Back to Journals » Journal of Pain Research » Volume 13
Central Sensitization-Related Changes in Brain Function Activity in a Rat Endometriosis-Associated Pain Model
Authors Zheng P, Jia S, Guo D, Chen S, Zhang W, Cheng A, Xie W, Sun G, Leng J, Lang J
Received 24 September 2019
Accepted for publication 17 December 2019
Published 13 January 2020 Volume 2020:13 Pages 95—107
DOI https://doi.org/10.2147/JPR.S232313
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Ping Zheng, 1 Shuangzheng Jia, 1 Dalong Guo, 2 Sikai Chen, 1 Wen Zhang, 1 Aoshuang Cheng, 1 Weijie Xie, 3 Guibo Sun, 3 Jinhua Leng, 1 Jinghe Lang 1
1Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, People’s Republic of China; 2Air Force Medical Center, PLA, Beijing, People’s Republic of China; 3Institute of Medicinal Plant Development, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100193, People’s Republic of China
Correspondence: Jinghe Lang Email [email protected]
Jinhua Leng Email [email protected]
Background: Pain sensitization processing in the central nervous system may be related to endometriosis-associated pain in patients. The purpose of this study was to understand the alterations in the abnormal pain response in central brain areas and explore the central sensitization mechanism of endometriosis-associated pain.
Methods: An endometriosis model was established in 40 Sprague-Dawley rats, and the rats underwent pain model assessment through behavioral tests. Twenty Sprague-Dawley rats underwent a sham operation as the control group. Thirteen pain rats and 8 control rats received Rs-fMRI examination to explore the brain functional activity areas, and the regional homogeneity (ReHo) method was used to analyze relevant functional signals among the whole brain. The states of neurons and expression of TRPV1 and NMDRA located in the abnormal ReHo signal brain regions were observed using Nissl staining, qRT-PCR and immunohistochemistry.
Results: The rats were divided into a pain group and a control group based on the different syndromes and behavioral assessments. We detected significant enhancement of ReHo signals in the anterior cingulate cortex, hippocampus, and thalamus and a reduction in the ReHo values in the basomedial amygdaloid nucleus (BM) and primary motor cortex (M1) in the pain rat group via Rs-fMRI examination. The number of Nissl bodies and apoptotic neurons was increased; moreover, the volume of neurons increased compensatorily in the cingulate cortex, thalamus and hippocampus in the pain group. TRPV1 and NMDRA were overexpressed in apoptotic neurons in the higher ReHo value brain regions in the endometriosis pain group.
Conclusion: These findings suggest that in rats with endometriosis-associated pain, ReHo signal enhancement was observed in the cingulate cortex, thalamus and hippocampus, which may be due to the increase in the number of apoptotic neurons or the compensatory increase in the volume of overactive neurons.
Keywords: endometriosis-associated pain, rat model, behavioral assessment, central sensitization, Rs-fMRI, regional homogeneity, TRPV1, NMDRA
Corrigendum for this paper has been published
Introduction
Endometriosis is a common gynecological disease in women of reproductive age. Approximately 70–80% of patients present different types of pain-related behaviors1 that are mainly characterized by chronic pelvic pain, dysmenorrhea, dyspareunia and defecation pain, etc., which seriously affect women’s health and quality of life and waste a large amount of medical resources; however, the efficacy of surgery and drug treatment is not significant.2 In addition, the pathogenesis of endometriosis-associated pain is currently entirely unclear. Berkley3 reported that ectopic endometrial growth develops autonomic and sensory innervations, which could contribute to the maintenance of ectopic growth and pain phenotype persistence. Coxon and Yan4,5 also proved that the density of nerve fibers is larger in ovarian endometriosis tissue than in normal ovarian tissue, including sensory nerve fibers, sympathetic and parasympathetic nerve fibers. However, the pain caused by ovarian endometriosis is significantly less than that caused by other types of endometriosis, such as deep infiltration endometriosis (DIE), which may cause fewer nerve fibers in ovarian endometriosis lesions than in other types. Interestingly, Stratton and Berkley6 found no direct proportion between the size and shape of local lesions and the degree of pain severity. In the clinic, laparoscopic surgery reveals extensive lesions over the pelvic and abdominal cavity in some patients with moderate pain. Hence, the central sensitization mechanism may explain why some patients suffer from hyperalgesia after long-term exposure to endometriosis-induced inflammation or this persistent pain status after the removal of the lesion or the pain relapse after the cessation of drug treatment.5,7,8
Berkley9 first proposed that endometriosis pain could have a widespread and varied influence on the activity of neurons throughout the central nervous system. The central sensitization mechanism in the nervous system is that afferent pain signals will be amplified by the central nerve to produce a continuous, amplified effect.10 Nociceptor input produces pain hypersensitivity, resulting in secondary alterations in brain activity, which can be detected by imaging techniques. Porpora et al11 observed a decrease in gray matter volume in certain areas of the cerebral cortex via cranial MRI in patients with long-term chronic pain, suggesting that changes in the pain center of the brain may be related to chronic pain.
Despite the above studies on chronic pain, due to the lack of reliable imaging evidence, the changes in the functions of various brain regions caused by central sensitization remain unclear at present. Therefore, Resting-state functional magnetic resonance imaging (Rs-fMRI) has become an important tool for studying living brain function. Due to its high temporal and spatial resolution, lack of radioactivity and repeatable detection, Rs-fMRI has been widely used in Alzheimer’s disease, epilepsy, attention-deficit hyperactivity disorder, schizophrenia, chronic pain disease and other diseases.12 Rs-fMRI can be used to simultaneously observe the activities of multiple brain regions and study the relationship between various functional regions; thus, it has become a popular modality for the study of the central sensitization mechanism. The core principle is that the excitation of brain neurons can cause the enhancement of local T2-weighted imaging signals, that is, T2-weighted imaging signals can reflect the activities of local neurons.13 Apkarian et al14 reviewed how localized brain activity in diverse brain regions creates and modulates the experience of acute and chronic pain states by Rs-fMRI. Furthermore, the regional homogeneity (ReHo) method, a data-driven analysis method, describes the degree of regional synchronization of fMRI time courses, which can effectively assess resting-state functional activity of the whole brain.15 This method could help better explain the endometriosis-related pain mechanism of central sensitization in brain function.
However, Rs-fMRI remains at a shallow level of description in the mechanism, diagnosis and prognosis of pain. Most previous studies are based on behavioral observation, and the evidence is insufficient. Additionally, the elaboration of the specific molecular mechanism is rarely reported. The abnormal activation of transient receptor potential vanilloid 1 (TRPV-1) and N-methyl-D-aspartate receptor (NMDAR) is reportedly involved in the mechanisms of central sensitization pain.16 TRPV-1 is a nonselective cationic channel on the surface of sensory nerve cells, and TRPV-1-positive nerve fiber cells are highly expressed in patients with endometriosis.17 TRPV1 is activated by pain and inflammation stimulation, thereby increasing the intracellular calcium ion concentration, releasing glutamate and activating NMDAR, which eventually leads to neuronal apoptosis through calcium overload, oxidative stress injury and apoptotic pathway activation,18 which may be the mechanism for the persistence of hyperalgesia. However, the relationship between TRPV-1 and NMDAR and the central sensitization mechanism has been poorly studied in endometriosis.
In our study, we hypothesized that we hypothesized that female rodents with endometriosis-associated pain will experience brain functional alterations. This finding explored the central sensitization mechanism using the endometriosis-associated pain model via the Rs-fMRI detection and expression of TRPV-1 and NMDAR in the corresponding brain regions. This study may lay the foundation of new treatment methods for endometriosis-associated pain.
Materials and Methods
Animals and Surgery
Sixty healthy female Sprague-Dawley (SD) rats, aged 8–9 weeks and weighing 230~250 g, were purchased from Beijing Vital Lihua Experimental Animals Co., Ltd. (license number SCXK (Beijing) 2016–0006). All rats were raised in the SPF standardized environment of the animal center of the Institute of Medicinal Plant Development, Peking Union Medical College and Chinese Academy of Medical Sciences.
After a week of acclimatization, the random number method was used to divide the rats into the model group (40 rats) and the sham operation group (20 rats); dies can be made according to the Berkely.19 The low abdomen of each rat was exposed via a ventral midline incision after anesthesia with 5% isoflurane. The endometriosis model (40 rats) was established through surgical procedures, including separation of the left side of the uterus, peripheral vascular ligation, and 1 cm tissue incision of the uterus. After the procedure, uterine tissue was washed with cold PBS in vitro. The isolated uterine biopsies were divided into four equally sized segments. Each small section was opened longitudinally and exposed to the endometrium, which was kept in cold PBS until implantation. The biopsies were sutured to the parietal peritoneum and mesentery using 4–0 vicryl sutures. The operation of control group performed exactly the same process as the experimental group except using the same size of abdominal adipose tissue as the implantation, which was placed in the same location as the model group after laparotomy of the 20 rats. Two weeks after the operation, the abdominal incision was healed completely, and all rats underwent laparotomy again to confirm successful establishment of the endometriosis model.
Pain Behavioral Assessment
Hot Plate Test
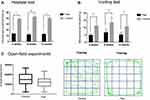
The hot plate test is a commonly used method to measure the thermal stimulus pain threshold in rodents.20,21 The hot plate instrument (purchased from Shanghai zhongshi dichuong technology development co., LTD, Shanghai, China. Model: zs-yls-6b) is mainly composed of a round heating plate with a diameter of approximately 25 cm at the bottom and a transparent plexiglass cylinder on the hot plate. All rats were tested 4 weeks, 8 weeks and 12 weeks after surgery. The temperature was set at 53.0 ± 0.1°C, and the rats were allowed to walk randomly on a hot plate for up to the maximum of 45 s (to avoid tissue damage). The timing of reactions including jumping, hind paw licking or flicking was recorded during the test. Each rat was tested only once in each session. The pain threshold was considered increased if the latency of foot licking was shorter.22 The operation process is shown in supplementary Figure 1.
Von Frey Test
Based on the research methods of Barrot and Gregory,23,24 the Von Frey test was adopted to value the mechanical pain threshold. The right plantar region of the rats was stimulated by Von Frey filaments (purchased in Shanghai jade research scientific instrument co., LTD. China) to observe the pain response. A single rat was placed on a metal mesh device (60 cm×40 cm×20 cm). After the rats became quiet, we stimulated the lateral sides of the right hind plantar side (innervation area of the sural nerve) and medial sides (innervation area of the saphenous nerve) by using different fold forces of the Von Frey filaments. Filaments of ascending strength were applied to the rats vertically until the fiber was bent into a C shape and maintained in place for 6~8 s. During the experiment, the fold forces were recorded automatically when the rats licked or withdrew the right hind paw. Mechanical pain threshold measurements were performed in all rats at 4 weeks, 8 weeks and 12 weeks from the surgery day. The operation process is shown in supplementary Figure 1.
Open Field Test
Rats with endometriosis-associated pain are more prone to anxiety than pain-free rats in an empty, open space. Thus, the open field test was used to evaluate the anxiety level and locomotor activity in rats.22,25 The open field test device comprises an open square box (100 cm × 100 cm ×40 cm), which is divided into a central zone and peripheral zones. The floor is smooth, and a camera is placed approximately 2 m away from the center at the top of the square box to measure the overall activity of rats in the open field. The locomotor activity tracking was recorded with SuperMaze software (provided by xinxin Shanghai). A single rat was kept in a quiet waiting area for approximately 30 min before the start of the experiment. Then, the rat was placed in a corner of the box and allowed to explore the field for 5 min. The overall distance and walking time in the open field were measured by the trajectory in video recordings. Rats with higher anxiety levels tend to spend more time in the periphery and less time in the central zone. The locomotor activity tracking of pain-free rats shows a more intricate pattern and an increase in overall distance compared with that of pain rats. All rats were tested 12 weeks after surgery. The operation process is shown in supplementary Figure 1.
Image Acquisition and ReHo Analysis
The second day after the pain behavior assessment, MRI data were acquired using a 7.0-T small animal scanner (Bruker Pharma Scan system) with a 38-mm-diameter birdcage coil (Bruker BioSpin, Ettlingen, Germany). All rats were anesthetized with 5% isoflurane. Each rat was loaded into a custom-made MR-compatible stereotactic holder with a bite bar to avoid head motion. The body was fixed to the holder with tape. During the MRI scan, the respiration rate, heart rate and body temperature of each rat were continuously monitored and recorded using a special instrument.
The T2-weighted High-resolution anatomical images were acquired using a rapid acquisition with relaxation enhancement (RARE) sequence [repetition time (TR) = 2000 ms; Echo time (TE) =30 ms; Scantime=1200 s; flip angle = 90°; field-of-view (FOV) = 32 × 32 mm2; slice thickness = 0.7 mm; slice number = 38;voxel size = 0.137 × 0.137 × 0.7mm]. Resting-state fMRI scans were acquired using a T2 weighted EPI sequence (TE = 15 ms; TR = 2000 ms; FOV = 30 × 30 mm2; matrix size = 64 × 64; slice thickness = 1 mm; slice number = 5200; voxel size = 0.313 × 0.313 ×1mm).26–28
Rs-fMRI data were preprocessed using Statistical Parametric Mapping 12 software (SPM12) (https://www.fil.ion.ucl.ac.uk/spm/). Preprocessing steps included discarding the first 10 volumes, slice timing correction, and motion correction. The Rs-fMRI images were aligned to their corresponding T2-weighted images and then normalized to the Paxinos and Watson rat brain atlas. To improve the accuracy of spatial normalization across rats, we further aligned Rs-fMRI data from each rat to the group-averaged Rs-fMRI images. Finally, the normalized images were linearly detrended, and the six-parameter motion curves were regressed. REST software (http://www.restfmri.sourceforge.net) was performed to calculate the ReHo value of each voxel in the whole brain, and then the ReHo maps were acquired from each rat. Finally, 8 mm Gaussian smoothing was carried out to improve the signal-to-noise ratio (SNR). Two-sample t-tests were used to identify significant differences in the ReHo value between the endometriosis-related pain group and the control group by SPM12 software. The voxel-level threshold was set at P < 0.001, uncorrected, with more than 20 contiguous voxels to a cluster threshold.
Nissl Staining and the Expression of TRPV1 and NMDAR
Nissl Staining
To detect the state of neuronal cells in the brain in endometriosis-associated pain rats, we sacrificed all rats at 12 weeks postoperation after Rs-fMRI detection, and their brains were dissected at 4°C and fixed rapidly for 48 h at 22°C with 10% formalin. The whole brain was cut into 4 parts by sagittal plane and embedded into wax blocks. Each part of the brain was made into 4 μm sections, which were washed with a graded ethanol series for 5 min and incubated in Nissl staining solutions (Institute of Medicinal Plant Development, Beijing, China) for 0.5 h at room temperature in accordance with the protocol. The state of neuronal cells in the brain was analyzed by a Direct Optical Microscope29 (Cambridge Research and Instrumentation Inc., Woburn, MA, USA).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from each specimen stored at −80°C using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc) according to the protocol. The RNA quality was measured by the A260/A280 ratio and agarose gel electrophoresis. Total RNA (500 ng) from each group was individually reverse transcribed into the reaction mixture in 20 μL using the GoScriptTM cDNA synthesis kit (A5000, Promega, USA). The reaction mix was incubated for 5 min at 25°C, 60 min at 42°C, and 15 min at 70°C. qRT-PCR was prepared using the GoTaq probe qPCR Master Mix (A6002, Promega, USA). Each PCR mixture followed the protocol and was performed using a Real‑Time PCR Quantification system (ABI 7500 fast; Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermal cyclic conditions used were as follows: initial denaturation and enzyme activation for 10 min at 95°C followed by 40 cycles (denaturation for 15 s at 95°C, annealing for 30 s at 60°C, extension for 45 s at 72°C), followed by melting curve analysis. Relative gene expression was determined by analyzing data using the 2−ΔΔCT method to adjust for expression of a housekeeping gene, GAPDH. All products obtained yielded the predicted melting temperature. All experiments were conducted in triplicate. The gene primers used are listed in Table 1.
 |
Table 1 The Primers of Gene |
Immunohistochemistry
Whole brain specimens were divided into 4 parts by sagittal plane from both endometriosis pain and sham rats. The tissue specimens were fixed in 4% formalin and embedded in paraffin at 12 weeks postoperation after Rs-fMRI examination. Immunohistochemical staining was performed using rat polyclonal antibodies Anti-NMDAR1 (1:1000; Abcam, Cambridge, MA, USA) and rabbit polyclonal antibodies Anti-VR1 (1:500, Abcam, Cambridge, MA, USA). Tissue sections were deparaffinized followed by dehydration with xylene and ethanol. The specific operation process of immunohistochemistry strictly followed the protocol. Goat immunoglobulin G (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a negative control. The number of stained cells and the intensity of staining were evaluated by two evaluators blinded to the specimen source. An H-score method was used to obtain a semiquantitative measure of expression.30
Statistics
SPSS 20.0 software was used for the statistical analysis, while GraphPad Prism7, Adobe Photoshop CS5 and Adobe Illustrator CC2015 were used for graph generation. Levene’s test for homogeneity was performed, the statistical description the mean ± standard deviation was used for statistical descriptions, and unpaired t-tests were used to compare the differences between the groups when the variance was irregular. The median value (P25~P75) was used for statistical description, and the Kruskal−Wallis test was used to identify the differences between the groups (when the variance was nonnormally distributed). P < 0.05 was considered statistically significant.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Laboratory Animal Ethics Committee of the Institute of Medicinal Plant Development, Peking Union Medical College, and conformed to the Guide for the Care and Use of Laboratory Animals (Permit Number: SYXK 2017–0020).
Results
Endometriosis-Like Lesions
The low abdomen of all rats was reopened to evaluate model establishment two weeks postoperation. All 40 rats exhibited endometriosis-like lesions by pathological verification (as shown in Figure 1). No corresponding cyst formation was found in the 20 rats in the sham control group.
Grouping and Behavioral Test Results
Twenty-eight rats were assigned to the endometriosis-related pain group in accordance with the behavioral results. The 20 sham surgery rats were allocated to the control group.
The hot plate test result showed that the latency of jumping, hind paw licking or flicking the paw in the endometriosis-related pain group was shorter than that in the control group at 4 weeks, 8 weeks and 12 weeks, respectively (See Figure 2A and Table 2). The Von Frey test results showed that the mechanical stimulation pain threshold of the pain group was significantly lower than that of the control group (as shown in Figure 2B and Table 3). The open field test indicated that the tracking of rats in the control group was disorderly, and the overall distance was significantly increased compared with that of the pain group. Additionally, the number of central entrances in the endometriosis pain group was significantly lower than that in the control group (as shown in Figure 2C and Table 4).
 |
Table 2 Comparison of Thermal Pain Threshold Time by Hotplate Test (s) |
 |
Table 3 Comparison of Pain Threshold of Mechanical Stimulation by Von Frey Test (g) |
 |
Table 4 Analysis of the Results of Open-Field Experiments in Rats |
Resting-State fMRI Data
We finally acquired the scanning parameters of 13 rats in the pain group and 8 rats in the control group to further analyze the data after preprocessing these rats. Compared with the sham control group, the endometriosis-associated pain group had significantly increased ReHo in the anterior cingulate cortex, area 2 (left); thalamic area (right); thalamic area (left); the CA1 field of the hippocampus, and brainstem (right); this group also exhibited significantly decreased ReHo in the basomedial amygdaloid nucleus (BM, left) and primary motor cortex (M1, right). The Numbers at the top and bottom of the color bar represent the t value of the statistical result. Besides, the Red presents the enhancement ReHo signal and the blue shows the decrease ReHo signal. The intermediate colors indicate that the ReHo signal has a strong to weak process from red to blue (P < 0.005, as shown in Figure 3 and Table 5).
 |
Figure 3 The ReHo value comparison in various brain regions. In comparison with the sham control, the endometriosis-associated pain group had significantly increased ReHo in the Anterior cingulate cortex, area 2 (left), thalamic area (right), thalamic area (left) and the field CA1 of hippocampus and brainstem (right), while significantly decreased ReHo in the basomedial amygdaloid nucleus (left) and primary motor cortex (right). The Numbers at the top and bottom of the color bar represent the t value of the statistical result. Besides, the Red presents the enhancement ReHo signal and the blue shows the decrease ReHo signal. The intermediate colors indicate that the ReHo signal has a strong to weak process from red to blue (P < 0.005, as shown in Table 5).Abbreviation: ReHo: regional homogeneity. |
 |
Table 5 Brain Regions Showing ReHo Differences in the Pain Group Compared to the Control |
The brain region activity in the anterior cingulate cortex, thalamic area, and CA1 field of the hippocampus was substantially increased in the ReHo value, which was closely related to pain perception and memory. Therefore, we decided to further examine the three brain regions to observe their morphological changes and the expression of related molecules.
Nissl Staining
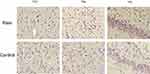
We found a large number of Nissl bodies and apoptotic neurons in the cingulate cortex, thalamus and hippocampus in the endometriosis-associated pain group. The nuclei were condensed and deformed, nucleoplasmic staining was deepened, and the volume of neurons around apoptotic cells was larger in the pain group than in the control group. Neuronal apoptosis was also rarely found in the control group, which is considered physiological. The number of normal neurons in the pain group was significantly lower than that in the control group (see Figure 4).
qRT-PCR
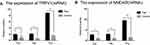
We extracted total RNA from all of the paired rat brain areas for validation. The relative fold changes in the expression of TRPV1 and NMDAR at the mRNA level located in the anterior cingulate cortex, hippocampus, and thalamus were significantly increased in the endometriosis-related pain group compared to those in the sham control group by qRT-PCR, as shown in Figure 5A and B.
Immunohistochemistry
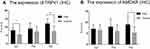
TRPV1 and NMDAR were both expressed in the cytoplasm and nuclei of central nervous system tissues and overexpressed in apoptotic neurons and partial Nissl bodies. However, the staining of TRPV1 in the pain group was stronger (dark brown color) than that in the control group in the anterior cingulate cortex (P<0.05) and hippocampus (P<0.001). There was no significant difference in the thalamus (Figure 6 and Figure 7A). The staining of NMDAR in the pain group was stronger than that in the control group in the hippocampus (P<0.05). There was no significant difference in the anterior cingulate cortex and thalamus (Figure 8 and Figure 7B).
Discussion
In human studies, the visual analogue scale (VAS) and McGill Pain Questionnaire (MPQ) can be used to quantitatively score pain in patients. Alternatively, the assessment of pain sensitization in rodent animals can be qualitatively analyzed through behavioral testing. Hence, we evaluated pain through behavioral experiments, such as hot plate, Von Frey and open field testing, to ensure the successful establishment of the model. We used a rat model of surgically induced endometriosis whose growths mimic those in women with endometriosis by autotransplanting,9 while the control group should not be treated with transplantation31 (because it is a normal rat mimic those in women without endometriosis). However, the control group was performed with adipose tissue transplantation and on-off abdominal suture, in order to balance the pain of the abdominal incision and the inflammatory reaction of local transplantation in the experimental group. The problem of postoperative pain, our solution is to test the pain threshold after the second surgery for 2 weeks. This period is to wait for the wound healing, the surgical pain almost relieved. Subsequently, Rs-fMRI was performed to identify the abnormal activation area, observe morphological changes and research the molecular mechanism in abnormal activation regions, with the aim of exploring the pathogenesis of endometriosis-related pain via the nervous system circuit beyond the limitation of local lesions.
Central sensitization is an important mechanism for the generation, memory and amplification of pain in endometriosis, which has been widely recognized.32,33 Central sensitization is the amplification of the pain transmission response of the spinal cord and the levels of the spinal cord (such as the thalamus, brainstem and cerebral cortex), including central sensitization at the level of the spinal cord. Pain is a result of damage on the threshold of stimulation, leading to conduction of A fibers and slow conduction C-fiber activation. The damage signal is spread to the spinal cord dorsal horn (dorsal root ganglion, DRG), pain signals spread to neurons, and preliminary integration occurs. Then, via axons, these neurons spread a signal, which is transferred to secondary neurons by the aforementioned structure on the thalamus (thalamic limbic system, namely, the cerebral cortex) for advanced integration. Patients with endometriosis are stimulated by focal neuroinflammatory factors for a long time, which activates local immune response and changes nerve potential conduction, leading to peripheral and central sensitization.34 Morotti et al35 found that patients with endometriosis suffered from chronic pelvic pain; in these patients, certain functional brain areas, such as the thalamus, putamen and insula, were significantly enhanced on fMRI, further strengthening the pain mechanism of central sensitization.
In our study, 28 endometriosis-induced rats were confirmed to have hyperalgesia responses and pain threshold decreases according to behavioral tests. The hot plate test is often used to evaluate the response to thermal pain stimulation, and the Von Frey test reflects the pain threshold measurement of rats to mechanical stimulation.36 Both experiments are often used to evaluate pain models in rats and mice and objectively evaluate the efficacy of analgesics.23,37 The open field test is used to evaluate the anxiety symptoms caused by pain in rats, which is a subjective perspective to evaluate the pain model.38 Based on the experimental results of this study, the heat pain in the endometriosis-associated pain group was generated in approximately 5–15 s, while the control rats felt the thermal pain in approximately 30 s or an even longer time. Von Frey filaments were used to stimulate the rat right hind plantar, and our results indicated that the number of grams of mechanical force was smaller in endometriosis-related pain rats than in the control rats, which suggested that the pain threshold of the endometriosis-related pain group was lower than that of the sham control group. Rats with pain exhibit less motion and exploration due to anxiety and are susceptible to irritability in the open environment.22 The open field test revealed that the overall distance of the tracking was higher in control rats than in endometriosis-related pain rats, and the number of entries into the central area was reduced. Therefore, the open field test further verified the reduction in the pain threshold caused by central sensitization in endometriosis rats. Compared with other experiments alone, our behavioral assessments are more persuasive and precise for evaluating pain models combined with the objective indicators of the hot plate test and Von Frey test and the subjective assessment of the open field test.
To further understand the mechanism of central sensitization, we studied the relationship between endometriosis-associated pain and brain functional activity using Rs-fMRI after thoroughly evaluating the pain model in animals. ReHo analysis (a fMRI analysis method) has recently been used to study pain and a functional disorder.39 Abnormal ReHo signals may provide a clue that is likely associated with a noncompensatory reaction or unbalanced local functionality of the entire brain network. Bao et al40 found that ReHo signals were reduced in the insula, middle cingulate cortex, and supplementary motor area in patients with abdominal pain. Using fMRI, Sawsan41 also found that the signal of brain functional activity in the insula and medial prefrontal cortex was enhanced in patients with endometriosis-related pain, and the excitatory neurotransmitter in this area was significantly increased. In our study, we found differences in spontaneous BOLD signals in several brain regions between endometriosis-related pain rats and sham control rats. In pain rats, the ReHo values were significantly increased in the anterior cingulate cortex, thalamic area, and CA1 field of the hippocampus. The reductions in the ReHo values in BM and M1 suggest a different reorganization of resting-state brain activities between endometriosis-related pain and sham control rats. This result may indicate an association between endometriosis-related pain and changes in brain activity. The increased synchronization of neuronal activities is possibly due to compensatory cortical reorganization in these brain regions with higher ReHo values.42 The results of this experiment suggest that the neurons are overactivated, pain-related excitatory neurotransmitters are metabolized vigorously, oxygen consumption is increased, and nerve conduction is enhanced in these brain areas with higher ReHo signals (anterior cingulate cortex, thalamus and hippocampus), resulting from the pain afferent from the local endometriosis lesions. The anterior cingulate cortex, thalamus and hippocampus are important components of pain networks in which there is a closed relationship with pain perception and memory; hence, they may be the key areas of central sensitization.
Central sensitization is the amplification and memory of pain and is associated with abnormal conduction of multiple pathways and plasticity of neurons.43 When the body experiences nociceptive stimuli, such as persistent pain, TRPV1 is activated and intracellular Ca2+ influx leads to the release of large quantities of excitatory neurotransmitter glutamine and the continuous increase in NMDAR, which mediates neuronal apoptosis through a series of complex biological processes. TRPV1 is involved in synaptic plasticity, neurotransmitter transmission, neuroinflammatory mediator release and other activities of neurons and glial cells.44,45 NMDAR is closely related to pain perception and pain memory.16,46 In our previous study, we found overexpression of TRPV1 in ectopic endometrial lesions.47 Our study also found that the expression of TRPV1 and NMDAR was strongly positive by qRT-PCR and immunohistochemistry in the higher ReHo signal brain areas of the anterior cingulate cortex, thalamus and hippocampus identified by Rs-fMRI scanning. In addition, the number of apoptotic neurons and Nissl bodies was increased in these brain areas as shown by Nissl staining. The partial neuronal cells were deformed, and the volume of these cells around the apoptotic cells was extended compensatorily in pain rats compared with that in control rats. The number of normal neurons in the pain group was significantly lower than that in the control group. Our result is similar to the results of Tian48 and Lee.16 In fact, the Nissl body, located in the cytoplasm (also called the granular endoplasmic reticulum), can produce protein. The number of Nissl bodies was increased, and the brain function activity was more vigorous. The hyperexpression of TRPV1 and NMDAR reflected that the functional activity was higher in these brain regions, which may be a manifestation of neuronal plasticity.
In future experimental studies, we will continue to pay attention to the subsequent reorganization and plasticity, changes in neurotransmitters, related genes and protein expression in these functional brain areas. The relevant brain regions will be dissected to explore the relationship between gene levels and the corresponding functional proteins in the pain mechanism of central sensitization. We will further explore new ideas for the nonhormonal treatment of endometriosis-associated pain from the perspective of molecular biology.
Through this study, the central sensitization mechanism of endometriosis-associated pain can be further elucidated. Endometriosis pain can result in abnormal activation in diverse brain regions and changes in neuronal function or the number or quality in these regions. Interventions in these brain regions could encompass targeted treatment for endometriosis-associated pain to alleviate the pain and improve patient quality of life.
Limitations
The results of this study are based on animal models; thus, the findings cannot be directly applied to human studies, and the number of animal models is insufficient. The sample size needs to be expanded or more methods need to be adopted to provide additional experimental evidence of preclinical research for nonhormonal treatment of endometriosis-related pain.
Acknowledgments
We would like to thank the funded by a grant from the National Natural Science Foundation of China (grant number 81471440), the National Key Research and Development Program of China (grant number 2017YFC1001200), and the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (grant number CAMS-2017-I2M-1-002). We would also like to thank Professor Dalong Guo for offering the fMRI data analysis.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors reported no conflicts of interest.
References
1. Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13(4):395–404. doi:10.1093/humupd/dmm010
2. Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci. 2008;1127:92–100. doi:10.1196/nyas.2008.1127.issue-1
3. Berkley KJ, Dmitrieva N, Curtis KS, et al. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci U S A. 2004;101(30):11094–11098. doi:10.1073/pnas.0403663101
4. Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018;51:53–67. doi:10.1016/j.bpobgyn.2018.01.014
5. Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14–24. doi:10.1016/j.ejogrb.2016.06.017
6. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346. doi:10.1093/humupd/dmq050
7. Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Annu Rev Physiol. 2011;73:163–182. doi:10.1146/annurev-physiol-012110-142158
8. Martinez B, Canser E, Gredilla E, et al. Management of patients with chronic pelvic pain associated with endometriosis refractory to conventional treatment. Pain Pract. 2013;13(1):53–58. doi:10.1111/papr.2012.13.issue-1
9. Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587–1589. doi:10.1126/science.1111445
10. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl)):S2–15. doi:10.1016/j.pain.2010.09.030
11. Porpora MG, Vinci V, De Vito C, et al. The role of magnetic resonance imaging-diffusion tensor imaging in predicting pain related to endometriosis: a preliminary study. J Minim Invasive Gynecol. 2018;25(4):661–669. doi:10.1016/j.jmig.2017.10.033
12. Hamilton JP, Chen G, Thomason ME, et al. Investigating neural primacy in major depressive disorder: multivariate granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry. 2011;16(7):763–772. doi:10.1038/mp.2010.46
13. Ogawa S, Lee TM, Kay AR, et al. Brain magnetic-resonance-imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87(24):9868–9872. doi:10.1073/pnas.87.24.9868
14. Apkarian AV, Bushnell MC, Treede R-D, et al. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi:10.1016/j.ejpain.2004.11.001
15. Pang R, Guo R, Wu X, et al. Altered regional homogeneity in chronic insomnia disorder with or without cognitive impairment. AJNR Am J Neuroradiol. 2018;39(4):742–747. doi:10.3174/ajnr.A5587
16. Lee J, Saloman JL, Weiland G, et al. Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. Pain. 2012;153(7):1514–1524. doi:10.1016/j.pain.2012.04.015
17. Kobayashi H, Yamada Y, Morioka S, et al. Mechanism of pain generation for endometriosis-associated pelvic pain. Arch Gynecol Obstet. 2014;289(1):13–21. doi:10.1007/s00404-013-3049-8
18. McAllister SL, McGinty KA, Resuehr D, et al. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1–3):255–264. doi:10.1016/j.pain.2009.09.022
19. Berkley KJ, Cason A, Jacobs H, et al. Vaginal hyperalgesia in a rat model of endometriosis. Neurosci Lett. 2001;306(3):185–188. doi:10.1016/S0304-3940(01)01906-1
20. Karl T, Pabst R, von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp Toxicol Pathol. 2003;55(1):69–83. doi:10.1078/0940-2993-00301
21. Bannon AW, Malmberg AB. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci. 2007. Chapter 8: p. Unit 8.9. doi:10.1002/0471142301.ns0809s41.
22. Li T, Mamillapalli R, Ding S, et al. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol Reprod. 2018;99(2):349–359. doi:10.1093/biolre/ioy035
23. Barrot M. Tests and models of nociception and pain in rodents. Neuroscience. 2012;211:39–50. doi:10.1016/j.neuroscience.2011.12.041
24. Gregory NS, Harris AL, Robinson CR, et al. An overview of animal models of pain: disease models and outcome measures. J Pain. 2013;14(11):1255–1269. doi:10.1016/j.jpain.2013.06.008
25. Aldad TS, Gan G, Gao X-B, et al. Fetal radiofrequency radiation exposure from 800-1900 MHz-rated cellular telephones affects neurodevelopment and behavior in mice. Sci Rep. 2012;2:312. doi:10.1038/srep00312
26. Hsu L-M, Liang X, Gu H, et al. Constituents and functional implications of the rat default mode network. Proc Natl Acad Sci U S A. 2016;113(31):E4541–7. doi:10.1073/pnas.1601485113
27. Khazipov R, Zaynutdinova D, Ogievetsky E, et al. Atlas of the postnatal rat brain in stereotaxic coordinates. Front Neuroanat. 2015;9:161. doi:10.3389/fnana.2015.00161
28. Yang P, Wang Z, Zhang Z, et al. The extended application of the rat brain in stereotaxic coordinates in rats of various body weight. J Neurosci Methods. 2018;307:60–69. doi:10.1016/j.jneumeth.2018.06.026
29. Aldad TS, Rahmani N, Leranth C, et al. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil Steril. 2011;96(1):175–179. doi:10.1016/j.fertnstert.2011.04.010
30. Huang G, Zhou J, Zhan W, et al. The neuroprotective effects of intraperitoneal injection of hydrogen in rabbits with cardiac arrest. Resuscitation. 2013;84(5):690–695. doi:10.1016/j.resuscitation.2012.10.018
31. Grummer R. Animal models in endometriosis research. Hum Reprod Update. 2006;12(5):641–649. doi:10.1093/humupd/dml026
32. Simis M, Reidler, JS, Duarte Macea, D, et al. Investigation of central nervous system dysfunction in chronic pelvic pain using magnetic resonance spectroscopy and noninvasive brain stimulation. Pain Pract. 2015;15(5):423–432. doi:10.1111/papr.12202
33. Yano M, Matsuda, A, Natsume, T, et al. Pain-related behavior and brain activation in cynomolgus macaques with naturally occurring endometriosis. Hum Reprod. 2018.
34. Aredo JV, Heyrana K, Karp B, et al. Relating chronic pelvic pain and endometriosis to signs of sensitization and myofascial pain and dysfunction. Semin Reprod Med. 2017;35(1):88–97. doi:10.1055/s-0036-1597123
35. Morotti M, Vincent K, Becker CM. Mechanisms of pain in endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:8–13. doi:10.1016/j.ejogrb.2016.07.497
36. Knazovicky D, Helgeson ES, Case B, et al. Replicate effects and test-retest reliability of quantitative sensory threshold testing in dogs with and without chronic pain. Vet Anaesth Analg. 2017;44(3):615–624. doi:10.1016/j.vaa.2016.08.008
37. Zhao T, Liu X, Zhen X, et al. Levo-tetrahydropalmatine retards the growth of ectopic endometrial implants and alleviates generalized hyperalgesia in experimentally induced endometriosis in rats. Reprod Sci. 2011;18(1):28–45. doi:10.1177/1933719110381928
38. Refsgaard LK, Hoffmann-Petersen J, Sahlholt M, et al. Modelling affective pain in mice: effects of inflammatory hypersensitivity on place escape/avoidance behaviour, anxiety and hedonic state. J Neurosci Methods. 2016;262:85–92. doi:10.1016/j.jneumeth.2016.01.019
39. Zhang B, Wang F, Dong H-M, et al. Surface-based regional homogeneity in bipolar disorder: a resting-state fMRI study. Psychiatry Res. 2019;278:199–204. doi:10.1016/j.psychres.2019.05.045
40. Bao C-H, Liu P, Liu H-R, et al. Differences in regional homogeneity between patients with Crohnʼs disease with and without abdominal pain revealed by resting-state functional magnetic resonance imaging. Pain. 2016;157(5):1037–1044. doi:10.1097/j.pain.0000000000000479
41. As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1–13. doi:10.1016/j.jpain.2015.09.008
42. Hong J-Y, Kilpatrick LA, Labus J, et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci. 2013;33(29):11994–12002. doi:10.1523/JNEUROSCI.5733-12.2013
43. Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16(11):1258–1266. doi:10.1038/nm.2231
44. Song C, Liu P, Zhao Q, et al. TRPV1 channel contributes to remifentanil-induced postoperative hyperalgesia via regulation of NMDA receptor trafficking in dorsal root ganglion. J Pain Res. 2019;12:667–677. doi:10.2147/JPR
45. Hong S-I, Nguyen T-L, Ma S-X, et al. TRPV1 modulates morphine-induced conditioned place preference via p38 MAPK in the nucleus accumbens. Behav Brain Res. 2017;334:26–33. doi:10.1016/j.bbr.2017.07.017
46. Zhao M-G, Toyoda H, Lee Y-S, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47(6):859–872. doi:10.1016/j.neuron.2005.08.014
47. Song N, Leng JH, Lang JH. Expression of transient receptor potentials of vanilloid subtype 1 and pain in endometriosis. Zhonghua Fu Chan Ke Za Zhi. 2012;47(5):333–336.
48. Tian GH, Tao -S-S, Chen M-T, et al. Electroacupuncture treatment alleviates central poststroke pain by inhibiting brain neuronal apoptosis and aberrant astrocyte activation. Neural Plast. 2016;2016:1437148. doi:10.1155/2016/1437148
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.