Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 7
Challenges of hemodialysis in a new renal care center: call for sustainability and improved outcome
Authors Oluyombo R, Okunola O, Olanrewaju T, Soje M, Obajolowo O, Ayorinde M
Received 9 April 2014
Accepted for publication 30 May 2014
Published 18 September 2014 Volume 2014:7 Pages 347—352
DOI https://doi.org/10.2147/IJNRD.S65835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Rotimi Oluyombo,1 Oluyomi O Okunola,2 Timothy O Olanrewaju,3 Michael O Soje,1 Omotola O Obajolowo,1 Margaret A Ayorinde1
1Renal Unit, Internal Medicine Department, Federal Medical Centre, Ido-Ekiti, Ekiti State, 2Renal Unit, Department of Internal Medicine, College of Health Sciences, Obafemi Awolowo University, Ile-Ife, Osun State, 3Renal Division, Internal Medicine Department, Faculty of Health Sciences, University of Ilorin, Ilorin, Kwara State, Nigeria
Background: Nephrologists are faced with enormous challenges in the management of patients with end-stage renal disease, especially in sub-Saharan Africa, where hemodialysis is the most common modality of renal replacement therapy in the region. Therefore, we reviewed our 3 years of experience with hemodialysis services in a tertiary hospital located in a rural community of South West Nigeria. This was with a view to presenting the profile of hemodialysis patients and the challenges they face in sustaining hemodialysis.
Methods: We reviewed the case records and hemodialysis registers for 176 patients over the 3 years from November 2010 to December 2013. The data were analyzed using Statistical Package for the Social Sciences version 20 software.
Results: Of the 176 patients, 119 (66.9%) were males. The mean age of the patients was 44.87±17.21 years. Most were semiskilled or unskilled (111; 63.5%) and 29 (16.5%) were students. Twenty-six (14.8%) had acute kidney injury in the failure stage. Chronic glomerulonephritis, hypertensive nephropathy, and diabetic nephropathy accounted for 45.3%, 23.3%, and 12.1%, respectively, of patients with end-stage renal disease. Only 6.8% of patients could afford hemodialysis beyond 3 months.
Conclusion: Sustainability of maintenance hemodialysis is poor in our environment. Efforts should be intensified to improve other modalities of renal replacement therapy, in particular kidney transplantation, which is cost-effective in the long-term. Also, preventive measures such as education for affected patients and the general population would assist in reducing the prevalence and progression to end-stage renal disease.
Keywords: end-stage renal disease, hemodialysis, sustainability, outcome
Introduction
Nephrologists are faced with enormous challenges in the management of patients with end-stage renal disease (ESRD), especially in sub-Saharan Africa. The main aim of medical care is to improve the health status of the population served. The interaction between demand and supply of services in health care influences the utilization of those services.1 For instance, nonavailability and high cost of services can prevent good health care delivery. ESRD is increasing both in incidence and prevalence, with the black population being at higher risk than whites;2,3 however, Africa contributes only 4.5% to the world’s total number of dialysis patients. Dialysis in ESRD prolongs survival, reduces morbidity, and improves quality of life. Hemodialysis is the most common modality used for renal replacement therapy in the African region. Very few countries in Africa enjoy reimbursement from government to fund renal replacement therapy.
Communicable diseases are the leading cause of death in Africa, and as a result of epidemiological transition, cardiovascular diseases are now becoming more prevalent.4,5 Both conditions contribute greatly to an increased incidence and prevalence of kidney disease. The prevalence of chronic kidney disease is high in Nigeria, and affects young and middle-aged people in their productive years.6,7 Unless there is decisive intervention, the trend of increasing prevalence and incidence of ESRD will continue because of the rising prevalence of hypertension and diabetes mellitus.
Nigeria has a population of about 160 million and a large proportion live in rural areas.8 Human development indices are poor, with a low gross domestic product per capita of USD2,221. About 70% of the population lives below USD1.25 per day9 and only 5.6% of the country’s budget is spent on health care. Nigeria has the third highest number (6.3 per million population) of patients on hemodialysis after South Africa and Kenya.10 Recently, there has been a proliferation of dialysis centers in Nigeria, owned by both private individuals and the government.
The provision of medical services for the treatment of kidney failure poses serious financial challenges for both patients and their families. This is because, apart from dialysis, patients still need other ancillary treatments like optimizing treatment of anemia with erythropoietin, mineral bone disease. This burden is borne by governments in developed countries, unlike the prevalent out-of-pocket practice in Nigeria. For example, 2%–3% of health care expenditure in the USA is spent on patients with ESRD, despite these patients representing a very small proportion (0.1%–0.2%) of the population.11 This is a great economic challenge for people with ESRD, their families, and attendant renal care professionals. Other forms of renal replacement therapy, which have been assessed to be cheaper and with better outcomes, such as peritoneal dialysis and kidney transplantation, are not well developed in Nigeria.
Interestingly, a mortality rate of 27% was reported for 143 patients over 10 years of kidney transplantation in Nigeria.12 However, patients on hemodialysis in the urban cities of Nigeria, eg, Port Harcourt13 and Uyo,14 had a mortality rate of 40% after 90 days after the commencement of hemodialysis and 63% after 1 year. The major challenge in these centers is the inability of patients to sustain hemodialysis. Therefore, it is not only the availability of hemodialysis but also the affordability of the service that should be addressed in order to improve outcomes for patients with ESRD. This is feasible if care for chronic kidney disease is incorporated into the national policy for management of noncommunicable diseases and inclusion in health insurance schemes with wide coverage of the population. Here we report on the challenges for patients with ESRD undergoing hemodialysis in a rural community, which add to the existing data regarding the need for further governmental support of renal replacement therapy and improvement of outcomes for patients with ESRD.
Materials and methods
We reviewed the case records of all admissions to the medical unit at the Federal Medical Center, Ido-Ekiti, Nigeria. Hemodialysis registers and the charts of 176 patients dialyzed over the 3-year period from October 2010 to November 2013 were reviewed. Information on patient sociodemographics, clinical characteristics, and relevant laboratory investigations were extracted.
Dialysis charts were reviewed for the vascular access method used, along with number of sessions and duration on hemodialysis. Information on the use of erythropoietin was also extracted. ESRD was established according to Kidney Disease Improving Global Outcomes guidelines. Diagnosis was based on established clinical criteria.15
Our dialysis center is located in Ido-Ekiti, a rural community in Ekiti State in the south west part of the country. Ekiti State has a population of about 3 million people (National Census, 2006) with a gross domestic product per capita of USD1,169. On average, a hemodialysis session costs USD140 when femoral vein catheterization is used for vascular access. The center is the first renal care facility in the state.
The data were analyzed using Statistical Package for the Social Sciences version 20 software (IBM Corporation, Armonk, NY, USA). Frequencies and means were calculated, and the chi-squared test and independent t-test were used to compare patients with ESRD and acute kidney injury for categorical and continuous variables, respectively.
Results
In total, 176 patients were dialyzed over 3 years. The male to female ratio was 67.2% to 32.8% (2:1). The age range was 13–86 years, with a mean age of 44.87±17.21 years. One hundred and twenty-six patients (84%) were aged 65 years or younger. The majority (36.7%) were unskilled workers and 16.4% were students. In total, there were 793 sessions of hemodialysis. Table 1 shows the clinical diagnosis and mean age of the patients. The mean age of those with acute kidney injury was 38.15±14.73 years. The mean hematocrit in patients with chronic renal failure was 23.86%±5.88%; 81.8% of patients had anemia, and 56% had blood transfusions between dialysis sessions. Of all patients on chronic hemodialysis, only 2% had an arteriovenous fistula while 88.7% used femoral catheterization for access.
  | Table 1 Clinical diagnosis and age of patients |
Acute kidney injury constituted 14.8% of diagnoses. Figure 1 shows the distribution of other clinical diagnoses. Chronic glomerulonephritis was most prevalent (45.3%), followed by hypertensive nephropathy (23.3%). The mean age of the patients with hypertensive nephropathy was 54.23±14.62 years and that of patients with chronic glomerulonephritis was 35.34±14.41 years (P<0.001, Table 1). The three leading causes of ESRD were chronic glomerulonephritis, hypertension, and diabetes mellitus (Figure 1). Figure 2 shows the three most common etiologies of ESRD in the zones of Nigeria as compared with other regions in Africa.
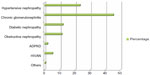  | Figure 1 Distribution of clinical diagnoses in patients with end-stage renal disease. |
  | Figure 2 Comparing the three common causes of end-stage renal disease in the geopolitical zones of Nigeria14–17 and other regions of Africa.29–32 |
There were more females than males with chronic glomerulonephritis (43.9% versus 35.3%, P=0.041) and diabetic nephropathy (12.3% versus 9.2%, P<0.001). However, more males than females had hypertensive nephrosclerosis (22.7% versus 14.0%, P=0.041) and human immunodeficiency virus-associated nephropathy (5.0% versus 3.5%).
Duration of hemodialysis was 1–190 days with a median duration of 7 days, while the number of dialysis sessions was 1–45, with a median of three sessions. Twenty-four patients (16.1%) had dialysis once and then could not continue, while 46.3% could afford hemodialysis three times a week for just 1 week. By the end of months 1 and 3, 73.8% and 93.2%, respectively, had discontinued hemodialysis. Only one patient (0.7%) was still dialyzing after 6 months.
Two patients (1.1%) had financial support from their employers, and the others funded their hemodialysis themselves. Eight patients (4.5%) were referred for kidney transplantation, five (62.5%) of whom underwent kidney transplantation, including the two patients with financial support.
Discussion
The mean age of our patients with chronic renal failure was 45.94±17.49 years and 75.5% were younger than 60 years, which is similar to other reports from Nigeria.15–17 In developing nations, as compared with developed countries, late presentation results in high rates of hemodialysis in young people because of delayed intervention.18–20 This particularly affects the young productive work force. In contrast, about 80% of patients with ESRD on hemodialysis, mainly the elderly, reside in countries with good access to health care.21 The demographic disparity of blacks being at higher risk of progressing to ESRD calls for more active intervention. Therefore, it is important to increase public awareness of kidney disease. Regular screening and intervention have been found to reduce the risk of ESRD.22,23
Anemia contributes to morbidity, mortality, and reduced quality of life in patients with ESRD. Adequate management of anemia in ESRD can be challenging because it depends on optimal use of medications and the adequacy and regularity of dialysis. The majority of the patients presented with anemia, also suggesting late presentation, which exposed them to a need for recurrent blood transfusions and the associated risks. The majority could not sustain erythropoietin therapy. Other groups in Nigeria have also reported a high prevalence of anemia at presentation. Patients with ESRD in developed countries also have anemia at presentation, but of less severity.24,25 It would be difficult to optimize treatment of anemia with erythropoietin because majority of the patients cannot afford the cost of the therapy. Tamiru et al reported improved 12-month survival when erythropoietin was used.23 In addition to regular use of erythropoietin (unless contraindicated), our unit also uses parenteral iron as part of management for anemia.
Another challenge in this study was appropriate vascular access for hemodialysis. An arteriovenous fistula was used in only 2% of patients, and less than 10% used a central venous catheter. Use of a catheter for vascular access has been associated with decreased short-term and long-term survival.23 The National Kidney Foundation Kidney Disease Outcome Quality Initiative recommends that use of a catheter for hemodialysis should be less than 10%.26 An arteriovenous fistula is preferred because it allows good blood flow and is associated with fewer complications. The cost of the procedure, inability to sustain hemodialysis, and late presentation significantly affect the choice of vascular access. At our center, insertion of nontunneled and tunneled catheters costs about USD110 and USD170, respectively. This adds to the high cost of maintenance hemodialysis. It also explains why the majority of our patients used a femoral catheter to sustain their few sessions. Similar experiences have been reported by other groups in Nigeria.14–17 Adequacy of dialysis is affected, coupled with the discomfort for patients as a result of repeated cannulations with each session of hemodialysis, putting them at risk of venous thrombosis, infection, and the complications of an arteriovenous fistula.
The majority of patients in our study did not have adequate hemodialysis. The major reason for this was nonavailability of funds to continue hemodialysis. Standard hemodialysis is prescribed three times weekly for 4–5 hours per session. The majority could afford hemodialysis for up to 2 weeks, and 46% were able to dialyze three times a week for just 1 week. Reports from other centers showed mortality rates of 40%–80% within 3 months of starting dialysis.13–16 Arogundade et al15 reported 87% mortality in the first month. In our study, the two patients who had the support of their employers were able to continue dialysis until they received a kidney transplant. This underscores the need for alternative strategies to enable ongoing management of ESRD. The scope of the local national health insurance scheme should be broadened to include hemodialysis for the less privileged, such as semiskilled workers, who constitute a significant number of patients requiring dialysis in our setting. Nonsustainability of hemodialysis contributes significantly to mortality in patients on chronic hemodialysis.13–15 In Africa, dialysis is subsidized in three countries, ie, South Africa, Mauritius, and Sudan.27
Peritoneal dialysis is an option for renal replacement therapy in developing countries because of its simple technical procedure and easy adaptability. However, our center, like many others in Nigeria, does not offer peritoneal dialysis. This is mainly because of the high cost of consumables and nonavailability of dialysis fluids. It has been reported that only 1.2% of adult patients with ESRD have continuous ambulatory peritoneal dialysis in Ile-Ife.15 Education, training, and subsidized supply of materials such as fluids are advocated in order to support the growth of peritoneal dialysis.28
Patients are being referred for kidney transplantation to other centers both within the country and outside, mainly India. In our study, all five patients referred for kidney transplant opted for India, and had living organ donation (both altruistic and related). As of the time of this study, three of these transplanted patients are being followed up in our clinic.
There is no cadaveric organ donation program in Nigeria. All kidney transplants involve living donors. As a result of a number of constraints, only a small proportion of patients requiring a transplant receive one. Mortality among recipients in a setting similar to ours was 30%.12 However, this is lower than the mortality resulting from discontinuation of hemodialysis. Thus, the obstacles to successful kidney transplantation, such as nonavailability of donors, should be removed in order to improve outcomes for patients with ESRD. There is thus a need for more concerted support.
Conclusion
Renal replacement therapy for patients with ESRD at our center has challenges similar to those previously reported in other settings in this subregion. The increasing prevalence of chronic kidney disease and incidence of ESRD should be tackled with accessible and affordable renal replacement therapy in order to reduce mortality. Therefore, it is imperative to increase utilization of this modality through subsidized services as the government establishes more renal care centers. The capacity building for other modalities for renal replacement therapy should be vigorously pursued in resource-constrained countries.
Acknowledgments
The authors acknowledge the nurses of the renal unit at the Department of Internal Medicine, Federal Medical Centre, under the leadership of Mrs Adekunle FTO, for their support and contribution to this research.
Author contributions
RO, TOO and OO Okunola were involved in the conception, design, drafting, and final approval of the article. MOS, MAA and OO Obajolowo were also involved in the design, drafting, and approval of the final copy of the article. All authors agreed to be accountable for all aspects of the work in this article.
Disclosure
The authors report no conflicts of interest in this work.
References
Tey NP, Lai SL. Correlates of and barriers to the utilization of health services for delivery in South Asia and sub-Saharan Africa. The Scientific World Journal. 2013;2013:423403. | |
Martins D, Agodoa L, Norris KC. Hypertensive chronic kidney disease in African Americans: strategies for improving care. Cleve Clin J Med. 2012;79:726–734. | |
Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol. 2008;19:1261–1270. | |
World Health Organization. The World Health Report 1997 – conquering suffering, enriching humanity. Available from: http://www.who.int/whr/1997/en/. Accessed July 15, 2014. | |
Forrester T, Cooper RS, Wheatherall D. Emergence of Western diseases in the tropical world: the experience with chronic cardiovascular diseases. Br Med Bull. 1998;54:463–473. | |
Oluyombo R, Ayodele OE, Akinwusi PO, et al. A community study of the prevalence, risk factors and pattern of chronic kidney disease in Osun State, South West Nigeria. West Afr J Med. 2013;32:85–92. | |
Ulasi II, Ijoma CK, Onodugo OD, Arodiwe EB, IfebunanduNA, Okoye JU. Towards prevention of chronic kidney disease in Nigeria: a community based study in South East Nigeria. Kidney Int Suppl. 2013;3:195–201. | |
The World Bank. World Bank Search. Available from: worldbank.org/data. Accessed March 17, 2014. | |
Human Development Reports. Human development indicators and thematic tables, 2013. Available from: hdr.undp.org/en/data. Accessed March 17, 2014. | |
Pozo ME, Leow JJ, Groen RS, Kamara TB, Hardy MA, Kushner AL. An overview of renal replacement therapy and health care personnel deficiencies in sub-Saharan Africa. Transplant Int. 2012;25:652–657. | |
United States Renal Data System. Annual Data Report 2012, Volume 2. Incidence, prevalence, patient characteristics and modality. Available from: http://www.usrds.org/2012/pdf/v2_ch1_12.pdf. Accessed May 19, 2014. | |
Arogundade FA. Kidney transplantation in low-resource setting: Nigeria experience. Kidney Int Suppl. 2013;3:241–245. | |
Alasia DD, Emem-Chioma P, Wokoma FS. A single center 7-year experience with end stage renal disease care in Nigeria – a surrogate for the poor state of ESRD care in Nigeria and other sub-Saharan African countries: advocacy for a global fund for ESRD care program in sub Saharan African countries. Int J Nephrol. 2012;2012:639653. | |
Ekrikpo UE, Udo AI, Ikpeme EE, Effa EE. Hemodialysis in an emerging center in a developing country: a two year review and predictors of mortality. BMC Nephrol. 2011;12:50. | |
Arogundade FA, Sanusi AA, Hazan MB, Akinsola A. Pattern, clinical characteristics and outcome of ESRD in Ile-Ife, Nigeria: is there a change in trend? Afr Health Sci. 2011;11:594–601. | |
Ulasi II, Ijoma CK. The enormity of chronic kidney disease in Nigeria: the situation in a teaching hospital in South-East Nigeria. J Trop Med. 2010;2010:501957. | |
Waziri B, Umar IA. A single-center 2-year experience with hemodialysis care in North Central Nigeria. Nigerian Association of Nephrology Conference Book of Abstracts. 2014;36. | |
Okunola O, Ayodele O, Akinwusi P, Gbadegesin B, Oluyombo R. Haemodialysis practice in a resource-limited setting in the tropics. Ghana Med J. 2013;47(1):4–9. | |
Udayaraj UP, Haynes R, Winearls CG. Late presentation of patients with end-stage renal disease for renal replacement therapy – is it always avoidable? Nephrol Dial Transplant. 2011;26:3646–3651. | |
Baer G, Lamiere N, Van Biessen W. Late referral of patients with end stage renal disease: an in-depth review and suggestions for further actions. NDT Plus. 2010;3:17–27. | |
World Health Organization. Preventing chronic diseases: a vital investment: WHO global report. Geneva, Switzerland: World Health Organization; 2005. | |
Gaziano TA, Galea G, Reddy KS. Scaling up interventions for chronic disease prevention: the evidence. Lancet. 2007;370:1939–1946. | |
Tamiru S, Gudina EK, Habte B, Derbew A, Agonafer T. Survival patterns on maintenance hemodialysis for end stage renal disease in Ethiopia: summary of 91 cases. BMC Nephrol. 2013;14:127. | |
Pisoni RL, Bragg-Gresham JL, Young EW, et al. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44(1):94–111 | |
Hörl WH, Macdougall IC, Rossert J, Rutkowski B, Wauters JP, Valderrabano F. Predialysis Survey on Anemia Management: Patient Referral. Am J Kidney Dis. 2003;41:49–61. | |
Schwab S, Besarab A, Beathard G, et al. NKF-KDOQI clinical practice guidelines for hemodialysis vascular access. Am J Kidney Dis. 1997; 30 Suppl 3:S137–S181. | |
Naidoo S, Naicker S, Malgas S, Paget G, Wadee S, Fabian J. Peritoneal dialysis in South Africa –a single centre experience. Indian Journal of Peritoneal Dialysis. 2008;13:18–21. | |
Abu-Aisha H, Elamin S. Peritoneal dialysis in Africa. Perit Dial Int. 2010;30:23–28. | |
Elamin S, Obeid W, Abu-Aisha H. Renal replacement therapy in Sudan, 2009. Available from: http://www.ajol.info/index.php/ajnt/article/view/58903. Accessed July 15, 2014. | |
Krzesinki J-M, Sumaili KE, Cohen E. How to tackle the avalanche of chronic kidney disease in sub-Saharan Africa: the situation in the Democratic Republic of Congo as an example. Nephrol Dial Transplant. 2007;22:332–335. | |
Afifi A. The Egyptian Renal Registry. 9th Annual Report for the Year 2008. Available from: http://www.esnonline.net/content/downloads/registry/2008.pdf. Accessed July 15, 2014. | |
Du Toit ED, Pascoe M, MacGregor K, Thomson PD, editors. Combined report on maintenance dialysis and transplantation in the Republic of South Africa. In: South African Dialysis and Transplantation Registry Report. Cape Town, South Africa: 1994. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
