Back to Journals » Risk Management and Healthcare Policy » Volume 16
Changes in the Incidence of Immune Thrombocytopenia in the Coronavirus Disease 2019 Era: A Nationwide Observational Study in Korea
Authors Choi YB, Jung HJ , Kim HR, Jeong SI
Received 19 January 2023
Accepted for publication 5 April 2023
Published 10 April 2023 Volume 2023:16 Pages 667—676
DOI https://doi.org/10.2147/RMHP.S403196
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Young Bae Choi,1,* Hyun Joo Jung,1,* Hae-Rim Kim,2 Soo In Jeong1
1Department of Pediatrics, Ajou University School of Medicine, Ajou University Hospital, Suwon, Korea; 2College of Natural Science, School of Statistics, University of Seoul, Seoul, Korea
*These authors contributed equally to this work
Correspondence: Soo In Jeong, Department of Pediatrics, Ajou University School of Medicine, Ajou University Hospital, 164, World Cup-Ro, Yeongtong-Gu, Suwon, 16499, Korea, Tel +82-31-219-5160, Fax +82-31-219-5169, Email [email protected]
Purpose: We investigated whether nonpharmaceutical interventions (NPI) to reduce the spread of coronavirus disease 2019 (COVID-19) was associated with a change in the incidence of immune thrombocytopenia (ITP).
Patients and Methods: Using the Korean Health Insurance Review and Assessment Services (HIRA) database, individuals newly diagnosed with ITP between January 2015 and December 2020 were identified. The NPI period was defined as February 2020 to December 2020. The ITP incidence in the NPI period was compared with the mean annual incidence during the same months in the pre-NPI period and the incidence predicted by the autoregressive integrated moving average model.
Results: In total, 25,723 patients were identified, and the overall annual incidence of ITP was 8.28 per 100,000 persons ([95% confidence interval (CI): 8.18– 8.39]. The ITP incidence in the NPI period was 6.60 per 100,000 person-years (95% CI: 6.37– 6.85), 0.77 times (95% CI: 0.74– 0.80) lower than that during the pre-NPI period [8.62/100,000 (95% CI: 8.50− 8.74)]. With the exception for patients aged ≥ 70 years, the ITP incidence was significantly lower in the NPI period than in the pre-NPI period. The most significant decline in the ITP incidence during the NPI period was observed in the 0– 9 years age group [25.76/100,000 vs 14.01/100,000, P < 0.001; incidence rate ratio (IRR): 0.54 (95% CI: 0.51– 0.58)]. The intravenous immunoglobulin-treated ITP incidence in the NPI period was 1.69/100,000 (95% CI: 1.58– 1.81), 0.79 times (95% CI: 0.73– 0.85) lower than that in the pre-NPI period 2.15/100,000 (95% CI: 2.09− 2.21)]. The incidence of steroid-treated ITP was lower in the NPI period than in the pre-NPI period (2.73/100,000 vs 2.2/100,000, P < 0.001), with an IRR of 0.80 (95% CI: 0.76– 0.83).
Conclusion: This nationwide study revealed a significant decrease in ITP incidence, particularly among children, after the implementation of NPI.
Keywords: purpura, thrombocytopenic, idiopathic, COVID-19, incidence, pandemic
Introduction
Immune thrombocytopenia (ITP) is an acquired autoimmune disease characterized by a low platelet count (<100,000/uL) caused by the destruction of platelets through an “antibody-mediated” process and impaired megakaryopoiesis, with a peak incidence in early childhood and after 75 years of age.1,2 Although the pathogenesis of ITP is not fully understood, it is considered to be a syndrome with multiple pathways leading to thrombocytopenia. While the primary trigger is unknown, infections can initiate an antiplatelet immune response that is initially caused by molecular mimicry or bystander stimulation and will later proceed despite infection resolution.3 ITP can occur as a primary disease or secondary to other conditions such as infections, malignancy, autoimmune diseases such as systemic lupus erythematosus, primary immunodeficiency, and drugs.1,4,5 In pediatric patients, upper respiratory infection often precedes ITP by 1–4 weeks in 50–65% of cases.6 Studies have reported an association between respiratory viral infections or viral gastroenteritis and childhood ITP.6–9
Since the start of the coronavirus disease 2019 (COVID-19) pandemic, many countries have implemented nonpharmaceutical interventions (NPI) to prevent the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the community. These NPI have significantly reduced the incidence of common respiratory viral infections such as influenza and respiratory syncytial virus infection.10–12 Following the implementation of NPI in Korea, a significant decrease in the diagnosis of infectious diseases, such as influenza, has been observed during periods of enforced social distancing.13–15 Given this context, the incidence of ITP in Korea may have decreased because of a possible decline in the transmission of infectious diseases after the implementation of NPI.
The main objective of this study was to investigate the potential association between the implementation of NPI to prevent the spread of COVID-19 and the incidence of ITP in different age groups in Korea. To achieve this goal, we analyzed data from the Korean Health Insurance Review and Assessment (HIRA) service databases and estimated the incidence of ITP before and after the implementation of NPI. By comparing the incidence for different age groups, we aimed to assess the impact of NPI on ITP incidence in Korea.
Materials and Methods
The Korean HIRA Databases
In this population-based retrospective study, we investigated ITP cases using HIRA data. The HIRA database includes claims for 50 million patients per year; these account for approximately 98% of the Korean population, as of 2014. It includes the beneficiary’s information, including age, sex, and address, as well as data regarding the healthcare services that were provided, such as diagnosis, procedures, surgical history, and prescription drugs.16 The HIRA databases are significant sources of real-world evidence (RWE) and are open to all investigators for academic purposes.
The institutional review board of Ajou University Hospital approved this study. The requirement for informed consent from each patient was waived because the HIRA provided secondary data that were entirely de-identified and anonymized before the authors gained access to the data. (IRB number: AJIRB-MED-KSP-21-309; HIRA Research Data: M2021052287).
Study Population
Diagnoses were coded according to the 7th Korean Classification of Diseases (KCD-7), a modified version of the International Classification of Disease-10 (ICD-10). The data of patients whose diagnosis during their first visit, between January 2015 and December 2020, was associated with ITP (KCD-7 codes D693) as the primary or related disease were extracted from the HIRA. For each year, we related the number of cases to the respective age groups to estimate the incidence per 100,000 patient-years for the corresponding months of 2015–2019 versus 2020. The NPI period was defined as time period from February 2020 to December 2020.
Statistical Analysis
The annual incidence was estimated as the number of patients who developed ITP divided by the relevant population during the relevant period. Data on the total South Korean population for the relevant year were estimated from the resident registration population available from the Korean Statistical Information Services (https://kosis.kr).
Descriptive analyses were used to compare differences between sex and age groups in the incidence of ITP. A 95% confidence interval (CI) for the incidence rate ratio (IRR) and differences in the ITP by sex and age groups were estimated. Trend-p values for the incidence of ITP, intravenous immunoglobulin (IVIG)-treated ITP, and steroid-treated ITP by year were estimated using the Cochran–Armitage test.
The ITP incidence in the NPI period was compared with the annual mean incidence during the same month in the pre-NPI period and the incidence predicted by the autoregressive integrated moving average (ARIMA) model with a two-sample test for equality of proportions. Statistical significance was defined as a P value of <0.05 for all analyses.
Statistical analyses were performed using R version 4.1.1 (R Project for Statistical Computing) and the SAS survey procedure (Version 9.4; SAS Institute, Cary, NC, USA).
Results
Overall ITP Incidence
A total of 25,723 patients, ie, 14,548 female (56.6%) and 11,175 male (43.4%) patients, who were newly diagnosed with ITP between January 2015 and December 2020 were identified in the database (Table 1). The overall annual incidence of ITP was 8.28 per 100,000 individuals (95% CI: 8.18–8.39). Female patients had a higher incidence than male patients [9.36/100,000 individuals (95% CI: 9.20–9.51) vs 7.21/100,000 individuals (95% CI: 7.08–7.34, P <0.001), with a female: male IRR of 1.29 (95% CI: 1.27–1.34). A peak incidence of 24.23/100,000 individuals [95% CI: 23.63–24.83] was observed in children aged 0–9 years, and the incidence was 6.82/100,000 individuals [95% CI: 6.73–6.92] in patients aged ≥10 years. The incidence was 3.55 times [95% CI: 3.45–3.65] higher in the 0–9 years age group than in the ≥10 years age group. Among the total number of patients, 6461 (25.1%) were treated with IVIG, and the incidence of IVIG-treated ITP was 2.08/100,000 person-years (95% CI: 2.03–2.13). A total of 8214 patients were treated with steroids (31.9%), and the incidence of steroid-treated ITP was estimated to be 2.65/100,000 person-years (95% CI: 2.51–2.79).
 |
Table 1 Demographic Factors of Newly Developed ITP Patients |
The incidence of ITP was 6.67/100,000 person-years [95% CI: 6.45–6.89] in 2020, which was lower than the mean annual incidence of 8.61/100,000 person-years [95% CI: 8.49–8.72] in the 2015–2019 period (P < 0.001; Table 1). The incidence of IVIG-treated ITP was 1.72/100,000 individuals [95% CI: 1.61–1.84] in 2020, which was lower than the mean annual incidence of 2.15/100,000 individuals [95% CI: 2.10–2.21] in the 2015–2019 period (P < 0.001). The incidence of steroid-treated ITP was 2.20/100,000 person-years (95% CI: 2.67–2.80) in 2020, which was lower than the mean annual incidence of 2.73/100,000 person-years (95% CI: 2.07–2.33] in the 2015–2019 period (P < 0.001).
The most significant decrease in the overall ITP incidence in 2020, relative to that in the period of 2015–2019, was observed in the 0–9 years age group [14.87/100,000 person-years (95% CI: 13.64–16.03) vs 25.92/100,000 person-years (95% CI: 25.26–26.60), P < 0.001; Figure 1).
Change in the Incidence of ITP Between the Pre-NPI and NPI Periods
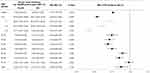
Figure 2 shows the incidence of ITP in all patients during the pre-NPI and NPI periods. The implementation of NPI led to a decrease in the incidence of ITP, especially in the 0–9 years age group. The overall incidence of ITP during the NPI period was 6.60 per 100,000 person-years (95% CI: 6.37–6.85), which was 0.77 times lower than the ITP incidence during the pre-NPI period [8.62/100,000 person-years (95% CI: 8.50–8.74); (Figure 3)]. With the exception of patients aged 70 years and older, the incidence of ITP was significantly lower in the NPI period than in the pre-NPI period (Figure 3). The most significant decline in ITP incidence during the NPI period compared with that in the pre-NPI period was observed in the 0–9 years age group, with an IRR of 0.54 (95% CI: 0.51–0.58), particularly in the 5–9 years age group [IRR: 0.43 (95% CI: 0.35–0.52)].
The overall incidence of IVIG-treated ITP was lower in the NPI period than in the pre-NPI period (1.69/100,000 vs 2.15/100,000, P < 0.001), with an IRR of 0.79 [95% CI: 0.73–0.85]. In the subgroup analysis by age group, a significantly lower incidence of IVIG-treated ITP was observed only in the 1–4 years [IRR: 0.54; 95% CI: 0.44–0.66] and 5–9 years [IRR: 0.45; 95% CI: 0.32–0.62; Figure 4] age groups.
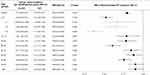
The overall incidence of steroid-treated ITP was lower in the NPI period than in the pre-NPI period (2.73/100,000 vs 2.2/100,000, P < 0.001), with an IRR of 0.80 (95% CI: 0.76–0.83). In the subgroup analysis by age group, a significantly lower incidence of steroid-treated ITP was observed only in the 1–4 years [IRR 0.54 (95% CI: 0.44–0.66)] and 5–9 years [IRR 0.45 (95% CI: 0.32–0.62); Figure 5] age groups.
Differences in Observed and Predicted ITP Incidence in Children
The ARIMA model was used to analyze the difference between the observed and predicted incidence of ITP during the NPI period in children aged 0–9 years, and it revealed a significant change in the ITP incidence during the NPI period. The ITP incidence was 14.01 per 100,000 person-years in the NPI period, which was lower than that in the pre-NPI period (25.80/100,000 person-years) and the predicted incidence of 21.67/100,000 [(95% CI: 14.89–28.45), P = 0.013; Figure 6A]. For subgroup analysis, the 0–9 years age group was divided into three groups as follows: <1 year, 1–4 years, and 5–9 years. In the <1 year age group, the ITP incidence during the NPI period (56.72/100,000 person-years) was lower than that during the pre-NPI period (78.8/100,000 person-years), but was similar to the predicted ITP incidence [66.63/100,000 person-years (95% CI: 34.39–98.87), P = 0.276; Figure 6B]. In the 1–4 years age group, the ITP incidence in the NPI period (19.54/100,000) was lower than that in the pre-NPI period (32.21/100,000 person-years) and the predicted ITP incidence [32.12/100,000 person-years (95% CI: 22.32–41.91), P = 0.006; Figure 6C]. In the 5–9 years age group, the ITP incidence in the NPI period (5.35/100,000 person-years) was lower than that in the pre-NPI period (12.68/100,000 person-years) and the predicted ITP incidence [12.67/100,000 person-years (95% CI, 7.31–18.04), P = 0.004; Figure 6D].
Discussion
Since NPI were implemented during the COVID-19 pandemic, multiple studies have reported a decrease in viral infections and the incidence of autoimmune-related diseases such as Kawasaki disease, which are presumed to have a viral etiology.17–19 Similarly, ITP has been suggested to be related to viral infection, and it is thought to be particularly relevant in children. Xu et al conducted a retrospective cohort study and reported that the ITP incidence decreased significantly after the beginning of the pandemic.20 Another single-center study reported that newly diagnosed thrombocytopenia in children decreased during the lockdown due to the pandemic, probably because of barrier measures that had reduced the incidence of viral triggers thought to be responsible for the vast majority of ITP cases involving children.21 In the present study, we analyzed the incidence of ITP and severe ITP by age group during a period of decreased viral infections due to NPI to predict the degree to which viral infections contribute to ITP in each age group.
The incidence of ITP varies according to factors such as age, sex, ethnicity, geographic regions of data collection, criteria for patient identification, and study design.1,6,22–24 In a recent study conducted in Korea, it was observed that the incidence of ITP was higher among children than among adults, with a peak among children under 5 years of age and a predominance among boys.22 In our study, a similar trend in sex distribution in the age groups was identified. During the NPI period, the overall incidence of ITP decreased significantly, particularly in the 1–4 and 5–9 years age groups. In the age group of 10–59 years, the ITP incidence during the NPI period decreased compared with that during the pre-NPI period. However, the decrease was not conspicuous in patients over 50 years of age, and there was no statistically significant change, particularly in the ≥70 years age group. This suggests that the ITP incidence during the NPI period decreased because of the decrease in viral infections among children, not because of the effect of NPI on the occurrence of ITP in older individuals. Alternatively, because hospital-seeking behavior was also reduced, the detection of mild ITP could have decreased in this age group.
The decrease in ITP incidence during the NPI period was relatively smaller in patients below 1 year of age than in patients older than 1 year of age. No significant difference was noted from the predicted incidence using the ARIMA model, suggesting that the role of viral infection affected by NPI in the occurrence of ITP in this age group is not as significant as that in the 1–4 and 5–9 years age groups. Moreover, no significant difference was noted in the ITP incidence between the pre-NPI and NPI periods in older individuals aged ≥ 70 years, suggesting that the occurrence of ITP due to etiologies affected by NPI, such as social distancing, was not the leading cause in this age group.
We also identified cases in which IVIG was administered. Since the Korean National Health Insurance approved IVIG administration only when the platelet count is less than 20,000/µL, IVIG-treated ITP cases can be considered to have a platelet count less than 20,000/µL, which is of great clinical importance. During the NPI period, the overall incidence of IVIG-treated ITP was significantly decreased [IRR 0.79, (95% CI 0.73–0.85); P < 0.001]. The incidence of IVIG-treated ITP showed markedly different patterns among different age groups. There was no significant difference in the incidence of IVIG-treated ITP in patients aged < 1 year. Conversely, in the 1–4 and 5–9 years age groups, there was a significant decrease in IVIG-treated ITP incidence after implementation of NPI. However, no significant difference was noted in the other groups of patients older than 10 years. These data indicate that the overall decrease in ITP incidence in patients below 1 year of age was due to a decrease in the incidence of less severe ITP that did not meet the criteria for IVIG treatment in this age group. This suggests that factors directly affected by NPI, such as viral infection, are related to less severe ITP. Therefore, other factors that are not affected by NPI should be considered the main cause of clinically significant ITP in this age group. Conversely, the incidence of IVIG-treated ITP also decreased along with the overall ITP incidence in the 1–4 and 5–9 years age groups. These findings suggest that NPI-associated factors, such as viral infection, are the main causes of ITP in these age groups, regardless of the severity. In the age group between 10 and 59 years, the overall ITP incidence decreased significantly, but the incidence of IVIG-treated ITP did not. This phenomenon leads to two inferences. First, the detection of less severe ITP may have decreased because of a decrease in hospital-seeking behavior along with social distancing in this age group. Second, factors affected by social distancing, such as viral infection, may also be the leading cause of less severe ITP in this age group. The decrease in ITP incidence in the older age group was lower than that in the younger age group. This suggests that NPI-related factors is not a significant cause of ITP in this age group.
Limitations
The strength of this study was that almost the entire nation would have been included because we analyzed the Korean Health Insurance Review data. However, this study has some limitations.
First, detailed clinical aspects, including laboratory findings, were not evaluated. Second, it is possible that autoimmune disease or malignancy, which can be mistaken for ITP, was included. However, because this study uses RWE databases intended to report trends under the same methodology, these limitations may not interfere with the purpose of the study. Third, it is speculated that hospital visits were avoided to minimize the risk of SARS-Cov-2 infection during the COVID-19 pandemic, and this could be associated with the decreased detection of asymptomatic ITP, particularly in children. However, even during the pandemic, it is difficult to expect that the treatment of significant ITP represented by medication-treated ITP, would have decreased. Therefore, the change in the incidence of significant ITP is meaningful.
Conclusion
This nationwide study revealed that the ITP incidence decreased significantly, especially in patients aged 1–4 and 5–9 years, after the implementation of NPI in Korea. Despite the limitations of this study, the observed changes in the overall ITP and treated ITP incidences during the NPI and pre-NPI periods are clinically meaningful. Further research is needed for a better understanding of the mechanisms underlying the relationship between NPI and ITP incidence.
Ethics Approval and Informed Consent
The institutional review board of Ajou University Hospital approved this study. The requirement for informed consent from each patient was waived because the HIRA provided secondary data that were entirely de-identified and anonymized before the authors gained access to the data (IRB number: AJIRB-MED-KSP-21-309; HIRA Research Data: M2021052287).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Moulis G, Palmaro A, Montastruc JL, Godeau B, Lapeyre-Mestre M, Sailler L. Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood. 2014;124(22):3308–3315. doi:10.1182/blood-2014-05-578336
2. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. doi:10.1182/blood-2010-08-302984
3. Audia S, Mahevas M, Samson M, Godeau B, Bonnotte B. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16(6):620–632. doi:10.1016/j.autrev.2017.04.012
4. Sahi PK, Chandra J. Immune thrombocytopenia: American Society of Hematology guidelines, 2019. Indian Pediatr. 2020;57(9):854–856. doi:10.1007/s13312-020-1966-8
5. Arnold DM, Kukaswadia S, Nazi I, et al. A systematic evaluation of laboratory testing for drug-induced immune thrombocytopenia. J Thromb Haemost. 2013;11(1):169–176. doi:10.1111/jth.12052
6. Lim JH, Kim YK, Min SH, Kim SW, Lee YH, Lee JM. Epidemiology and viral etiology of pediatric immune thrombocytopenia through Korean public health data analysis. J Clin Med. 2021;10(7):1356. doi:10.3390/jcm10071356
7. Elalfy MS, Nugent D. Viruses, anti-viral therapy, and viral vaccines in children with immune thrombocytopenia. Semin Hematol. 2016;53(Suppl 1):S70–2. doi:10.1053/j.seminhematol.2016.04.021
8. Ai Q, Yin J, Chen S, Qiao L, Luo N. Rotavirus-associated immune thrombocytopenic purpura in children: a retrospective study. Exp Ther Med. 2016;12(4):2187–2190. doi:10.3892/etm.2016.3582
9. Yenicesu I, Yetgin S, Ozyurek E, Aslan D. Virus-associated immune thrombocytopenic purpura in childhood. Pediatr Hematol Oncol. 2002;19(6):433–437. doi:10.1080/08880010290097233
10. Poole S, Brendish NJ, Clark TW. SARS-CoV-2 has displaced other seasonal respiratory viruses: results from a prospective cohort study. J Infect. 2020;81(6):966–972. doi:10.1016/j.jinf.2020.11.010
11. Huang QS, Wood T, Jelley L, et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun. 2021;12(1):1001. doi:10.1038/s41467-021-21157-9
12. Qi Y, Shaman J, Pei S. Quantifying the impact of COVID-19 nonpharmaceutical interventions on influenza transmission in the United States. J Infect Dis. 2021;224(9):1500–1508. doi:10.1093/infdis/jiab485
13. Kim KY, Kim JS, Lee YK, Kim GY, Jung BK. Changes in respiratory pathogens before and after the COVID-19 pandemic (2018–2021). Biomed Res Int. 2022;2022:1324052. doi:10.1155/2022/1324052
14. Lee H, Lee H, Song KH, et al. Impact of public health interventions on seasonal influenza activity during the COVID-19 outbreak in Korea. Clin Infect Dis. 2021;73(1):e132–e140. doi:10.1093/cid/ciaa672
15. Noh JW, Lee WR, Kim LH, Cheon J, Kwon YD, Yoo KB. Influence of COVID-19-related interventions on the number of inpatients with acute viral respiratory infections: using interrupted time series analysis. Int J Environ Res Public Health. 2023;20(4):2808. doi:10.3390/ijerph20042808
16. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal Use of HIRA data. J Korean Med Sci. 2017;32(5):718–728. doi:10.3346/jkms.2017.32.5.718
17. Hwangbo J, Lee JS, Choe SA, Choe YJ. Impact of social distancing on Kawasaki disease-associated hospitalization, South Korea. Pediatr Infect Dis J. 2021;40(10):e383–e384. doi:10.1097/INF.0000000000003202
18. Kang JM, Kim YE, Huh K, et al. Reduction in Kawasaki disease after nonpharmaceutical interventions in the COVID-19 era: a nationwide observational study in Korea. Circulation. 2021;143(25):2508–2510. doi:10.1161/CIRCULATIONAHA.121.054785
19. Jeong SI, Jung HJ. Effect of a regional outbreak of coronavirus disease 2019 on Kawasaki disease in Korea. Risk Manag Healthc Policy. 2022;15:739–745. doi:10.2147/RMHP.S359781
20. Xu S, Hong V, Sy LS, et al. Changes in incidence rates of outcomes of interest in vaccine safety studies during the COVID-19 pandemic. Vaccine. 2022;40(23):3150–3158. doi:10.1016/j.vaccine.2022.04.037
21. Ceglie G, Musolino A, Clemente V, et al. Impact of restrictive measures due to the COVID-19 pandemic on the incidence of immune thrombocytopenia in children: an Italian single center experience. Pediatr Hematol Oncol. 2023;40(2):192–195. doi:10.1080/08880018.2022.2095473
22. Lee JY, Lee JH, Lee H, et al. Epidemiology and management of primary immune thrombocytopenia: a nationwide population-based study in Korea. Thromb Res. 2017;155:86–91. doi:10.1016/j.thromres.2017.05.010
23. Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the general practice research database. Br J Haematol. 2009;145(2):235–244. doi:10.1111/j.1365-2141.2009.07615.x
24. Park SH, Kwak SG, Kim JY. Incidence and prevalence of immune thrombocytopenia under the copayment waiver policy for pediatric patients in Korea: data from the National Health Claims Database. Lupus. 2021;30(4):655–660. doi:10.1177/0961203321995247
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.