Approval and Informed Consent
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Meizhou People’s Hospital (Huangtang Hospital), Meizhou Academy of Medical Sciences (Ethical approval number: 2016-A-29). Before BRCA1/2 mutation testing, written informed consent was obtained from all the patients.
Consent for Publication
The author confirms that its publication has been approved by all co-authors.
Acknowledgments
The authors would like to thank the other colleagues from the Department of Medical Oncology and the Center for Precision Medicine, Meizhou People’s Hospital (Huangtang Hospital) for their helpful comments on the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; drafted or wrote or substantially revised or critically reviewed the article; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agreed to take responsibility and be accountable for the contents of the article.
Funding
This study was supported by the Scientific Research Cultivation Project of Meizhou People’s Hospital [grant number: PY-C2020013, awarded to Dr. Qiuming Wang] and the Medical and Health Research Project of Meizhou City (Guangdong Province, China) [grant number: 2016-B-33, awarded to Dr. Qiuming Wang]. The funding sources had no involvement in the study design, collection, analysis, and interpretation of data, writing of the manuscript, or in the decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li H, Zheng RS, Zhang SW, et al. Incidence and mortality of female breast cancer in China, 2014. Chin J Oncol. 2018;40(3):166–171.
2. Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Chin J Oncol. 2019;41(1):19–28.
3. Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–e289. doi:10.1016/S1470-2045(13)70567-9
4. DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi:10.3322/caac.21583
5. Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003–2005: a population-based study. Int J Cancer. 2015;136(8):1921–1930. doi:10.1002/ijc.29227
6. Newman LA. Breast cancer disparities: socioeconomic factors versus biology. Ann Surg Oncol. 2017;24(10):2869–2875. doi:10.1245/s10434-017-5977-1
7. Williams F, Jeanetta S, James AS. Geographical location and stage of breast cancer diagnosis: a systematic review of the literature. J Health Care Poor Underserved. 2016;27(3):1357–1383. doi:10.1353/hpu.2016.0102
8. Samilia OG, Lava T, Oindrila B, et al. Breast cancer presentation, surgical management and mortality across the rural-urban continuum in the national cancer database. Ann Surg Oncol. 2020;27(6):1805–1815. doi:10.1245/s10434-020-08376-y
9. McCullough LE, Flowers CR. Identifying and addressing disparities in survival outcomes for rural patients with cancer. JAMA Netw Open. 2018;1(4):e181243. doi:10.1001/jamanetworkopen.2018.1243
10. Jubair S, Alkhateeb A, Abou Tabl A, et al. A novel approach to identify subtype-specific network biomarkers of breast cancer survivability. Netw Model Anal Health Inform Bioinform. 2020;9(1):43. doi:10.1007/s13721-020-00249-4
11. Kwong A, Shin VY, Ho JC, et al. Comprehensive spectrum of BRCA1 and BRCA2 deleterious mutations in breast cancer in Asian countries. J Med Genet. 2016;53(1):15–23. doi:10.1136/jmedgenet-2015-103132
12. Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24(5):628–663. doi:10.1038/s41591-018-0009-7
13. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi:10.1056/NEJMoa1706450
14. Kim EK, Park SY, Kim SW. Clinicopathological characteristics of BRCA-associated breast cancer in Asian patients. J Pathol Transl Med. 2020;54(4):265–275. doi:10.4132/jptm.2020.04.07
15. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi:10.3322/caac.21388
16. Chan DSM, Abar L, Cariolou M, et al. World Cancer Research Fund International: continuous update project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30(11):1183–1200.
17. Ellingjord-Dale M, Vos L, Tretli S, et al. Parity, hormones and breast cancer subtypes - results from a large nested case-control study in a national screening program. Breast Cancer Res. 2017;19(1):10. doi:10.1186/s13058-016-0798-x
18. Leso V, Ercolano ML, Cioffi DL, et al. Occupational chemical exposure and breast cancer risk according to hormone receptor status: a systematic review. Cancers (Basel). 2019;11(12):E1882. doi:10.3390/cancers11121882
19. White AJ, DeRoo LA, Weinberg CR, et al. Lifetime alcohol intake, binge drinking behaviors, and breast cancer risk. Am J Epidemiol. 2017;186(5):541–549. doi:10.1093/aje/kwx118
20. Gao Y, Huang Y, Song F, et al. Urban-rural disparity of overweight/obesity distribution and its potential trend with breast cancer among Chinese women. Oncotarget. 2016;7(35):56608–56618. doi:10.18632/oncotarget.10968
21. Wen D, Wen X, Yang Y, et al. Urban rural disparity in female breast cancer incidence rate in China and the increasing trend in parallel with socioeconomic development and urbanization in a rural setting. Thorac Cancer. 2018;9(2):262–272. doi:10.1111/1759-7714.12575
22. Kang SY, Kim YS, Kim Z, et al. Basic findings regarding breast cancer in Korea in 2015: data from a breast cancer registry. J Breast Cancer. 2018;21(1):1–10. doi:10.4048/jbc.2018.21.1.1
23. Lin WZ, Lin YC, Zeng D, et al. Clinical and pathological features of 1920 patients with breast cancer in Chaoshan area. Chin J Cancer Prev Treat. 2009;16(24):1905–1908.
24. Linghu RX, Si W, Li Y. Epidemiological and clinicopathological characteristics of patients with breast cancer: a retrospective analysis of 3 846 case. Acad J PLA Postgrad Med Sch. 2015;36(10):1017–1021.
25. Nakamura K, Okada E, Ukawa S, et al. Characteristics and prognosis of Japanese female breast cancer patients: the BioBank Japan project. J Epidemiol. 2017;27(3S):S58–S64.
26. Argolo DF, Hudis CA, Iyengar NM. The impact of obesity on breast cancer. Curr Oncol Rep. 2018;20(6):47. doi:10.1007/s11912-018-0688-8
27. Wang L, Zhou B, Zhao Z, et al. Body-mass index and obesity in urban and rural China: findings from consecutive nationally representative surveys during 2004–18. Lancet. 2021;398(10294):53–63. doi:10.1016/S0140-6736(21)00798-4
28. Park B, Choi JY, Sung HK, et al. Attribution to heterogeneous risk factors for breast cancer subtypes based on hormone receptor and human epidermal growth factor 2 receptor expression in Korea. Medicine (Baltimore). 2016;95(14):e3063. doi:10.1097/MD.0000000000003063
29. Gao X, Nan X, Liu Y, et al. Comprehensive profiling of BRCA1 and BRCA2 variants in breast and ovarian cancer in Chinese patients. Hum Mutat. 2020;41(3):696–708. doi:10.1002/humu.23965
30. Liu Y, Wang H, Wang X, et al. Prevalence and reclassification of BRCA1 and BRCA2 variants in a large, unselected Chinese Han breast cancer cohort. J Hematol Oncol. 2021;14(1):18. doi:10.1186/s13045-020-01010-0
31. Wong-Brown MW, Meldrum CJ, Carpenter JE, et al. Prevalence of BRCA1 and BRCA2 germline mutations in patients with triple-negative breast cancer. Breast Cancer Res Treat. 2015;150(1):71–80. doi:10.1007/s10549-015-3293-7
32. Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394–2405. doi:10.1056/NEJMoa2105215
33. Schettini F, Corona SP, Giudici F, et al. Clinical, radiometabolic and immunologic effects of olaparib in locally advanced triple negative breast cancer: the OLTRE window of opportunity trial. Front Oncol. 2021;11:686776.
34. Eikesdal HP, Yndestad S, Elzawahry A, et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann Oncol. 2021;32:240–249. doi:10.1016/j.annonc.2020.11.009
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
