Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Cesarean scar pregnancy treated by curettage and aspiration guided by laparoscopy
Authors Shu S, Luo X, Wang Z, Yao Y
Received 4 April 2015
Accepted for publication 3 June 2015
Published 3 August 2015 Volume 2015:11 Pages 1139—1141
DOI https://doi.org/10.2147/TCRM.S86083
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Deyun Wang
Shan-rong Shu, Xin Luo, Zhi-xin Wang, Yu-hong Yao
Department of Obstetrics and Gynecology, The First Affiliated Hospital of JiNan University, HuangPu Road West, Guangzhou, People’s Republic of China
Abstract: Pregnancy in a cesarean scar is the rarest form of an ectopic pregnancy. The treatment for cesarean scar pregnancy mainly includes systemic methotrexate and uterine artery embolization. Here, we reported a case of cesarean scar pregnancy treated by curettage and aspiration guided by laparoscopy. The treatment plan included two phases. Three days after a combination of methotrexate and mifepristone was administered, the gestational sac was removed under laparoscopy, which enabled a successful treatment for the unruptured ectopic pregnancy in a previous cesarean scar and made it possible to preserve the reproductive capability of the patient.
Keywords: cesarean scar pregnancy, laparoscopy, curettage and aspiration
Introduction
Implantation of a pregnancy within the scar of a previous cesarean delivery is the rarest form of ectopic pregnancy. The incidence of cesarean scar pregnancy (CSP) ranges from 1:1,800 to 1:2,216,1,2 which is more common than previously thought. Patients with CSP have the risk of uterine rupture and severe life-threatening hemorrhage, which may lead to hysterectomy and cause dramatic consequences for their future reproductive capacity. Because of the rarity of the condition, most CSPs in the literature are case report or small case series; therapeutic protocols have not been universally established. The treatments for CSP include methotrexate (MTX) alone or in conjunction with bilateral uterus artery embolization (UAE), dilation and curettage (D&C) suction after UAE, or regular D&C alone.3 However, these treatments have various shortcomings as follows: 1) regular suction curettage alone creates a predisposition to uncontrollable aggressive massive hemorrhage, even necessitating an inevitable conversion to hysterectomy – it is reported that the failure rate is 70%.4 2) D&C after UAE has the high cost of UAE, its ischemic complications, such as bladder necrosis and low abdominal or limb pain, and the requirement for the procedure to be performed by experienced interventional radiologists.3 3) UAE with the delivery of intravascular MTX before occlusion takes a long time for the gestational sac to be spontaneously reabsorbed, usually several months to 1 year. Also, despite the fact that chemoembolization can obstruct local blood flow around the lesion, there is a risk of massive hemorrhage with the gradual reestablishment of collateral circulation, which necessitates further treatment or even emergency hysterectomy.3 Moreover, several cycles of systemic administration of MTX cause fever, adverse effect on hepatic and renal function, and so on.
Hence, we reported a case of CSP which was treated by curettage and aspiration guided by laparoscopy after systemic MTX injection accompanied with mifepristone. We described the propaedeutics and highlighted the intervention procedure and patient evolution.
Case report
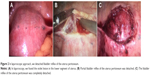
The patient was a 29-year-old multiparous woman in her seventh pregnancy, who had underwent two times of cesarean delivery, and the last cesarean delivery was performed 3 years ago. She was admitted to our hospital with a complaint of genital bleeding that had started 20 days ago. Routine ultrasound examination revealed a 2.1 cm ×1.1 cm ×1.5 cm gestational sac containing a secondary yolk sac inside the lower anterior wall of the uterus, and the myometrial thickness between the gestational sac and bladder wall was 3 mm. The gestational sac was surrounded by rich blood flow signal, and the fetal cardiac activity was 120 bpm (shown in Figure 1). Serum beta-human chorionic gonadotropin (β-HCG) level was 29,560 IU/L. For the existence of the heart beat and considering the possibility of uterine rupture, the patient was informed of the severity of the situation. Given the advanced presentation and location, local therapy was first chosen before systemic treatment or a potentially difficult surgical approach was performed. So, MTX was administrated by intragluteal injection with the dosage of 20 mg/d for 5 days, and also the mifepristone was orally administered. The serum β-HCG was monitored every 3 days. On the seventh day after the administration of MTX and mifepristone, transvaginal ultrasonography was taken, which showed the blood flow around the gestational sac was significantly decreased and the β-HCG has dropped to 11,100 IU/L. Because the patient wanted to preserve her reproductive capacity and want to be discharged from the hospital, we decided to perform curettage and aspiration guided by laparoscopy for the reduced blood flow around the gestational sac and the decreased β-HCG value. In laparoscopic approach, we detached bladder reflex of the uterus peritoneum (shown in Figure 2) and sutured the uterus scar (shown in Figure 3), then we performed curettage and aspiration under laparoscopy as usual. Once the uterus perforation occurred, we sutured the perforation immediately under the laparoscopy, which could significantly decrease the chance of hysterectomy. Fortunately, the procedure was successfully performed with little bleeding, and the gestational sac was eliminated completely, which was confirmed by postoperative ultrasonography and pathological examination. After operation, oxytocin and misoprostol were used to facilitate the uterus contraction, we carefully monitored the bleeding amount. Lastly, the patient uneventfully recovered. After discharged from hospital, serum β-HCG levels were followed to ensure complete resolution of the ectopic pregnancy.
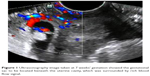  | Figure 1 Ultrasonography image taken at 7 weeks’ gestation showed the gestational sac to be located beneath the uterine cavity, which was surrounded by rich blood flow signal. |
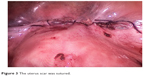  | Figure 3 The uterus scar was sutured. |
Discussion
With the growing number of cesarean deliveries, the CSP was becoming more common than we thought. Vaginal ultrasonography was the preferred diagnostic method for CSP. The ultrasonography criteria to diagnose the CSP were the gestational sac being partly in the anterior part of uterine isthmus, leading to the discontinuity of anterior wall of uterus. In addition, the myometrial layer between the bladder and gestational sac was thin and attenuated, which was usually <0.5 cm. The presence of blood flow signal around the gestational mass revealed by Doppler ultrasound helped the diagnosis of CSP. In the current case, the diagnosis was based on the history of cesarean section and those ultrasonography criteria.
CSPs were usually at high risk of uterine rupture and massive bleeding that could necessitate hysterectomy.5 So, it was suggested that CSP should be terminated once the diagnosis was made, but conservative treatment might be an option for those patients, who had mild symptoms or without symptoms, without viable embryo or gestation sac, and rapidly decreased serum β-HCG level. In our case, the ultrasonography showed the existence of heart beat, and with the patient’s informed consent, we first used MTX and mifepristone to decrease the viability of the embryo. Considering the severe adverse side effects on the blood system and digestive system of using MTX and mifepristone for longer periods, we used these drugs only for 5 days. But the usage of these drugs for longer period could significantly kill the embryo and reduce the blood flow around the gestational sac, which contributed to curettage and aspiration.
Compared with other management options for CSP such as conservative management or blind uterine curettage and aspiration, the greatest advantage of curettage and aspiration guided by laparoscopy was that this method seemed to decrease the risk of uterine rupture and reduce the failure rate for CSP. The estimated blood loss of this procedure is 50 mL, which is less than other managements. It was reported the blood loss for uterine curettage combined with MTX and bilateral arterial embolization was 100–3,000 and 50–1,500 mL, respectively.6,7 Also, it was different from several cycles of systemic MTX administration, which had adverse effects such as leukopenia, alopecia, stomatitis, nausea or vomiting, or liver or renal dysfunction. Compared with bilateral uterus embolization, it did not block uterine arterial blood flow and had no effect on ovary function. Future pregnancy and management after CSP was rarely reported. In our case, we will pay attention to the patient regarding this situation.
Shortly, curettage and aspiration guided by laparoscopy was a promising form of treatment for CSP, especially for those who wished to have shorter period of hospitalization.
Acknowledgments
This work was supported by Cultivating Innovation Fund of Ji Nan University (11614305), Medical Research Foundation in Guangdong Province and the National Natural Science Foundation of China (no 81402143).
Disclosure
The authors report no conflicts of interest in this work.
References
Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373–1381. | ||
Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3):247–253. | ||
Litwicka K, Greco E. Caesarean scar pregnancy: a review of management options. Curr Opin Obstet Gynecol. 2011;23(6):415–421. | ||
Arslan M, Pata O, Dilek TU, Aktas A, Aban M, Dilek S. Treatment of viable cesarean scar ectopic pregnancy with suction curettage. Int J Gynaecol Obstet. 2005;89(2):163–166. | ||
Michener C, Dickinson JE. Caesarean scar ectopic pregnancy: a single centre case series. Aust N Z J Obstet Gynaecol. 2009;49(5):451–455. | ||
Wang JH, Xu KH, Lin J, Xu JY, Wu RJ. Methotrexate therapy for cesarean section scar pregnancy with and without suction curettage. Fertil Steril. 2009;92(4):1208–1213. | ||
Zhang XB, Zhong YC, Chi JC, et al. Caesarean scar pregnancy: treatment with bilateral uterine artery chemoembolization combined with dilation and curettage. J Int Med Res. 2012;40(5):1919–1930. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.