Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Cerebral Arteriovenous Malformation Treatment by Full Transcranial Neuroendoscopic Approaches
Authors Cai Q, Ji B, Li Z, Wang W, Liu J, Chen Z
Received 24 April 2020
Accepted for publication 13 July 2020
Published 6 August 2020 Volume 2020:16 Pages 1899—1905
DOI https://doi.org/10.2147/NDT.S259800
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Qiang Cai, Baowei Ji, Zhiyang Li, Wenju Wang, Junhui Liu, Zhibiao Chen
Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, People’s Republic of China
Correspondence: Qiang Cai; Baowei Ji Tel +86-15072358160
; +86-18062565552
Email [email protected]; [email protected]
Background: Traditionally, cerebral arteriovenous malformations (cAVMs) are surgically treated with microscopy, and no cases receive full neuroendoscopy. We operated on two small cAVMs with hematoma under a neuroendoscope, and the two patients obtained excellent results.
Objective: To explore the feasibility of treating cAVMs with a full transcranial neuroendoscopic approach.
Methods: The clinical data and operative techniques were collected and described for two patients who were diagnosed with cAVMs with hematoma and treated via a full transcranial neuroendoscopic approach.
Results: In the two patients, the hematomas were successfully evacuated, and the AVMs were removed simultaneously; both patients had achieved excellent recoveries at discharge.
Conclusion: To our knowledge, this is the first report in the literature to successfully treat cAVMs with hematoma with a full transcranial neuroendoscopic approach, showing that this approach is feasible and may be an alternative for small ruptured cAVMs. However, while the feasibility of attempting this approach was demonstrated, it was difficult to certify its obvious advantage. Nevertheless, we believe that this approach might be difficult for middle and large AVMs due to the shortcomings of neuroendoscopy. More cases and practice are needed to confirm the merits and limitations of this new approach for AVMs.
Keywords: cerebral arteriovenous malformations, transcranial neuroendoscopic approach, hematoma
Introduction
Cerebral arteriovenous malformations (cAVMs) are still a challenge because of their annual bleeding rates (approximately 2–4%) and relatively high morbidity and mortality rates.1–3 Although the treatment for cAVMs includes microsurgery, radiosurgery, endovascular surgery and multimodal approaches, microsurgery is still the most common treatment due to its effectiveness in regard to an immediate cure. Microsurgery might be the first and only choice for some patients with ruptured cAVMs, especially those with life-threatening hematoma.
To date, all microsurgery procedures for cAVMs have been performed under a microscope or an endoscopic-assisted microscope,4–6 and no full neuroendoscopic approach has been reported. For the first time in the world, we attempted to dissect cAVMs via a full transcranial neuroendoscopic approach in two ruptured cAVMs, and the results were excellent, suggesting that this approach is feasible and may provide an alternative approach.
Case Report
Case 1
History and Diagnosis
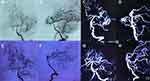
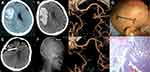
A 13-year-old girl initially presented with sudden onset of headache, which was accompanied by nausea and vomiting. A computed tomography (CT) scan revealed a small hematoma in the left posterior medial temporal gyrus and a subarachnoid hemorrhage. After two days of conservative therapy in a county-level hospital, she was transferred to our hospital. No obvious positive signs were found from the neurological examination. CT angiography (CTA) and digital subtraction angiography (DSA) showed a small AVM in the posterior medial temporal gyrus, a supplying artery that is a branch of the posterior cerebral artery, and the vein drained into the superior petrosal sinus (Spetzler-Martin grade II, 1+0+1=2). After discussion with the interventional surgeon and radiologist, she underwent craniotomy with her family’s consent.
Surgical Procedure
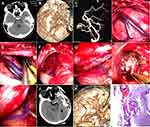
The line for a horseshoe skin incision was drawn at the back of the temporal lobe, and craniotomy was performed. After opening the dura mater, a neuroendoscope was employed to evacuate the hematoma in the temporal lobe, and then the intracranial pressure decreased. The dilated and red-colored draining veins were followed retrogradely, and an AVM nidus was discovered under the neuroendoscope through the working channel created by the endoport. Under the visualization of the neuroendoscope, the feeding artery as well as its small vessel branches and communicating venules were isolated, cauterized and divided, and then the draining vein became darker in color. Then, the nidus of the AVM was circumferentially dissected from the adjacent brain parenchyma. Finally, the venous outflow was disconnected, and the wound was closed (Figure 1A–L).
Postoperative Course
Immediately after surgery, the girl was taken to the intensive care unit (ICU) and transferred to the ward the next day. No complications occurred, and the rest of the postoperative course was uneventful before she was discharged on postoperative day 14. One month later, the patient returned to our hospital for follow-up. DSA was performed, and the AVM had disappeared (Figure 2A–H). At the 3-month follow-up, the patient had returned to school without any neurological deficits.
Case 2
History and Diagnosis
A 67-year-old man presented with sudden headache and left-sided weakness and was sent to a county-level hospital. A CT scan showed a medium-sized hematoma in the right frontal lobe, and the patient was immediately transferred to our hospital. The consciousness level declined to drowsiness, there was left-sided hemiplegia, and the muscle power grade decreased to 0–1. A repeat CT scan and CTA were performed preoperatively, showing that the hematoma was enlarged and that there was a small AVM in the left frontal lobe (Spetzler-Martin grade II, 1+1+0=2). After discussion with the interventional surgeon and his family, an emergency craniotomy was performed (Figure 3A–C).
Surgical Procedure
The line for a linear skin incision (approximately 7 cm) was drawn parallel to the Sylvian fissure, and then a craniotomy was performed. After opening the dura mater, the intracranial pressure was found to be high. The hematoma was evacuated under the direct visualization of the neuroendoscope, and then the intracranial pressure decreased. After carefully checking the cavity of the hematoma, the AVM nidus was identified. Then, the feeding artery and the draining vein were located and isolated, and after cauterizing and cutting the feeding arteries, the draining vein immediately became darker. Then, the core of the AVM was circumferentially dissected from the adjacent brain parenchyma, and the venous outflow was disconnected (Figure 3D–H and 4A–H).
Postoperative Course
The patient was sent to the ICU and recovered consciousness the next day. No other complications occurred, and the rest of the postoperative course was uneventful. In fact, the muscle power grade on the left side increased to 4 before discharge.
During these two operations, the neuroendoscope was held by an assistant who adjusted the angle and direction at any time according to the surgical procedure. At the same time, a microscope was prepared in case of excessive intraoperative bleeding.
This study was approved by the ethics committee of Renmin Hospital of Wuhan University (Wuhan, China; ID:2017K-C043). Written informed consent for publication was obtained from the patients or their family members. All procedures in human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Discussion
cAVMs are not benign, with an average mortality rate over 40 years of up to 25%.7 The estimated prevalence rate of AVMs ranges from 0.02% to 0.2%,8,9 and the most common type is intracerebral hemorrhage, with an annual bleeding rate of approximately 2.2% for unruptured AVMs and 4.5% for ruptured AVMs.10 AVM is a dynamic disease, and the treatment may differ based on whether the patient is symptomatic, the Spetzler-Martin grade, whether the case is an emergency and so on. The final goal of cAVM treatment is the prevention of hemorrhage and the complete disappearance of the nidus on angiography. This can be achieved by surgical excision, endovascular embolization, radiosurgical thrombo-obliteration or a combination of these modalities. To compare these treatments, van Beijnum et al analyzed 13,698 AVM patients and found that angiographic nidus obliteration was achieved in approximately 96% of microsurgical resections, 38% of stereotactic radiosurgeries and 13% of endovascular treatments.11 Mortality or morbidity occurred in 7.4% of patients in the microsurgical group, 5.1% of patients in the radiosurgical group, and 6.6% of patients in the endovascular group. In regard to the complication of intracranial hemorrhage, the rates were 1.4 per 100 person-years overall, 0.18 after microsurgery, 1.7 after radiosurgery and 1.7 after embolization.11 Therefore, microsurgery plays a key role in the management of cAVMs, especially for ruptured cAVMs or cAVMs with hematoma.
In recent years, neuroendoscopic techniques have been developed so quickly that they have been applied in a broad range in modern neurosurgeries. These techniques have tremendous advantages in terms of evacuating hematomas resulting from hypertension, trauma and even aneurysms due to their excellent illumination and high-definition vision.12–14 To date, no cAVM excisions under neuroendoscopy have been reported because cAVMs are embedded in the brain parenchyma, because there is no room for an endoscope during the operation, and because of the complex anatomy of an AVM.
However, several authors have attempted to use endoscope-assisted microscopy to remove brain vascular malformations. In 1994, Otsuki et al reported their initial experience with endoscopic resection in four patients with angiographically occult vascular malformations (AOVMs).5 They utilized a stereotactic system combined with endoscopy and a fiber-guided yttrium aluminum garnet (YAG) laser to remove AOVMs that were relatively small but located deep in brain vascular malformations. The histological diagnosis of the AOVMs was cavernous angioma in three patients and nonspecific hematoma in one patient, who was considered to have an obliterated cryptic AVM. Because no significant complications were observed in these patients, the authors concluded that endoscopy combined with stereotactic laser surgery was a safe and promising treatment for small AOVMs located in deep or complex neural structures. In 1999, Yamada et al employed endoscope-assisted microscopy to treat twenty-five consecutive cases of AVM.6 They inserted an endoscope into the subarachnoid space to interrupt the communicating venules around the major draining vein. Additionally, for intraventricular AVMs, the endoscope was inserted into the ventricle and provided visualization for microsurgery. Twenty-three cases of AVMs were totally resected, and two cases of capsulocaudatothalamic AVMs decreased in size sufficiently following radiosurgery. They believed that endoscope-assisted microsurgery enhanced the magnification, illumination and technical precision while dissecting the core vessels of AVMs. In 2015, Dale Ding described a case in which an endoscope was used to ligate an anterior cranial fossa (ACF) dural arteriovenous fistula (DAVF) with an interhemispheric approach.4 This patient was a 53-year-old woman who was incidentally diagnosed with an ACF DAVF. Angiography showed that the ACF DAVF was supplied by ethmoidal branches of the bilateral ophthalmic arteries and that it drained into the left inferior frontal cortical vein. The initial attempt with endovascular treatment failed due to significant stenosis of the cavernous sinus. Then, they introduced an endoscope into the interhemispheric fissure to visualize the arterialized draining vein, dissecting to the point of fistulization and ligating the proximal portion of the draining vein. They concluded that the endoscope could provide excellent visualization and could safely facilitate surgical treatment in this patient with an ACF DAVF.
The main aim of the treatment for ruptured cAVMs with hematoma is to first remove the hematoma and control acute bleeding and then to remove the AVM during the same emergency surgery, if possible. In our report, we described two patients with ruptured cAVMs with hematoma in whom the hematoma was first evacuated and then the AVM was excised with a purely transcranial neuroendoscopic approach. These two patients had an excellent recovery, and no complications occurred, which demonstrates that this approach is a safe and feasible option for treating ruptured cAVMs. However, this approach was limited by several shortcomings, which were, apart from the lack of adequate instrumentation to date, the restrained vision and difficult visualization of the angioarchitecture of the whole AVM, as well as the unfeasibility of intraoperative indocyanine-green video-angiography and being only achievable with a microscope.15
Conclusion
For the first time, we successfully treated cAVMs with hematoma via a purely transcranial neuroendoscopic approach, showing this approach is feasible and may be an alternative option for small ruptured cAVMs. However, while the feasibility of attempting this approach was demonstrated, it is difficult to certify its obvious advantage. Nevertheless, we believe that this approach might be difficult for the treatment of middle and large AVMs due to the shortcomings of neuroendoscopy. More cases and practice are needed to confirm the merits and limitations of this new approach for AVMs.
Ethical Approval
This study was approved by the ethics committee of Renmin Hospital of Wuhan University (Wuhan, China;ID:2017K-C043). Written informed consent for publication was obtained from the patients or their family members. All procedures in human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no potential conflicts of interest in this work.
References
1. Gross BA, Du R. Natural history of cerebral arteriovenous malformations: a meta-analysis. J Neurosurg. 2013;118:437–443. doi:10.3171/2012.10.JNS121280
2. Hernesniemi JA, Dashti R, Juvela S, Vaart K, Niemela M, Laakso A. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008;63:823–829. doi:10.1227/01.NEU.0000330401.82582.5E
3. Yamada S, Takagi Y, Nozaki K, Kikuta K, Hashimoto N. Risk factors for subsequent hemorrhage in patients with cerebral arteriovenous malformations. J Neurosurg. 2007;107:965–972. doi:10.3171/JNS-07/11/0965
4. Ding D, Starke RM, Crowley RW, Liu KC. Interhemispheric approach for endoscopic ligation of an anterior cranial fossa dural arteriovenous fistula. J Clin Neurosci. 2015;22:1969–1972. doi:10.1016/j.jocn.2015.06.003
5. Otsuki T, Jokura H, Nakasato N, Yoshimoto T. Stereotactic endoscopic resection of angiographically occult vascular malformations. Acta Neurochir Suppl. 1994;61:98–101. doi:10.1007/978-3-7091-6908-7_17
6. Yamada S, Iacono RP, Mandybur GT, et al. Endoscopic procedures for resection of arteriovenous malformations. Surg Neurol. 1999;51:641–649. doi:10.1016/S0090-3019(99)00021-X
7. Kondziolka D, Humphreys RP, Hoffman HJ, Hendrick EB, Drake JM. Arteriovenous malformations of the brain in children: a forty year experience. Can J Neurol Sci. 1992;19:40–45. doi:10.1017/S0317167100042517
8. Al-Shahi R, Fang JS, Lewis SC, Warlow CP. Prevalence of adults with brain arteriovenous malformations: a community based study in Scotland using capture-recapture analysis. J Neurol Neurosurg Psychiatry. 2002;73:547–551. doi:10.1136/jnnp.73.5.547
9. Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359:863–873. doi:10.1016/S0140-6736(02)07946-1
10. Bertani R, Abi-Aad KR, Perret C, AlMekkawi AK, Monteiro R. Is intraoperative ultrasound a valuable tool for brain arteriovenous malformation diagnosis and treatment? A case report. Cureus. 2019;11:e5888.
11. van Beijnum J, van der Worp HB, Buis DR, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA. 2011;306:2011–2019. doi:10.1001/jama.2011.1632
12. Cai Q, Guo Q, Li Z, et al. Minimally invasive evacuation of spontaneous supratentorial intracerebral hemorrhage by transcranial neuroendoscopic approach. Neuropsychiatr Dis Treat. 2019;15:919–925. doi:10.2147/NDT.S195275
13. Cai Q, Zhang H, Zhao D, et al. Analysis of three surgical treatments for spontaneous supratentorial intracerebral hemorrhage. Medicine (Baltimore). 2017;96:e8435. doi:10.1097/MD.0000000000008435
14. Cai Q, Zhang W, Ji B, Ding X, Chen Z, Chen Q. Basal ganglion hematoma evacuation and clipping of middle cerebral artery aneurysm by neuroendoscopy: a case report. Medicine (Baltimore). 2018;97:e0606. doi:10.1097/MD.0000000000010606
15. Messina R, Bozzi MT, Chiumarulo L, Tacconi L, Signorelli F. Ruptured tentorial arteriovenous fistula: endoscopic-assisted microsurgical disconnection using indocyanine green videoangiography guidance. World Neurosurg. 2020;134:377. doi:10.1016/j.wneu.2019.11.024
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.