Back to Journals » Drug Design, Development and Therapy » Volume 16
Changes of Optical Coherence Tomography Biomarkers in Macular Edema Secondary to Retinal Vein Occlusion After Anti-VEGF and Anti-Inflammatory Therapies
Authors Ding X, Hu Y, Yu H, Li Q
Received 29 November 2021
Accepted for publication 18 February 2022
Published 15 March 2022 Volume 2022:16 Pages 717—725
DOI https://doi.org/10.2147/DDDT.S351683
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Xuefei Ding,1,* Yijun Hu,2,3,* Honghua Yu,2 Qiuming Li1
1Department of Ophtalmology, Henan Eye Hospital, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 2Department of Ophthalmology, Guangdong Eye Institute, Guangdong Provincial People’ s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, People’s Republic of China; 3Refractive Surgery Center, Aier Institute of Refractive Surgery, Guangzhou Aier Eye Hospital, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiuming Li, Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, No. 1, Jianshe Road, Zhengzhou, 450052, People’s Republic of China, Tel +86 15837188476, Email [email protected] Honghua Yu, Department of Ophthalmology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No. 106, Zhongshan Second Road, Guangzhou, 510080, People’s Republic of China, Email [email protected]
Purpose: This study aimed to assess the short-term changes of macular microstructures following anti-VEGF and anti-inflammatory therapies in patients with macular edema secondary to retinal vein occlusion (RVO-ME).
Patients and Methods: In this retrospective study, 70 eyes of 70 patients with RVO-ME were divided into the anti-VEGF (Group A, 35 eyes) and anti-inflammatory (Group B, 35 eyes) treatment groups. All patients underwent best-corrected visual acuity (BCVA) assessment, intraocular pressure (IOP) assessment, slit lamp, fundus fluorescein angiography (FA), scanning laser ophthalmoscopy (SLO), and spectral-domain optical coherence tomography (SD-OCT). Group A received intravitreal injection of 0.05 mL anti-VEGF antibodies (Lucentis® or Aflibercept®) monthly for 3 consecutive months, while Group B received 0.7 mg dexamethasone (Ozurdex®) single intravitreal injection. BCVA and SD-OCT biomarkers were recorded at baseline and 3 months after the first injection. Changes of BCVA and SD-OCT biomarkers following these treatments were compared between the two groups.
Results: BCVA and SD-OCT biomarkers, except choroidal thickness, in both groups were significantly improved after treatment (all P < 0.01). At 3 months, the height of serous retinal detachment (SRD) was markedly lower (P = 0.006), with significantly less hyperreflective dots (HRD, P = 0.037) in Group B compared with Group A. Other SD-OCT biomarkers and BCVA were not significantly different between the two groups (all P > 0.05).
Conclusion: Anti-VEGF and anti-inflammatory therapies are both effective in RVO-ME, with improvement in BCVA and SD-OCT biomarkers. Anti-inflammatory therapy may be more effective than anti-VEGF therapy in SRD and HRD resolution.
Keywords: macular edema, SD-OCT, biomarkers, anti-VEGF therapy, anti-inflammatory therapy
Introduction
Retinal vein occlusion (RVO) is one of the most common visual impairments due to abnormal retinal vessels. The incidence rates of CRVO and BRVO are 0.1–0.3% and 0.4–2.7%, respectively.1–8 Macular edema (ME) is a predominant complication in patients with RVO, which manifests as blurred and deformed vision.9,10 The majority of CRVO patients have macular edema, and 5–15% BRVO patients develop ME. In total, RVO-ME is estimated to affect 1.36–4.47 million individuals in the world.6,11
Multiple therapies are available for RVO-ME. Over the past decade, intravitreal drugs have become the first-line treatment of ME. Anti-VEGF drugs have shown excellent efficacy in treating ME in RVO patients in the BRAVO and CRUISE trials.12,13 Haller et al also demonstrated the great effect of DEX in improving visual acuity and macular anatomy in RVO patients with ME through 12 months of observation.14 However, criteria for choosing anti-VEGF (AVF) or anti-inflammatory (AIM) therapy remain unspecified.
At present, spectral-domain optical coherence tomography (SD-OCT) is the most effective non-invasive examination method for assessing macular edema and enables ophthalmologists to recognize structural biomarkers of the macula. Each microstructure for morphological categorization of the macula can be distinguished by OCT. Moreover, as shown previously, disorganization of retinal inner layers (DRIL), external limiting membrane (ELM) disruption, ellipsoid zone (EZ) disruption, hyperreflective dot (HRD) amounts, and serous retinal detachment (SRD) height are all predictors of BCV after treatment.15–18 Eldeeb et al demonstrated that the change of DRIL extent is firmly correlated with BCVA improvement or decline.19 Additionally, studies have proposed that HRD might be activated microglia, so their number may indicate the inflammatory level in ME patients.20 Evaluating OCT biomarkers after AVF and AIM is important for selecting the optimal treatment for patients with RVO-ME.
In this study, we evaluated SD-OCT-derived microstructural parameters in the macula to compare changes in SD-OCT-related biomarkers of RVO-ME after AVF and AIM.
Patients and Methods
Study Design
This was a retrospective, comparative and randomized study, carried out at the the First Affiliated Hospital of Zhengzhou University. Patients with RVO-ME who received intravitreal injections from November 28, 2018 to May 14, 2021 were included. The study was approved by the institutional review board (ID: 2021-KY-0423) and conducted following the Declaration of Helsinki. Because of anonymization and the retrospective design, the institutional review board waived the need for written informed consent.
Study Population
Seventy eyes of 70 patients with RVO-ME were enrolled in this study. Among these cases, 33 and 37 were classified as non-ischemic and ischemic, respectively. Ischemic was defined as the presence of 5 disc areas of retinal non-perfusion in BRVO, and 10 disc areas of retinal non-perfusion in CRVO.10,21 The study included 35 patients treated with AVF (Lucentis or Eylea) injection and 35 administered AIM (Ozurdex) injection. Treatment-naive ME patients due to RVO with CMT ≥250µm who received 3 AVF injections or 1 AIM injection at baseline, with a minimum follow-up of 4 months (from initial RVO-ME diagnosis) were included. Those with other retinal diseases (diabetic retina disease, myopia and rhegmatogenous retinal detachment), glaucoma, refractive interstitial turbidity (cataract or vitreous opacity), eye tumor, and optic nerve diseases were excluded.
Clinical Study Protocol
Patients were treated with anti-VEGF injections (Group A) or anti-inflammatory injections (Group B) according to EURETINA.10 Group A patients received injections of 0.05 mL AVF antibodies monthly for three consecutive months according to the 3+pro re nata (PRN) schedule. Patients in Group B received a 0.7-mg DEX injection in the first month. All patients had exhaustive ophthalmic examinations, including BCVA, IOP, OCT, SLO, and FFA assessments, before and three months after the first injection.22,23
Image Analysis
OCT images were acquired using 6-mm line (vertical and horizontal) scans centered on the fovea by SD-OCT in high resolution mode (Heidelberg Spectralis OCT, Heidelberg Engineering, Germany). Within a 2 mm radius of the fovea, the discontinuous length of each retinal layer structure (inner layer, ELM and EZ), total areas of the intraretinal cyst (IRC), hyperreflective dot (HRD) number, serous retinal detachment (SRD) height and the presence of fovea bulge were manually measured by the same experienced ophthalmologist twice (Xuefei Ding), and the averages were used for analysis.
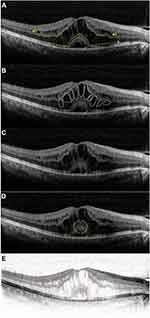
DRIL was defined as a condition in which boundaries between the ganglion cell-inner plexiform layer (GCL-IPL) complex, inner nuclear layer (INL) and outer plexiform layer (OPL) could not be recognized in OCT images24 (Figure 1Aa1 and a2). ELM is a linear conflux of adherens junctions between Muller cells and photoreceptors, which serves as a barrier between the inner retina and the subretinal area (Figure 1Ab). The EZ is displayed as the second hyperreflective band in OCT scans, and represents a biomarker of photoreceptors25 (Figure 1Ac). IRC manifests as a stretched and circular low-reflection area in the neural retina26 (Figure 1B). SRD is displayed as a non-reflective cavity in the subretinal with no shadow of artifact in the B-scan of OCT27 (Figure 1C). Foveal bulge (FB) appears as a bulge at the central in the EZ line28 (Figure 1D). HRDs were defined as particles with 20–40-µm diameters and equal or higher reflectivity than the RPE29 (Figure 1E). All scans were processed with Image J 1.48 (National Institutes of Health, USA).
Statistical Analysis
Statistical analysis was performed with SPSS 26.0 (SPSS. Inc., USA). A normality test was used for all variables. Data were expressed as mean±SD with normal distribution, or as medium (interquartile range) otherwise. Data conforming to normal distribution were compared by a parametric test, while non-normally distributed data were compared with a non-parametric test. Paired t-test and the Wilcoxon signed-rank test were applied to evaluate changes in all biomarkers and BCVA in the same group. Independent samples t-test and the Mann–Whitney test were used to compare baseline data and treatment effects between the two groups. Spearman correlation analysis was performed to assess all parameters, and multiple linear regression was performed for changes of BCVA and eligible biomarkers of the macula, respectively. P<0.05 was considered statistically significant.
Results
Patient Characteristics
In this study, 70 eyes of 70 patients with ME secondary to RVO were included. Considering the different drugs, the patients were divided into Group A (AVF) and Group B (AIM). There were 17 (48.5%) male patients in Group A and 20 (57.1%) in Group B. Mean ages in Groups A and B were 53.69±12.29 years and 51.26±14.21, respectively. Totally 19 (54.2%) cases were branch retinal vein occlusion (BRVO) in Group A versus 18 (51.4%) in Group B. Totally 16 (45.7%) cases belonged to the non-ischemic type in Group A versus 17 (48.5%) in Group B. There were no significant differences (all P>0.05) between the two groups in all patient characteristics. Baseline characteristics of OCT images were shown in Table 1.
 |
Table 1 Baseline Characteristics of the Patients |
BCVA
Mean BCVA values in both groups at baseline and after treatment are listed in Table 2. After injection, BCVA values in Groups A and B were significantly improved (both P<0.001). Compared with pretreatment values, mean BCVA increased from 0.79±0.50 to 0.22 (0.31) in group A, and from 0.93±0.64 to 0.40 (0.60) in Group B. BCVA values after injection had no significant differences between the two groups.
 |
Table 2 Variation of BCVA and Optical Biomarkers After Anti-VEGF or Anti-Inflammatory Treatment |
Biomarkers of SD-OCT Images
All optical biomarkers derived from SD-OCT images are shown in Table 2. The inter-measurement correlation coefficients were all higher than 0.950 (P<0.05). Both groups showed significantly improved CMT, CT, SRD height, total area of IRC, length of disruption structures (DRIL, ELM and EZ), and amounts of HRDs after injection (all P<0.01).
After treatment, CMT, CT, DRIL, ELM disruption, EZ disruption, IRC and FB were not significantly different between the two groups (P=0.162, P=132, P=0.454, P=0.451, P=0.303, P=0.829 and P=0.388; respectively).
However, the number of HRDs after injection was significantly different between group A and group B (P=0. 037<0. 05). Furthermore, the HRDs in group A (48. 43±21. 80) was significantly higher than it in group B (38. 29±17. 98). Similarly, the change of SRD was significantly different between the two groups (P=0. 006), with a mean change in SRD of 112. 00 (238.00) µm for group A and 0. 00 (162.00) µm for group B.
Correlations Between BCVA Improvement After Treatment and Changes of OCT Biomarkers
Spearman correlation analysis was performed to identify the associations of changes of OCT biomarkers after treatment with ΔBCVA. ΔCMT and ΔSRD height were correlated to ΔBCVA (coefficients of 0.32 and 0.38, respectively; both P<0.05). However, ΔHRDs was not significantly correlated with ΔBCVA (coefficient of 0.01, P >0.05).
Furthermore, multivariate linear regression analysis of ΔCMT and ΔSRD height with ΔBCVA in all patients showed that ΔSRD was the most powerful predictor of ΔBCVA (standard β=0. 34, r2=0.12, forward stepwise method).
Correlations of ΔCMT After Treatment and Changes of OCT Biomarkers
Spearman correlation analysis was conducted in all patients to identify the effect of CMT improvement on microstructures in the macula. ΔIRC, ΔSRD, ΔELM and ΔEZ were significantly correlated with ΔCMT (coefficients of 0.49, 0.56, 0.31 and 0.50, respectively; all P<0.05). In addition, multivariate linear regression analysis showed that ΔIRC and ΔSRD were significant predictors of ΔCMT (standard β values of 0.31 and 0.67, respectively; r2=0.53, forward stepwise method).
Discussion
Several studies have investigated the modification of microstructures in ME after intravitreal injection.26,30–35 Meanwhile, very little is known about the differences between anti-VEGF and anti-inflammatory therapies in healing the microstructures of eyes with RVO-ME. This study included 70 cases of RVO-ME and compared the effects of anti-VEGF and anti-inflammatory treatments on microstructural disruption of the macula. The results demonstrated that both anti-VEGF and anti-inflammatory therapies were effective in improving BCVA and macular anatomic outcomes in patients with RVO-ME in the short term. Additionally, anti-inflammatory therapy was more likely than anti-VEGF therapy to perform well in treating SRD and HRDs.
In a literature review, we found that AIM drugs perform better than AVF drugs in treating SRD in DME patients. Ceravolo et al reported that 88% of patients treated with DEX had a complete resolution of SRD. In addition, Demircan et al concluded that SRD height decrease is significantly greater in patients injected DEX than those injected ranibizumab.26,36,37 However, few studies have focused on differences between the AIM and AVF responses of SRD height caused by RVO. We evaluated SRD changes in patients with RVO-ME to explore whether SRD could be a biomarker guiding a proper treatment. Consistent with previous reports, this study demonstrated that DEX treated RVO-ME patients had improved SRD compared with the AVF group (P<0.05). In Group B, the cure rate of DEX for SRD reached 100%, and SRD height in Group B decreased from 0.00 (162.00) µm to 0.00 (0.00) µm. The mechanism of SRD formation is nonetheless still uncertain, although previous studies have proposed several reasons. The liquid balance of the retina is maintained by the blood-retinal barrier (BRB), including internal and external barriers. On the one hand, the internal barrier of the retina is formed by capillary endothelial cells and the associated tight junctions, which could be injured by ischemia. As a result, lipids and proteins leaked from the capillaries accumulate in the subretinal fluid, forming SRD. On the other hand, RPE, the external barrier of the retina, might be impaired by retinal venous disorders. When pro-inflammatory mediators are released in response to ischemia, RPE would be impaired by inflammatory factors, including VEGF.37,38 RPE dysfunction causes choroidal vessel leakage and a reduction of RPE transport function, promoting the formation of SRD. Damage to Müller cells could also lead to increased VEGF.39 Noma et al showed that VEGF and soluble intercellular adhesion molecule-1 (sICAM-1) might contribute to the development of SRD.40 Ozurdex is a slow-release dexamethasone system in the vitreous cavity. Not only do corticosteroids reduce vascular permeability and protect the RPE by inhibiting inflammatory factors, such as VEGF, IL-6 and MCP, they also promote the production of the tight junction protein BRB.41 Therefore, the inflammatory pathway plays an important role in the formation of SRD, which can be regarded as an optic biomarker of anti-inflammatory therapy.
Another note of caution is that HRDs responded better to treatment in group B compared to group A (P=0.037). HRDs in Group B changed from 58.11±19.30 to 38.29±17.98. Consistent with this study, Do et al demonstrated the superiority of DEX in the treatment of HRDs.42 HRDs are defined as particles with 20–40µm diameters and equal or higher reflectivity than the RPE, differing from exudation. The origin of HRDs is controversial. At present, some scholars believe that HRDs in RVO-ME are a manifestation of activated microglial migration under inflammation, as was shown in animal models.43 Others proposed that HRDs are lipid extravasation or degenerated RPE cells.44 HRDs could rapidly disappear after injection, which indicated that they are not related to lipid extravasation. Moreover, in the early stage of the disease, HRDs were observed gathering in the inner layers of the retina. With the prolonged duration of ME, HRDs moved to the external layers of the retina, corresponding to the migration of microglia.35,45 Consequently, we inferred that proinflammation plays a critical role in the conformation of HDRs. Anti-inflammatory has better ability to decrease the number of HRDs than anti-VEGF. However, the effect of reduced HRDs numbers on treatment effect needs further discussion.
We also assessed other microstructures of the macula. In both groups, DRIL, disruption of ELM, and disruption of the EZ were all improved after treatment. Multiple studies have reported that these biomarkers of the macula are associated with prognostic visual acuity. ELM works as a barrier to high-molecular weight polymers, which form the junctional complex between photoreceptors and Müller cells. The EZ reflects the integrity of photoreceptors. In agreement with previous studies, this analysis found positive correlations between ΔCMT and ΔELM and ΔEZ. On one hand, thickened macula stretched ELM, creating physical damage. On the other hand, Jain et al showed ICAM-1 levels, increased in patients with higher macular thickness, are significantly correlated with the disruption of ELM and IS-OS. As mentioned above, we inferred that the disruptions of ELM and EZ might be consistent with CMT. Furthermore, ELM and EZ affect each other in the pathogenesis of ME. Saxena et al revealed that ELM restoration occurs before the EZ, defining ELM as a novel retinal barrier maintaining the water-liquid balance of the retina.17 The EZ consists of bountiful mitochondria, which provide energy to photoreceptors. When ELM and EZ are disrupted, the energy balance and liquid balance would be broken. Consequently, the visual function of patients could decline. We found that both AVF and AIM could decrease CMT, with no significant differences between the two treatments. As CMT decreased, the traction from ME on ELM and EZ was relieved. The disruptions of ELM and EZ would be no longer aggravated and begin the repair process. Therefore, similar to CMT, the recoveries of ELM and EZ disruptions had no significant differences between the two treatment groups. However, the duration and extent of ELM and EZ disruptions play important roles in final visual defect and visual acuity.
DRIL is defined as the condition in which boundaries between the ganglion cell-inner plexiform layer (GCL-IPL) complex, inner nuclear layer (INL) and outer plexiform layer (OPL) could not be recognized. Sun et al proposed that DRIL might reflect a phenomenon in which bipolar axons snap as they pull from the macular edema. When cells located in inner layers are disrupted, the ability of signal transduction decreases, leading to visual function decline.46 Besides the mechanical stresses of the macular edema, foveal avascular zone enlargement and capillary nonperfusion contribute to DRIL.15,47 However, with CMT reduction the traction of ME to the inner layers of the retina was reduced, which relieves DRIL to some extent. The persistence of hypoxia and ischemia in the macula negatively affects the recovery of DRIL. Consequently, unlike ELM and EZ, DRIL was not significantly correlated with CMT.
The present study had some notable limitations, with the first being the retrospective design. Next, patients with ME due to RVO were not submitted to optical coherence tomography angiography (OCTA), and the situation of the perfusion in each layer of the macula could not be assessed, as well as the associations of prognostic BCVA with OCTA parameters. Further studies could perform OCTA in patients with ME to observe the relationships among anatomical damages, perfusion and visual function. Furthermore, the follow-up period was not long enough. The present conclusion just reflected the short-term changes of optical biomarkers. Moreover, RVO is a vascular disease associated with systematic metabolic factors,48,49 so aqueous humor samples, vitreous fluid samples, and serum samples should also be collected and analyzed.
Conclusions
In conclusion, both anti-VEGF and anti-inflammatory could facilitate the restoration of macular edema. SRD and HRDs resolution may be better in eyes treated with anti-inflammatory therapy. Finally, ELM and EZ disruptions are significantly correlated with CMT.
Data Sharing Statement
The datasets used and analyzed are available from the corresponding authors upon reasonable request.
Ethics Approval and Informed Consent
The study was approved by the institutional review board of the First Affiliated Hospital of Zhengzhou University (ID: 2021-KY-0423) and conducted following the Declaration of Helsinki. Because of anonymization and the retrospective design, the institutional review board waived the need for written informed consent.
Consent for Publication
The details of any images, videos, recordings, etc can be published.
Acknowledgments
Xuefei Ding and Yijun Hu are co-first authors for this study.
Author Contributions
XD and YH contributed equally to this work. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Medical Science and Technology Program of Henan, China (2018010003) (QL) and the National Natural Science Foundation of China (81870663 and 82171075) (HY).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313–321. doi:10.1016/j.ophtha.2009.07.017
2. Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124(5):726–732. doi:10.1001/archopht.124.5.726
3. Liu W, Xu L, Jonas JB. Vein occlusion in Chinese subjects. Ophthalmology. 2007;114(9):1795–1796. doi:10.1016/j.ophtha.2007.03.010
4. Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126(4):513–518. doi:10.1001/archopht.126.4.513
5. Arakawa S, Yasuda M, Nagata M, et al. Nine-year incidence and risk factors for retinal vein occlusion in a general Japanese population: the Hisayama Study. Invest Ophthalmol Vis Sci. 2011;52(8):5905–5909. doi:10.1167/iovs.11-7775
6. Laouri M, Chen E, Looman M, Gallagher M. The burden of disease of retinal vein occlusion: review of the literature. Eye. 2011;25(8):981–988. doi:10.1038/eye.2011.92
7. Zhou JQ, Xu L, Wang S, et al. The 10-year incidence and risk factors of retinal vein occlusion: the Beijing eye study. Ophthalmology. 2013;120(4):803–808. doi:10.1016/j.ophtha.2012.09.033
8. Song P, Xu Y, Zha M, Zhang Y, Rudan I. Global epidemiology of retinal vein occlusion: a systematic review and meta-analysis of prevalence, incidence, and risk factors. J Glob Health. 2019;9(1):010427. doi:10.7189/jogh.09.010427
9. Ho M, Liu DT, Lam DS, Jonas JB. Retinal vein occlusions, from basics to the latest treatment. Retina. 2016;36(3):432–448. doi:10.1097/IAE.0000000000000843
10. Schmidt-Erfurth U, Garcia-Arumi J, Gerendas BS, et al. Guidelines for the management of retinal vein occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2019;242(3):123–162. doi:10.1159/000502041
11. Jonas JB, Monés J, Glacet-Bernard A, Coscas G. Retinal vein occlusions. Dev Ophthalmol. 2017;58:139–167.
12. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a Phase III study. Ophthalmology. 2010;117(6):1102–1112.e1. doi:10.1016/j.ophtha.2010.02.021
13. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124–1133.e1. doi:10.1016/j.ophtha.2010.02.022
14. Haller JA, Bandello F, Belfort R
15. Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132(11):1309–1316. doi:10.1001/jamaophthalmol.2014.2350
16. Xie J, Cheng XL, Li XL. Optical coherence tomography in macular edema. Chin J Ocul Fundus Dis. 2004;20(3):152–155.
17. Saxena S, Meyer CH, Akduman L. External limiting membrane and ellipsoid zone structural integrity in diabetic macular edema. Eur J Ophthalmol. 2021;32(1)15–16.
18. Narayanan R, Stewart MW, Chhablani J, et al. Baseline morphological characteristics as predictors of final visual acuity in patients with branch retinal vein occlusions: MARVEL report no 3. Indian J Ophthalmol. 2018;66(9):1291–1294. doi:10.4103/ijo.IJO_342_18
19. Eldeeb M, Chan EW, Sun V, Chen JC. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol. 2018;186:167–168. doi:10.1016/j.ajo.2017.10.037
20. Chung YR, Lee SY, Kim YH, Byeon HE, Lee K. Hyperreflective foci in diabetic macular edema with serous retinal detachment: association with dyslipidemia. Acta Diabetol. 2020;57(7):861–866. doi:10.1007/s00592-020-01495-8
21. Branch Vein Occlusion Study Group. Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. Arch Ophthalmol. 1986;104(1):34–41. doi:10.1001/archopht.1986.01050130044017
22. Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52(1):80–86. doi:10.1167/iovs.10-5285
23. Larsen M, Waldstein SM, Priglinger S, et al. Sustained benefits from ranibizumab for central retinal vein occlusion with macular edema: 24-month results of the CRYSTAL study. Ophthalmol Retina. 2018;2(2):134–142. doi:10.1016/j.oret.2017.05.016
24. Sun JK, Radwan SH, Soliman AZ, et al. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes. 2015;64(7):2560–2570. doi:10.2337/db14-0782
25. Saxena S, Akduman L, Meyer CH. External limiting membrane: retinal structural barrier in diabetic macular edema. Int J Retina Vitreous. 2021;7(1):16. doi:10.1186/s40942-021-00284-x
26. Suciu CI, Suciu VI, Nicoara SD. Optical Coherence Tomography (Angiography) Biomarkers in the Assessment and Monitoring of Diabetic Macular Edema. J Diabetes Res. 2020;2020:6655021. doi:10.1155/2020/6655021
27. Turgut B, Gul FC, Demir T, Celiker U. The causes of serous macular detachment excluding central serous chorioretinopathy. Clinical Optometry. 2010;2:51–54. doi:10.2147/OPTO.S10344
28. Fujihara-Mino A, Mitamura Y, Inomoto N, et al. Optical coherence tomography parameters predictive of visual outcome after anti-VEGF therapy for retinal vein occlusion. Clin Ophthalmol. 2016;10:1305–1313. doi:10.2147/OPTH.S110793
29. Mo B, Zhou HY, Jiao X, Zhang F. Evaluation of hyperreflective foci as a prognostic factor of visual outcome in retinal vein occlusion. Int J Ophthalmol. 2017;10(4):605–612. doi:10.18240/ijo.2017.04.17
30. Lotery A, Clemens A, Tuli R, et al. Effectiveness and safety of ranibizumab in patients with central retinal vein occlusion: results from the real-world, global, LUMINOUS study. Eye. 2021. doi:10.1038/s41433-021-01702-y
31. Muftuoglu IK, Tokuc EO, Sümer F, Karabas VL. Evaluation of retinal inflammatory biomarkers after intravitreal steroid implant and Ranibizumab injection in diabetic macular edema. Eur J Ophthalmol. 2021;112067212110294. doi:10.1177/11206721211029465
32. Zur D, Iglicki M, Busch C, et al. OCT biomarkers as functional outcome predictors in diabetic macular edema treated with dexamethasone implant. Ophthalmology. 2018;125(2):267–275. doi:10.1016/j.ophtha.2017.08.031
33. Ceravolo I, Oliverio GW, Alibrandi A, Bhatti A, Trombetta CJ. The application of structural retinal biomarkers to evaluate the effect of intravitreal ranibizumab and dexamethasone intravitreal implant on treatment of diabetic macular edema. Diagnostics. 2020;10(6):413. doi:10.3390/diagnostics10060413
34. Do JR, Park SJ, Shin JP, Park DH. Assessment of hyperreflective foci after bevacizumab or dexamethasone treatment according to duration of macular edema in patients with branch retinal vein occlusion. Retina. 2021;41(2):355–365. doi:10.1097/IAE.0000000000002826
35. Bayat AH, Akpolat Ç, Livan H, Bölükbaşı S, Elçioğlu MN. Comparison of the effects of aflibercept and dexamethasone in central retinal vein occlusion with serous retinal detachment. Clin Exp Optom. 2021;1–6. doi:10.1080/08164622.2021.1927676
36. Demircan A, Ozkaya A, Alkin Z, Kemer B, Yesilkaya C, Demir G. Comparison of the effect of ranibizumab and dexamethasone implant on serous retinal detachment in diabetic macular edema. J Fr Ophtalmol. 2018;41(8):733–738. doi:10.1016/j.jfo.2018.03.004
37. Celık E, Doğan E, Turkoglu EB, Çakır B, Alagoz G. Serous retinal detachment in patients with macular edema secondary to branch retinal vein occlusion. Arq Bras Oftalmol. 2016;79(1):9–11. doi:10.5935/0004-2749.20160004
38. Küçük B, Sirakaya E, Karaca C. Comparison of ranibizumab versus aflibercept in treating macular edema among patients with serous retinal detachment secondary to branch retinal vein occlusion. Ocul Immunol Inflamm. 2021;29(2):403–410. doi:10.1080/09273948.2019.1681474
39. Eichler W, Yafai Y, Keller T, Wiedemann P, Reichenbach A. PEDF derived from glial Müller cells: a possible regulator of retinal angiogenesis. Exp Cell Res. 2004;299(1):68–78. doi:10.1016/j.yexcr.2004.05.020
40. Noma H, Funatsu H, Mimura T, Tatsugawa M, Shimada K, Eguchi S. Vitreous inflammatory factors and serous macular detachment in branch retinal vein occlusion. Retina. 2012;32(1):86–91. doi:10.1097/IAE.0b013e31821801de
41. Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005;80(2):249–258. doi:10.1016/j.exer.2004.09.013
42. Do JR, Park SJ, Shin JP, Park DH. Assessment of hyperreflective foci after bevacizumab or dexamethasone treatment according to duration of macular edema in patients with branch retinal vein occlusion. Retina. 2021;41(2):355-365. doi:10.1097/IAE.0000000000002826
43. Ebneter A, Kokona D, Schneider N, Zinkernagel MS. Microglia activation and recruitment of circulating macrophages during ischemic experimental branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2017;58(2):944–953. doi:10.1167/iovs.16-20474
44. Fonollosa A, Zarranz-Ventura J, Valverde A, Becerra E, Zapata MA. Predictive capacity of baseline hyperreflective dots on the intravitreal dexamethasone implant (Ozurdex®) outcomes in diabetic macular edema: a multicenter study. Graefes Arch Clin Exp Ophthalmol. 2019;257(11):2381–2390. doi:10.1007/s00417-019-04446-4
45. Vujosevic S, Bini S, Midena G, Berton M, Pilotto E, Midena E. Hyperreflective intraretinal spots in diabetics without and with nonproliferative diabetic retinopathy: an in vivo study using spectral domain OCT. J Diabetes Res. 2013;2013:1–5. doi:10.1155/2013/491835
46. Sun JK, Radwan SH, Soliman AZ, et al. Neural Retinal Disorganization as a Robust Marker of Visual Acuity in Current and Resolved Diabetic Macular Edema. Diabetes. 2015;64(7):2560-2570. doi:10.2337/db14-0782
47. Berry D, Thomas AS, Fekrat S, Grewal DS. Association of disorganization of retinal inner layers with ischemic index and visual acuity in central retinal vein occlusion. Ophthalmol Retina. 2018;2(11):1125–1132. doi:10.1016/j.oret.2018.04.019
48. Hu Y, Atik A, Yu H, et al. Serum heparanase concentration and heparanase activity in patients with retinal vein occlusion. Acta Ophthalmol. 2017;95(1):e62–66. doi:10.1111/aos.13170
49. Hu Y, Yu Y, Bu Z, et al. Increased systemic heparanase in retinal vein occlusion is associated with activation of inflammation and thrombophilia. Retina. 2020;40(2):345–349. doi:10.1097/IAE.0000000000002374
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.