Back to Journals » Cancer Management and Research » Volume 12
Changes and Influential Factors of Chemotherapy Usage for Non-Small Cell Lung Cancer Patients in China: A Multicenter 10-Year (2005–2014) Retrospective Study
Authors Xing PY, Wang SZ, Shi JF, Wang L, Hui ZG, Ren JS , Liu SM, Qiao YL , Dai M, Li JL
Received 13 March 2020
Accepted for publication 9 June 2020
Published 20 July 2020 Volume 2020:12 Pages 6033—6044
DOI https://doi.org/10.2147/CMAR.S253789
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Pu-Yuan Xing,1,* Shou-Zheng Wang,1,* Ju-Fang Shi,2 Le Wang,2 Zhou-Guang Hui,3 Jian-Song Ren,2 Shang-Mei Liu,4 You-Lin Qiao,5 Min Dai,2 Jun-Ling Li1
1Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 2Office of Cancer Screening, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 3Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 4Department of Pathology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 5Department of Epidemiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun-Ling Li; Ju-Fang Shi Email [email protected]; [email protected]
Background: Chemotherapy has improved the survival of non-small cell lung cancer (NSCLC) patients over the past few decades. However, there have not been any epidemiological studies on chemotherapy for Chinese NSCLC patients.
Patients and Methods: The patients diagnosed as primary lung cancer between January 1, 2005, and December 31, 2014, in eight hospitals from eight provinces in China were retrospectively reviewed. Demographic and clinical data were extracted from medical history systems. Chi-square test and logistic regression were used to analyze the changes of chemotherapy usage and influential factors.
Results: A total of 7184 lung cancer cases were eligible, among which 6481 NSCLC cases were included in this analysis. Among stage I/II patients, the percentages of receiving adjuvant chemotherapy did not change significantly between the earlier (28.5%) and the latter five years (25.7%) (p = 0.1288). Among stage IIIA patients, the percentages of chemotherapy usage did not change significantly between the earlier and the latter five years in neo-adjuvant (7.5% vs 5.6%, p = 0.1478) and adjuvant (23.1% vs 26.8%, p = 0.1129) treatment. The proportions of first-line platinum-based doublets for stage IIIB/IV patients changed significantly over the 10 years (p < 0.0001). Patients from provinces with inferior gross domestic product, with lower medical reimbursement rates and without smoking history were more likely to use the docetaxel/paclitaxel doublets, comparing with the gemcitabine doublets.
Conclusion: From 2005 to 2014, there was no significant change in the chemotherapy pattern of early NSCLC. Economic factors mainly contributed to the significant changes in the first-line chemotherapy regimen selection for advanced patients.
Keywords: non-small cell lung cancer, chemotherapy, neo-adjuvant chemotherapy, adjuvant chemotherapy, epidemiology
Introduction
Lung cancer has been the leading cause of cancer-related mortality both worldwide and in China.1–3 The estimated age-standardized incidence rates of lung cancer flattened out among the whole population from 2005 to 2014 in China, fluctuating around 47 per 100,000 in males and 21 per 100,000 in females.4,5 Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancers, most of which are squamous carcinoma and adenocarcinoma.6
Over the past two decades, surgical resection has remained the primary and recommended treatment for early stage NSCLC patients, particularly for those with good performance status (PS).7 However, it’s important to note that for these patients, adjuvant chemotherapy has been shown to improve survival in patients with completely resected NSCLC,8,9 as well as neo-adjuvant chemotherapy.10
Unfortunately, the majority of patients have been at advanced stage (stage IIIB/IV) when diagnosed as NSCLC and the prognosis of untreated advanced patients was pretty poor, with a median survival of only 4–5 months.11,12 However, the treatment efficacy for these patients has been improved since the introduction of platinum-based chemotherapy in the mid-1990s.13,14 As the development of the third-generation platinum-based regimens, consisting of platinum plus docetaxel/paclitaxel or gemcitabine or pemetrexed or vinorelbine, the platinum-based doublets chemotherapy have been established as the standard first-line treatment for patients without driver mutations, with 1-year survival rates ranging from 31% to 36%.14 Except for the pemetrexed and cisplatin combination that has demonstrated selectively superior efficacy in non-squamous NSCLC in the first-line treatment,15 platinum-based doublets regimens have demonstrated similar response rates and survival benefits.14
Hitherto, some studies conducted in developed countries have described trends of the usage of chemotherapy for lung cancers and revealed that several epidemiological factors could influence its usage.12,16–18 A comprehensive understanding of the trends of treatment patterns and their potential influential factors, especially the impact of epidemiological characteristics among NSCLC patients, could lead to more equitable evidence-based care. However, there have not been any analogous large-sample studies in China yet. Herein, we conducted a hospital-based, multicenter, retrospective clinical epidemiological survey in various geographic and socioeconomic areas in China to illustrate the trends of chemotherapy usage among NSCLC patients and explore the correlations between demographic, tumor or economic characteristics and the usage of chemotherapy.
Patients and Methods
Study Design and Sampling Methods
This study was a hospital-based, multicenter, retrospective, clinical epidemiological survey of randomly selected primary lung cancer cases over a 10-year (2005–2014) interval in China via medical chart review.
The hospital selection and case sampling methods have been previously described in detail.19,20 To obtain the study population, China was stratified into seven geographic regions (north, northeast, central, south, east, northwest and southwest) according to the traditional administrative district definition by the National Bureau of Statistics. One or two cancer hospitals of the highest level in each region were selected by means of convenience sampling, and a total of eight hospitals from eight provinces across China were finally included. One month was randomly selected from each year between 2005 and 2014 for each province, and all inpatient primary lung cancer cases were reviewed in each hospital. January and February were excluded from selection due to the potential confounding effects of the Chinese New Year vacation. A minimum of 100 inpatient primary lung cancer cases were collected in each hospital each year. If there were not enough eligible cases in the selected month, cases from the next month would be reviewed for supplement and the total sample was no less than 8000 cases.
Patient Selection
The patients first diagnosed by pathology as primary lung cancer between January 1, 2005, and December 31, 2014 were reviewed. The patient selection and inclusion criteria have been previously described in detail.19 All the patients enrolled in this study were required to meet the following four inclusion criteria: (i) primary NSCLC was confirmed by pathology; (ii) the main treatment was received in the hospital where the survey was conducted (patients only receiving diagnosis or undergoing follow-up without any treatment were excluded); (iii) sociodemographic information was available; (iv) clinical characteristics including staging and pathology, and medical information including diagnosis and treatment were available.
Data Collection and Quality Control
As described previously,19 data were extracted using a questionnaire including demographic information, clinical characteristics and medical service usage. Local clerks with an educational background of clinical medicine or public health were responsible for the data extraction. They all had undergone training before screening cases according to the inclusion/exclusion criteria via the medical system in local hospitals and recording the results into a designed form according to the designated protocol.
Multilevel quality control was conducted to ensure the accuracy of the data. Local clerks checked on each other, and data managers checked on the clerks. All variables were double-entered to computer-based database (EpiData 3.1) by two different data-input clerks, and then sent to the center team based at the National Cancer Center for the logic check.
Data Analysis
As part of the whole epidemiological project, we extracted NSCLC cases from the database for analysis. According to the average gross domestic product (GDP) level over these 10 years, the provinces included were divided into a superior group (Zhejiang, Liaoning, Shanxi and Hunan) and an inferior group (Guangxi, Anhui, Gansu and Yunnan).19 Descriptive analyses were used to demonstrate changes in the first-line chemotherapy regimens among the whole advanced NSCLC patients and subgroups. Changes in patient demographics, clinical characteristics and treatment selection over the 10-year period were analyzed using chi-square tests. Factors associated with administration of adjuvant chemotherapy among stage I/II patients, neo-adjuvant or adjuvant chemotherapy among stage IIIA patients and chemotherapy among stage IIIB/IV patients were analyzed using chi-square tests and binary unconditional logistic regression. Factors influencing the selection of first-line chemotherapy regimens among stage IIIB/IV patients were assessed using multinomial logistic regression. For all tests, two-sided p values < 0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
From 2005 to 2014, a total of 7184 primary lung cancer cases were extracted into the database, among which 6481 cases (90.2%) were NSCLC patients. The changes in demographic and clinical characteristics of these cases between the former and the latter five years are summarized in Table 1. Except for patient distribution of provinces with different GDP level, all the other demographic and clinical characteristics included changed significantly over the 10 years.
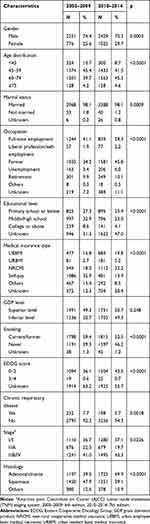 |
Table 1 Characteristics of Non-Small Cell Lung Cancer Patients Over 10 Years (2005–2014) |
Stage I/II
A total of 645 (27.0%) patients in stage I/II received adjuvant chemotherapy. The percentage of patients receiving post-surgical adjuvant chemotherapy in the former five years was not significantly different from that of the latter five years (p = 0.1288). Chi-square tests indicated that the usage of adjuvant chemotherapy was found to be associated with age of diagnosis, occupation, educational level, medical insurance type, GDP level of the province, Eastern Cooperative Oncology Group (ECOG) score and stage of disease (Table 2). When included in logistic regression analyses, patients at age 60 years or older [60–74 and ≥75 vs <45: odds ratio (OR): 0.568, 95% confidence interval (CI): 0.403–0.800 and OR: 0.131, 95% CI: 0.057–0.301, respectively], without job or working as a farmer (farmer, unemployment and retirement vs full-time employment: OR: 0.735, 95% CI: 0.592–0.912, OR: 0.504, 95% CI: 0.304–0.834 and OR: 0.666, 95% CI: 0.476–0.932, respectively), with insurance type of urban resident basic medical insurance (URBMI), new rural cooperative medical insurance (NRCMI) or self-pay [URBMI, NRCMI and self-pay vs urban employee basic medical insurance (UEBMI): OR: 0.573, 95% CI: 0.344–0.955, OR: 0.741, 95% CI: 0.569–0.965 and OR: 0.576, 95% CI: 0.439–0.755, respectively], from inferior GDP level provinces (inferior vs superior: OR: 0.319, 95% CI: 0.258–0.395) and with ECOG score of 3–4 points (3–4 vs 0–2: OR: 0) were less likely to receive post-surgical chemotherapy, while administration of adjuvant chemotherapy was significantly more common among patients at stage IIB (stage IIB vs stage I: OR: 1.348, 95% CI: 1.083–1.678).
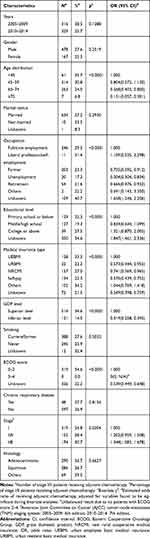 |
Table 2 Factors Associated with Receipt of Adjuvant Chemotherapy in Stage I/II Non-Small Cell Lung Cancer Patients |
Stage IIIA
There were 1355 stage IIIA patients included. Factors associated with administration of neo-adjuvant or adjuvant chemotherapy in stage IIIA NSCLC patients are summarized in Table 3. Chi-square tests revealed that gender, educational level, GDP level of the province, smoking history, ECOG score and histology were associated with both neo-adjuvant and adjuvant chemotherapy in stage IIIA NSCLC patients.
 |
Table 3 Factors Associated with the Usage of Neo-Adjuvant and Adjuvant Chemotherapy in Stage IIIA Patients |
Patient age, occupation, medical insurance type and stage of disease were only associated with adjuvant chemotherapy. When included in logistic regression analyses, female patients (female vs male: OR: 0.493, 95% CI: 0.270–0.900), patients without smoking history (non-smoker vs smoker: OR: 0.591, 95% CI: 0.364–0.959) and patients with ECOG score 3–4 (3–4 vs 0–2: OR: 0) were less likely to receive neo-adjuvant chemotherapy, while patients from inferior GDP level provinces (inferior vs superior: OR: 2.291, 95% CI: 1.472–3.564) and patients with squamous carcinoma (squamous vs adenocarcinoma: OR: 2.504, 95% CI: 1.475–4.251) were more likely to receive neo-adjuvant chemotherapy.
The usage of adjuvant treatment was significantly less common among patients at age 75 years or older (≥75 vs <45: OR: 0.168, 95% CI: 0.048–0.582), working as a farmer (farmer vs full-time employment: OR: 0.708, 95% CI: 0.522–0.959), with insurance type of URBMI or self-pay (URBMI and self-pay vs UEBMI: OR: 0.318, 95% CI: 0.120–0.845, OR: 0.639, 95% CI: 0.438–0.933, respectively), from inferior GDP level provinces (inferior vs superior: OR: 0.290, 95% CI: 0.218–0.386), with ECOG score 3–4 (3–4 vs 0–2: OR: 0), with N1 or N0 disease (N1 and N0 vs N2: OR: 0.487, 95% CI: 0.322–0.737, OR: 0.292, 95% CI: 0.124–0.687, respectively) and with squamous carcinoma (squamous vs adenocarcinoma: OR: 0.535, 95% CI: 0.412–0.696).
Stage IIIB/IV
A total of 2736 stage IIIB/IV NSCLC patients were included into the analysis. In this group, the percentage of patients receiving chemotherapy did not change significantly over the 10 years (p = 0.1566) (Table 4). Factors associated with the usage of chemotherapy among stage IIIB/IV patients included patient age, marital status, occupation, educational level, medical insurance type, GDP level of provinces, smoking history, ECOG score and stage of disease. When included in logistic regression analyses, patients at age 75 years or older (≥75 vs <45: OR: 0.468, 95% CI: 0.305–0.718), not married (not married vs married: OR: 0.392, 95% CI: 0.197–0.779), from inferior GDP level provinces (inferior vs superior: OR: 0.723, 95% CI: 0.607–0.861), without smoking history (non-smoker vs smoker: OR: 0.843, 95% CI: 0.715–0.994) and with ECOG score 3–4 (3–4 vs 0–2: OR: 0.515, 95% CI: 0.271–0.978) were less likely to receive chemotherapy. The usage of chemotherapy was more common among patients working as a farmer (farmer vs full-time employment: OR: 1.607, 95% CI: 1.321–1.954), with insurance type of NRCMI (NRCMI vs UEBMI: OR: 1.640, 95% CI: 1.264–2.128) and with stage IV disease (IV vs IIIB: OR: 1.640, 95% CI: 1.382–1.948).
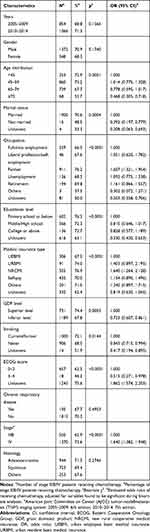 |
Table 4 Factors Associated with the Usage of Chemotherapy in Stage IIIB/IV Non-Small Cell Lung Cancer Patients |
Changes in the Usage of First-Line Chemotherapy for Stage IIIB/IV Patients
The proportions of the usage of four main first-line platinum-based doublets regimens each year are displayed in Figure 1, including platinum plus docetaxel/paclitaxel or gemcitabine or pemetrexed or vinorelbine/vincristine. The proportion of the vinorelbine/vincristine doublets continuously reduced from 40.12% in 2005 to 5.80% in 2014, while the proportion of the pemetrexed doublets increased from 0.60% in 2005 to 22.22% in 2014. Subgroup analyses further illustrated that the usage of the pemetrexed doublets mainly increased among non-squamous cases, rising dramatically from 1.72% in 2008 to 12.12% in 2009, and gradually ascended to 32.61% in 2014 (Figure 2A), while remained relatively low among squamous carcinoma cases (Figure 2B). The proportions of each regimen varied significantly in the latter five years compared with the former five years (p < 0.0001) (Supplemental Table 1).
 |
Figure 1 Trends of the usage of four main first-line platinum-based doublets regimens over the 10 years. |
 |
Figure 2 Trends of the usage of four main first-line platinum-based doublets regimens over the 10 years stratified by histological types. (A) Non-squamous NSCLC; (B) squamous NSCLC. |
Factors Affecting the Selection of the Four First-Line Platinum-Based Doublets
Except the platinum plus gemcitabine regimen, the other three platinum-based doublets regimens all experienced significant changes in usage between the former and the latter five years (Supplemental Table 2). The usage of docetaxel/paclitaxel doublets and gemcitabine doublets increased significantly, while the usage of the vinorelbine/vincristine doublets decreased significantly. In bivariate analyses, the usage of these four regimens was found to be significantly associated with gender, occupation, educational level, medical insurance types, GDP level of the province, smoking history, disease stage and histology types.
When included in logistic regression analyses, the platinum plus docetaxel/paclitaxel regimen was more likely to be used among patients with insurance type of NRCMI (NRCMI vs UEBMI: OR: 1.678, 95% CI: 1.205–2.337) and squamous carcinoma (squamous vs adenocarcinoma: OR: 1.308, 95% CI: 1.049–1.630), and less likely among patients after retirement (retirement vs full-time employment: OR: 0.613, 95% CI: 0.398–0.944). The platinum plus gemcitabine regimen was more likely to be used among patients with squamous NSCLC (squamous vs adenocarcinoma: OR: 1.593, 95% CI: 1.293–1.961), and less likely among female patients (female vs male: OR: 0.780, 95% CI: 0.626–0.972), patients with an educational level of middle/high school (middle/high school vs primary school or below: OR: 0.650, 95% CI: 0.507–0.833), patients from inferior GDP level provinces (inferior vs superior: OR: 0.599, 95% CI: 0.492–0.730) and patients without smoking history (non-smoker vs smoker: OR: 0.754, 95% CI: 0.619–0.917). The platinum plus pemetrexed regimen was more likely to be used among female patients (female vs male: OR: 1.831, 95% CI:1.350–2.485), patients with an educational level of middle/high school (middle/high school vs primary school or below: OR: 1.884, 95% CI: 1.262–2.813), patients from inferior GDP level provinces (inferior vs superior: OR: 1.469, 95% CI: 1.068–2.022), patients without smoking history (non-smoker vs smoker: OR: 2.032, 95% CI: 1.496–2.761) and patients with stage IV disease (stage IV vs stage IIIB: OR: 2.317, 95% CI: 1.557–3.449), and less likely among patients with insurance type of self-pay (self-pay vs UEBMI: OR: 0.489, 95% CI: 0.303–0.788) and patients with squamous disease (squamous vs adenocarcinoma: OR: 0.074, 95% CI: 0.040–0.137). The platinum plus vinorelbine/vincristine regimen was more likely to be used among patients with an educational level of middle/high school (middle/high school vs primary school or below: OR: 1.430, 95% CI: 1.073–1.906) and patients with insurance type of self-pay (self-pay vs UEBMI: OR: 1.895, 95% CI: 1.301–2.761), while less likely among patients with stage IV disease (stage IV vs stage IIIB: OR: 0.597, 95% CI: 0.470–0.760).
In further multinomial logistic regression analyses (Supplemental Table 3), comparing with the gemcitabine doublets, patients diagnosed during the latter five years (2010–2014 vs 2005–2009, p = 0.0033), with insurance type of NRCMI (NRCMI vs UEBMI, p = 0.0257), from inferior GDP level provinces (inferior vs superior, p = 0.0072) and without smoking history (non-smoker vs smoker: p = 0.0088) tended to receive docetaxel/paclitaxel doublets treatment. Comparing with the gemcitabine doublets, the administration of the vinorelbine/vincristine doublets was less common among patients diagnosed during the latter five years (2010–2014 vs 2005–2009, p < 0.0001), patients with stage IV disease (stage IV vs stage IIIB, p < 0.0001) and patients with squamous disease (squamous vs adenocarcinoma, p = 0.0123), while more common among female patients (female vs male, p = 0.0249), patients with an educational level of middle/high school (middle/high school vs primary school or below, p = 0.0067) and patients from inferior GDP level provinces (inferior vs superior, p < 0.0001).
Discussion
Our study was the first multicenter, large sample epidemiological study of the chemotherapy treatment for NSCLC patients in China over the past decades. Our study illustrated the trends of neo-adjuvant or adjuvant chemotherapy treatment for early stage NSCLC patients and first-line chemotherapy treatment for advanced NSCLC patients from 2005 to 2014 in China and revealed the correlations between demographic, tumor or economic characteristics and the usage of chemotherapy.
During the study period, the usage of neo-adjuvant or adjuvant chemotherapy for stage I/II and IIIA patients remained stable between the former and the latter five years. However, several sociodemographic characteristics may still influence the usage of the adjuvant chemotherapy. According to the International Adjuvant Lung Cancer Trial (IALT), patients at age 70 years or older might ultimately reap less benefit from adjuvant chemotherapy (trend p value of 0.08),21 while another observational cohort study demonstrated that postoperative chemotherapy could benefit patients up to 80 years old.22 Our results revealed that during the clinical practice in the real world in China patients at age 60 years or older in stage I/II and patients at age 75 years or older in stage IIIA were significantly less likely to receive postoperative adjuvant chemotherapy, which indicated that the usage of adjuvant chemotherapy might be relatively conservative among elderly patients in China.
The treatment of patients at stage IIIA with N2 nodal involvement was the most controversial issue in the management of NSCLC. Several studies indicated that neo-adjuvant chemotherapy might benefit selected patients with N2 disease.23,24 In our study, patients with N2 nodal involvement were more likely to take neo-adjuvant chemotherapy, but the result was not significant. This may result from the relatively small sample of the stage IIIA patients.
For stage IIIB/IV NSCLC patients, platinum-based chemotherapy has generated a plateau, with an overall response rate between 25% and 35% and a median survival of 8–10 months. However, platinum-based doublets are still established as first-line treatment among patients with good performance status (PS 0–1) and without driver mutations. These doublets can prolong survival, improve symptom control and yield superior quality of life in comparison to best supportive care.6
Except for pemetrexed doublets, which are only approved to be used in non-squamous NSCLC patients, the other three doublets are all approved to be used in both squamous and non-squamous NSCLC patients in the first line. Several studies indicated that there was no significant difference in overall survival between gemcitabine doublets, paclitaxel/docetaxel doublets and vinorelbine doublets.25–30 However, during clinical practice in the real world, we found a tendency of unbalanced choices of these regimens.
One regimen that experienced a consecutive reduction during the study period was the vinorelbine/vincristine and platinum combination. The amount of this combination in the latter five years was significantly lower than that of the former five years. Cost-effectiveness analysis may be able to explain this phenomenon. Measured by quality-adjusted life year, vinorelbine doublets do not cost least but benefit patients least. Thus vinorelbine doublets can neither be the optimal effective selection nor a cost-effective selection.31
Multinomial logistic regression analyses suggested that patients from inferior GDP provinces and patients with insurance type of NRCMI (insurance with lower medical reimbursement rate) were more likely to use the docetaxel/paclitaxel doublets compared with the gemcitabine doublets. Cost-effectiveness analysis may partly explain the results as well. For patients with squamous disease, the paclitaxel doublets were the minimum cost options and therefore represented the initial high cost-effective treatment, and paclitaxel’s preferred alternative favored docetaxel over gemcitabine, as docetaxel’s greater effectiveness seemed to overweigh the additional acquisition cost.31 One single-center cost-effectiveness study on chemotherapy for advanced NSCLC conducted in China also suggested that the gemcitabine doublets became the most expensive regimen among the four third-generation platinum-based regimens after price cuts since 2006.32
Our study also revealed that the usage of pemetrexed doublets increased remarkably in 2009 in non-squamous NSCLC patients, which was in accordance with the development of pemetrexed and the approval of pemetrexed to be used in locally advanced or metastatic non-squamous NSCLC in 2008.33 The significant survival advantage of pemetrexed doublets over gemcitabine doublets among non-squamous NSCLC patients contributed to its growing usage among these patients.15
There are several limitations in our study as well. First, although our study was conducted nationwide and selected patients using convenience sampling, there might be still bias in patient selection due to the relatively high hospital level. Second, target therapies have become increasingly important in the first-line treatment for advanced NSCLC patients. However, because we only enrolled inpatients, outpatients treated with oral target therapies were omitted. Third, data quality is partly dependent on the quality of the medical history documentation, which lacks a standard to assess. Finally, as a retrospective study without follow-up, we are not able to evaluate whether the changes in the treatment choices have led to different survival benefits.
Conclusion
Our study demonstrated the changes of the usage of chemotherapy for NSCLC patients in China from 2005 to 2014. The usage of neo-adjuvant or adjuvant chemotherapy among early stage patients did not vary significantly over these ten years. Economic factors mainly contributed to the significant changes in the first-line chemotherapy regimen selection for advanced patients.
Ethics and Consent Statements
This study was conducted as a part of a broader National Key Public Health Program of China–Cancer Screening Program in Urban China, which was approved by the Ethics Committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences (Approval No. 15-071/998). Because it is a retrospective study that contained no identifiable data, informed consent was waived. This study was conducted in accordance with the Declaration of Helsinki. Patients’ privacy and personal identity information were well protected.
Acknowledgments
The authors thank De-Bin Wang, Yun-Chao Huang, Xian-Zhen Liao, Xiao-Jing Xing, Ling-Bin Du, Li Yang, Yu-Qin Liu and Yong-Zhen Zhang for their contributions to the field data collection. The authors thank Jie He, Kai Zhang for their support to the overall program management. The authors thank Ye Zhang for his contribution to the questionnaire design. The authors thank all the members of this survey from the National Cancer Center of China, field provinces, and expert panel.
Disclosure
The authors declare that they have no conflict of interest with respect to this research study and paper.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
3. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19.
4. Ren JS, Chen WQ, Shin HR, et al. A comparison of two methods to estimate the cancer incidence and mortality burden in China in 2005. Asian Pacific j Cancer Prevention. 2010;11(6):1587–1594.
5. Yang L, Parkin DM, Ferlay J, Li L, Chen Y. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol, Biomarkers Prevention. 2005;14(1):243–250.
6. Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 4.2017. J Nat Comprehensive Cancer Network. 2017. doi:10.6004/jnccn.2017.0050
7. Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S. doi:10.1378/chest.12-2359
8. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360.
9. Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–727. doi:10.1016/S1470-2045(06)70804-X
10. Song WA, Zhou NK, Wang W, et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer: an updated meta-analysis of 13 randomized control trials. J Thoracic Oncol. 2010;5(4):510–516. doi:10.1097/JTO.0b013e3181cd3345
11. Rapp E, Pater JL, Willan A, et al. Chemotherapy can prolong survival in patients with advanced non-small-cell lung cancer–report of a Canadian multicenter randomized trial. J clin oncol. 1988;6(4):633–641. doi:10.1200/JCO.1988.6.4.633
12. Lang K, Marciniak MD, Faries D, et al. Trends and predictors of first-line chemotherapy use among elderly patients with advanced non-small cell lung cancer in the United States. Lung Cancer. 2009;63(2):264–270. doi:10.1016/j.lungcan.2008.05.003
13. Group N-sCLCC. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small cell lung cancer collaborative group. BMJ. 1995;311(7010):899–909. doi:10.1136/bmj.311.7010.899
14. Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi:10.1056/NEJMoa011954
15. Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J clin oncol. 2008;26(21):3543–3551. doi:10.1200/JCO.2007.15.0375
16. Patel N, Adatia R, Mellemgaard A, Jack R, Moller H. Variation in the use of chemotherapy in lung cancer. Br J Cancer. 2007;96(6):886–890. doi:10.1038/sj.bjc.6603659
17. Hardy D, Liu CC, Xia R, et al. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115(10):2199–2211. doi:10.1002/cncr.24248
18. Kaniski F, Enewold L, Thomas A, Malik S, Stevens JL, Harlan LC. Temporal patterns of care and outcomes of non-small cell lung cancer patients in the United States diagnosed in 1996, 2005, and 2010. Lung Cancer. 2017;103:66–74. doi:10.1016/j.lungcan.2016.11.020
19. Li Y, Shi J, Yu S, et al. Effect of socioeconomic status on stage at diagnosis of lung cancer in a hospital-based multicenter retrospective clinical epidemiological study in China, 20052014. Cancer Med. 2017;6(10):2440–2452. doi:10.1002/cam4.1170
20. Shi JF, Wang L, Wu N, et al. Clinical characteristics and medical service utilization of lung cancer in China, 20052014: overall design and results from a multicenter retrospective epidemiologic survey. Lung Cancer. 2019;128:91–100. doi:10.1016/j.lungcan.2018.11.031
21. R A, A D, JP P, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28(1):35–42. doi:10.1200/JCO.2009.23.2272
22. Wisnivesky JP, Smith CB, Stuart P, et al. Survival and risk of adverse events in older patients receiving postoperative adjuvant chemotherapy for resected stages II-IIIA lung cancer: observational cohort study. BMJ. 2011;343(jul14 1):d4013. doi:10.1136/bmj.d4013
23. Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst. 1994;86(9):673–680. doi:10.1093/jnci/86.9.673
24. Martins RG, D’Amico TA, Jr LB, et al. The management of patients with stage IIIA non-small cell lung cancer with N2 mediastinal node involvement. J Nat Comprehensive Cancer Network Jnccn. 2012;10(5):599. doi:10.6004/jnccn.2012.0062
25. Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J clin oncol. 2002;20(21):4285–4291. doi:10.1200/JCO.2002.02.068
26. Martoni A, Marino A, Sperandi F, et al. Multicentre randomised phase III study comparing the same dose and schedule of cisplatin plus the same schedule of vinorelbine or gemcitabine in advanced non-small cell lung cancer. Eur j Cancer. 2005;41(1):81–92. doi:10.1016/j.ejca.2004.08.029
27. Smit EF, van Meerbeeck JP, Lianes P, et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European organization for research and treatment of cancer lung cancer group–EORTC 08975. J clin oncol. 2003;21(21):3909–3917. doi:10.1200/JCO.2003.03.195
28. Chen YM, Perng RP, Shih JF, et al. A randomised Phase II study of weekly paclitaxel or vinorelbine in combination with cisplatin against inoperable non-small-cell lung cancer previously untreated. Br J Cancer. 2004;90(2):359–365. doi:10.1038/sj.bjc.6601526
29. Chen YM, Perng RP, Shih JF, Tsai CM, Whang-Peng J. A randomized phase II study of docetaxel or vinorelbine in combination with cisplatin against inoperable, chemo-naive non-small-cell lung cancer in Taiwan. Lung Cancer. 2007;56(3):363–369. doi:10.1016/j.lungcan.2007.01.011
30. Thomas P, Robinet G, Gouva S, et al. Randomized multicentric phase II study of carboplatin/gemcitabine and cisplatin/vinorelbine in advanced non-small cell lung cancer GFPC 99-01 study (Groupe francais de pneumo-cancerologie). Lung Cancer. 2006;51(1):105–114. doi:10.1016/j.lungcan.2005.10.004
31. Brown T, Pilkington G, Bagust A, et al. Clinical effectiveness and cost-effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer: a systematic review and economic evaluation. Health Technol Assess. 2013;17(31):1–278. doi:10.3310/hta17060
32. Li B, Li MY, Sun LL, Wang J, Zheng YQ, Hao J. Impact of anticancer drugs price cut on physician’s prescription choices on first-line chemotherapy regimens and health expenditure for advanced non-small cell lung cancer in China. J Thorac Dis. 2016;8(10):2832–2842. doi:10.21037/jtd.2016.09.35
33. Cohen MH, Justice R, Pazdur R. Approval summary: pemetrexed in the initial treatment of advanced/metastatic non-small cell lung cancer. Oncologist. 2009;14(9):930–935. doi:10.1634/theoncologist.2009-0092
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
