Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Central nervous system toxicity of metallic nanoparticles
Authors Feng X, Chen A, Zhang Y, Wang J, Shao L , Wei L
Received 28 November 2014
Accepted for publication 29 April 2015
Published 3 July 2015 Volume 2015:10(1) Pages 4321—4340
DOI https://doi.org/10.2147/IJN.S78308
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Xiaoli Feng,1 Aijie Chen,1 Yanli Zhang,1 Jianfeng Wang,2 Longquan Shao,1 Limin Wei2
1Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China; 2School and Hospital of Stomatology, Wenzhou Medical University, Wenzhou, People’s Republic of China
Abstract: Nanomaterials (NMs) are increasingly used for the therapy, diagnosis, and monitoring of disease- or drug-induced mechanisms in the human biological system. In view of their small size, after certain modifications, NMs have the capacity to bypass or cross the blood–brain barrier. Nanotechnology is particularly advantageous in the field of neurology. Examples may include the utilization of nanoparticle (NP)-based drug carriers to readily cross the blood–brain barrier to treat central nervous system (CNS) diseases, nanoscaffolds for axonal regeneration, nanoelectromechanical systems in neurological operations, and NPs in molecular imaging and CNS imaging. However, NPs can also be potentially hazardous to the CNS in terms of nanoneurotoxicity via several possible mechanisms, such as oxidative stress, autophagy, and lysosome dysfunction, and the activation of certain signaling pathways. In this review, we discuss the dual effect of NMs on the CNS and the mechanisms involved. The limitations of the current research are also discussed.
Keywords: nanomaterials, neurotoxicity, blood–brain barrier, autophagy, ROS
Introduction
Nanomaterials (NMs) are materials that have structural components smaller than 1 μm in at least one dimension.1 Nanotechnology, a new and exciting field, has offered scientists the opportunity to control matter on small dimensions, and has opened many possibilities for creating new products, such as new clothing materials, packaging materials, and lightweight building materials. Nanotechnology has also led to revolutionary advances in medical applications. Numerous medical products contain NMs, including sunscreens in which NMs are used to provide UV protection while remaining transparent on the skin, and drugs in which NMs are used as the means of drug delivery to the target organ, such as Doxil and Abraxane.
NMs are composed of nanoparticles (NPs), which are particles with at least one dimension smaller than 1 μm and potentially as small as atomic and molecular length scales (~0.2 nm).2,3 Because of their unique size and high surface area, after surface modifications, many NPs are capable of bypassing or crossing the blood–brain barrier (BBB). Several animal studies have provided direct evidence that NPs could reach and accumulate in the brain parenchyma, including the striatum and hippocampus. Kao et al4 observed the translocation of zinc oxide (ZnO) NPs into the brain following in vivo nasal administration in a Sprague Dawley rat model. Transferrin-containing gold NPs can enter and accumulate in the brain parenchyma after systemic administration in mice through a receptor-mediated transcytosis pathway.5 Furthermore, the accumulation of 100 nm PS-COOH NPs within the lysosomes of the in vitro BBB was observed by Raghnaill et al.6 Iron oxide (IO) NPs, ZnO NPs, and titanium dioxide (TiO2) NPs have also been found to be translocated to the brain in various animal models.7–9
Because of their distinguishing features, NPs are increasingly used in diagnosing, monitoring, and treating diseases in the human central nervous system (CNS). For example, NPs are used as a drug carrier to help drugs cross the BBB in CNS disease treatment, in nanoscaffolds for axonal regeneration, in nanoelectromechanical systems (NEMSs) in neurological operations, and in molecular imaging and CNS imaging. With the increasing applications of nanotechnology in human life, the likelihood of people coming in contact with NMs has increased considerably. The biological safety evaluation of NMs is becoming increasingly important, particularly in terms of neurotoxicity. NPs can be accepted by the body in multiple ways, such as through the digestive tract, olfactory nerve, or sensory nerve. Following uptake, NPs can be translocated into the secondary target organs, including the brain, potentially damaging the CNS and inducing neurotoxic effects.10 Recent studies suggested that NPs are able to induce considerable neurotoxicity in animals. For example, ZnO NPs were found to attenuate learning and memory ability in rats.8 Hu et al11 observed the accumulation of TiO2 NPs in the mouse hippocampus after intragastric administration, and this accumulation led to hippocampal apoptosis and impairment in spatial recognition memory. Although many NPs tested to date have dose-dependent neurotoxic effects, some NPs have protective functions toward the CNS. For example, fullerenol was found to play a neuroprotective role, preventing hydrogen peroxide and cumene hydroperoxide-induced damage in rat hippocampal slices.12 An in vitro study indicated that carboxyfullerenes (a malonic acid C60 derivative) could eliminate both the superoxide anion and hydrogen peroxide (H2O2), and were effective inhibitors of lipid peroxidation. Carboxyfullerenes exhibited strong neuroprotective effects against excitotoxic, apoptotic, and metabolic insults in cortical cell cultures. They were also able to protect mesencephalic dopaminergic neurons from both 1-methyl-4-phenylpyridinium injury and 6-hydroxydopamine-induced degeneration.13 This review focuses mainly on the dual effects of NPs on the CNS and the possible mechanism behind these effects.
Main sources of NP entry into human CNS
Nanostrategies for disease diagnosis and therapy
Nanotechnology has created the possibility of cell-specific drug delivery using NPs. Because of their special properties, NPs can be used to improve drug delivery. Table 1 provides a list of nanodrugs approved by the US Food and Drug Administration (FDA).
The early diagnosis and successful treatment of brain diseases are of great significance. Rapidly emerging nanotechnologies have provided many novel methods for diagnosing and treating CNS diseases, such as nanodrug delivery systems targeting CNS diseases, nanoscaffolds used for axonal regeneration, NEMSs in neurology operations, and NPs in molecular imaging and CNS imaging. The nanotechnology applications for targeting CNS diseases and nonCNS diseases enable the BBB to be overcome through systemic administration. In the following sections, the applications of nanotechnology targeting the CNS will be described in detail.
NP-based drug delivery systems
A variety of formidable obstacles hinder the entry of drugs into the CNS. These obstacles include physiological barriers, such as the BBB and the blood–cerebrospinal fluid barrier, as well as various efflux transporter proteins. In current clinical applications, ~98% of chemical drugs and 100% of protein peptide drugs cannot directly enter the brain. Because of their small size and functionalization potential, NPs can be utilized as carriers for drug delivery through the human physiological barriers. For example, the solubility of a poorly soluble drug can be improved by utilizing a drug delivery system containing both hydrophilic and hydrophobic elements. NPs have significant advantages over other currently available drug delivery systems for the delivery of drugs across the BBB. Intensive research and development is being conducted to exploit the biological effects of engineered NMs for therapeutic applications in the CNS.
The NMs used in drug-delivery systems can be divided into two types: soft NMs (lipid- or polymer-based) and hard NMs (often metals, metal oxides, or ceramics). Soft NMs have been widely applied in clinics for over a decade, and many new formulations are currently undergoing clinical trials.14 In contrast, hard NPs are not being widely implemented in clinical settings despite their substantial scientific improvements.15 Some current NP-based strategies for brain-targeted drug delivery are listed in Table 2. The preparation of a flake–shell microcapsule is illustrated in Figure 1.
  | Table 2 Examples of NP-based carriers for brain-targeted drug delivery |
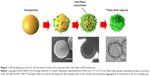  | Figure 1 Self-templating process for the formation of silica microcapsules with a thin flake–shell architecture. |
Nanoscaffolds for neuroregeneration: application of NPs in neural tissue engineering (neural tissue regeneration)
A large proportion of the world’s population suffer from traumatic injuries to the brain and spinal cord, which lead to permanent disability and thus a significant global health burden. However, only a few effective treatments are available because the CNS is refractory to axonal regeneration and relatively inaccessible to most drugs.16 Nanoscaffolding is a medical process used for tissue regrowth, including limbs and organs. A nanoscaffold is a three-dimensional nanoscale structure composed of polymer fibers that allows damaged cell adherence and helps rebuild missing tissue. As the tissue grows, the scaffold is absorbed into the body and disappears completely.17 Advances in nanotechnology have increased the potential for axonal regeneration after a brain injury. As a new class of NMs, both single-walled carbon nanotubes (CNTs) and multiwalled CNTs (MWCNTs) have been increasingly used as scaffolds for neuronal growth and, more recently, for neural stem cell growth and differentiation.18 CNT scaffolds have the ability to promote neuron growth, differentiation, and survival, and allow the modification of their electrophysiological properties.19 For example, the mammalian visual system model is a self-assembling peptide nanofiber scaffold designed by Ellis-Behnke et al20 and has the ability to regenerate axons at the site of an acute injury and knit the brain tissue together. Furthermore, a poly(l-lactic acid)/ammonium persulfate doped-polypyrrole composite fibrous scaffold with moderate conductivity produced by Yu et al21 can promote neuronal growth and neurite outgrowth in an intensity- and time-dependent manner when stimulated with a moderate current intensity for an appropriate duration.
Nanoelectromechanical systems
NEMSs and microelectromechanical systems (MEMSs) are devices manufactured at the nanoscale and microscale, respectively. Such systems are typically composed of a variety of refined miniaturized electrical and mechanical apparatuses, such as actuators, beams, sensors, pumps, resonators, and motors.22 NEMSs and MEMSs are capable of monitoring mechanical and physiological variables, such as intracranial pressure, cerebrospinal fluid (CSF) pulsatility, weight load, and strain. Lee et al23 have investigated the feasibility of incorporating MEMS technology to develop microactuators that essentially clear away calcium deposits and cell debris from the catheter surface.
NPs in molecular imaging and CNS imaging
NPs can be conjugated with specific cell markers and antibodies, enabling the selective targeting of pathological tissues. In fact, it has already been demonstrated that NP-enhanced imaging of the CNS at the subcellular level significantly increases the precision of localizing the intracranial neoplasms.
As a widely used imaging technology, magnetic resonance imaging (MRI) is playing an increasingly important role in the oncological imaging of the CNS. For in vivo brain imaging, the selection of contrast agents for MRI detection is critical because MRI is relatively insensitive to exogenous agents. Manganese-enhanced MRI (MEMRI), which uses manganese as a T1 contrast neural tracer for MRI, is increasingly of interest for use in animal studies. Because high doses of manganese induce cellular toxicity, a critical issue for the eventual extension of MEMRI for use in humans is minimizing the dose required. Magnetic NPs (MNPs) have been proven to be outstanding contrast agents for MRI and excellent carriers for drug delivery.24 Superparamagnetic IO NPs (SPIONPs) and paramagnetic contrast agents, such as gadolinium or perfluorocarbons, have also been considered major players in tracking single or clustered labeled cells in target tissues.25 Positron emission tomography (PET) is another functional brain imaging tomography technique. Because of its high sensitivity in detecting molecular tracers, PET has become a powerful imaging modality in the diagnosis, therapy, monitoring, and imaging of the gene expression of diverse reporter genes and probes. For instance, labeled lipid NPs have been designed to study the in vivo distribution of liposome-encapsulated hemoglobin using PET.26 Stockhammer et al27 utilized PET to target intratumorally injected MNPs in patients with glioblastoma.
Possible routes responsible for the transport of NPs to the CNS are 1) translocation to the lymphatic and circulatory system; 2) activity of the mucociliary escalator and subsequent oral exposure; and 3) translocation via nerves, such as the olfactory or trigeminal nerve.28 Among these potential pathways, the sensory nerve pathway, which initiates from the regions of the brain and terminates in the nasal cavity at the olfactory epithelium or respiratory epithelium, is the major route for the brain delivery of certain NPs following intranasal administration.29,30
The distribution of NPs in the brain is related to the mechanism of uptake to the brain, eg, via the olfactory nerve to the amygdala, via the trigeminal nerve to the trigeminal nucleus and thalamus, or via the BBB to different parts of the brain. After uptake, NPs can permeate into other parts of the brain by simple diffusion and then travel in the direction of the convection of the interstitial fluid and the CSF flow.31
Potential hazards of NPs to the CNS: nanoneurotoxicity
For the past several years, nanotechnology has benefited humankind in scores of ways. With its increasing use in many aspects of human life, the potential hazards of NMs have begun to garner greater attention.
The effect of the NPs on biosystems is determined by various factors. Particle size, shape, and ability to interact with the surrounding tissue greatly influence the toxicity of NMs. NPs may cause phagocytic cells to “overload”, leading to a defensive fever and reduced body immunity. NPs may be unable to degrade, and they may accumulate in the organs owing to their slow degradation rates. Because of their high surface area, NPs exposed to the organism will affect enzymes and proteins and may disturb biological processes in the body. Unlike other damaged tissues, neurons cannot be repaired via regeneration.32 Moreover, most drugs cannot pass the BBB into the brain, which makes it difficult to mediate damage to neurons. Thus, a comprehensive and systemic evaluation of nanoneurotoxic effects is particularly important for reducing or preventing CNS damage.
An increasing amount of research on the toxicity of NPs is being carried out by researchers all over the world. Hirst et al33 concluded that the biodistribution and potential applications of CeO2 NPs are related to their hazard assessment. Silver NPs have been found to be transferred to the blood and distributed throughout the brain after subcutaneous injection in rats. Furthermore, swollen astrocytes, BBB destruction, and neuronal degeneration have also been observed in the same test rats.34 Ma et al35 reported that a high-dose administration of nanoparticulate anatase TiO2 could lead to brain injury and change some glias into filamentous shapes and others into inflammatory cells. The concentration of nanoparticulate anatase TiO2 in the brain was highly correlated with the nanoparticulate anatase TiO2 dosages used. In a study by Wang et al36 the fatty degeneration of the hippocampus and brain lesions were observed in ICR mice after the injection of a nanosized TiO2 suspension via the gastrointestinal tract. Boyes et al37 suggested that the NPs could be 1) located in the luminal wall of brain microvascular endothelial cells or the pericytes, 2) located in the astrocyte foot processes, or 3) maintained by the basement membrane.
In “Relationship between NMs and CNS dysfunction” section, we will review the possible relationship between NMs and CNS diseases and the possible mechanisms of nanoneurotoxicity.
Relationship between NMs and CNS dysfunction
An increasing number of people are reported to suffer from neurodegenerative diseases, such as Alzheimer disease (AD), Parkinson disease (PD), Huntington disease (HD), and primary brain tumors. The exact cause of these diseases is still unknown, but we suspect that NPs from both nanodrugs and environmental NP pollutants may play a role in aggravating these diseases.38 Although there is a lack of evidence regarding the relationship between NPs and CNS diseases in human studies to date, animal research suggests possible links. For example, Mirsattari et al39 reported a case of a 71-year-old man who developed myoclonic status epilepticus and went into a coma after daily ingestion of colloidal silver for 4 months. High levels of silver were found in the plasma, erythrocytes, and CSF of the patient. Unfortunately, plasmapheresis was not effective in this patient, and he persisted in vegetative state until his death 5.5 months later. Silver products may induce irreversible neurologic toxicity, which is related to poor outcomes. Another study in mice indicated that TiO2 NPs accumulate in the mouse hippocampus by intragastric administration, leading to hippocampal apoptosis and impairment in spatial recognition.11 In vivo studies have shown that the neurotoxic effects of TiO2 NPs on mice are likely caused by the disturbed homeostasis of trace elements, enzymes, and neurotransmitter systems.40 In addition, silver NPs have been found to have the ability to induce damage to the BBB and astrocytes and cause neuronal degeneration in rats after subcutaneous injection.34
The impact of NPs on offspring is another concern because the CNS exhibits considerable plasticity in the early stage of life and could be significantly influenced by environmental invasions encountered during the fetal period.41 Unfortunately, studies have revealed that NPs entering the maternal body during gestation may harm fetal development through direct or indirect mechanisms. Even small amounts of particles in the maternal blood can translocate to the fetal compartment. Neurodevelopmental studies have observed that both male and female offspring exhibit differential phenotypes after prenatal insults by NPs. Furthermore, Hougaard et al42 found that the inhalation of TiO2 NPs led to long-term lung inflammation in mating adult mice, and their offspring exhibited abnormal neurobehavior as a result of the gestational exposure to NPs.
One study showed that TiO2 NPs administered subcutaneously to pregnant mice translocated to the offspring and affected the genital system of the male offspring by reducing their daily sperm production. The cranial nervous system was also affected, and an increased number of apoptosis cells was observed in the olfactory bulb of the brain.43 A subsequent study by the same author showed that the mice fetuses that were exposed to TiO2 NPs prenatally exhibited an increased level of dopamine and its metabolites in the prefrontal cortex and neostriatum. This result highlights the possibility that prenatal exposure to TiO2 NPs might affect the development of the central dopaminergic system in mouse offspring.44 Shimizu et al45 also found that maternal exposure to TiO2 NPs influenced the expression of functional genes related to brain development in mice. Similar in vivo studies showed that prenatal exposure to TiO2 NPs affects the synaptic plasticity in the offspring’s hippocampal dentate gyrus area, revealing the hidden toxicity of TiO2 NPs in CNS. These findings indicate that the developmental brain is vulnerable to TiO2 NP exposure, especially during the lactation period.46 In addition, Mohammadipour et al47 observed reduced hippocampal cell proliferation and decreased spatial memory, inhibitory memory, and learning ability in rat offspring after the maternal administration of TiO2 NPs during pregnancy. In that study, rats were exposed to 100 mg/kg body weight of TiO2 NPs every day, which is equivalent to ~6,000 mg/60 kg of body weight for humans, far lower than the LD50 (dose required to kill 50% of a population of test animals) of TiO2 for rats (12,000 mg/kg body weight) per the 1969 guidelines of the World Health Organization (WHO). Although this dose was less than the dose reported by the WHO, it still had side effects on the hippocampus. Therefore, long-term applications of NPs, such as the use of TiO2 NPs in pregnant women in dentistry, must be considered with great caution. NMs may harm not only the mother but also the offspring. This type of effect is serious and may last for a lifetime.
Possible mechanisms for NP neurotoxicity: nanoneurotoxicity
Because of their small size, NPs can easily reach the brain and are taken up by the brain cells, such as neurons and glia. The well-accepted mechanisms of NP uptake by cells include pinocytosis, endocytosis dependent on caveolae and lipid raft composition, clathrin-dependent endocytosis and phagocytosis.48 The intracellular sites of localization of NPs vary depending on the cell type and the uptake mechanism. NPs may enter the endothelial cell monolayer and accumulate along the endolysosomal pathway, affecting the normal morphology and function of the BBB itself. The intercellular interactions between NPs and biological molecules, such as DNA, proteins, and lipids, could have many consequences, including oxidative stress, conformational alteration, increased membrane permeability, mutations, signaling pathway activation, ionic exchange disorder, enzyme failure, and new protein epitope exposure.49 After interacting with NPs, various mediators released from the microglia and astrocyte induce inflammation, apoptosis, and oxidative stress in the brain. Because nervous tissue regeneration is limited, most of the nerve damage is irreversible.28 From this perspective, it is critical to investigate the mechanisms of nanoneurotoxicity, and an increasing number of studies are being conducted to discover the underlying mechanisms. The interaction between NPs and the biosystem is also determined by the type of NPs. Soft NPs, such as liposomes, and hard NPs, such as metal oxides, disturb the biosystem through different mechanisms. The possible mechanisms discussed in here are the most commonly proposed in the current literature and include oxidative stress, immune system dysfunction, and autophagy dysfunction.
Oxidative stress
Reactive oxygen species (ROS) are potentially highly reactive molecules that contain an oxygen atom; examples include the superoxide radical (O2−) and H2O2.50 Under physiological conditions, ROS are present in every cell, being produced by the mitochondrial and cytoplasmic oxidation processes. A low to moderate ROS concentration is essential to maintain normal physiological processes. However, an excessive production of ROS induced by oxidative stress would be extremely damaging. The CNS is particularly susceptible to oxidative stress because of its high oxygen consumption, its weak antioxidative ability, and the terminal-differentiation feature of neurons.51 Oxidative stress has been established as a crucial contributor to acute-CNS-injury-related neurodegenerative diseases, such as DNA damage, which can impair the viability of cerebral cells.52 An increasing number of studies show that ROS play a critical role in neurodegenerative diseases, such as AD and PD. Moreover, ROS contribute to neuronal apoptosis, which is a key mechanism in brain development.53 Furthermore, ROS can regulate neuronal ion channels, kinases, and transcription factors.54,55 ROS generated from the NADPH oxidase 2 (Nox2) system also contribute to long-term memory dysfunction.56
ROS are capable of inducing membrane damage that affects the structure and function of inner proteins, lipid denaturation, and structural alteration of DNA. DNA oxidation is of the utmost concern because it can lead to mutations and alterations in gene expression. Due to the lack of DNA repair enzymes, mitochondrial DNA appears to be more susceptible to ROS-induced mutations. Protein oxidation may cause the aggregation of insoluble protein, which serves as the molecular basis of some diseases, especially neurodegenerative pathologies.57 There is an increasing amount of evidence indicating that NPs can cause cytotoxicity due to their large surface area, which is capable of inducing ROS, a key factor that generates cellular stress and disease pathogenesis.58 However, the impact of NP-induced ROS on the CNS is unclear. Several types of NMs, such as quantum dots and metal-oxide NPs, have been shown to cause the overexpression of intracellular ROS.59 NPs can provoke oxidative stress through multiple interactions, as reviewed in what follows (Figure 2).
One thing that needs to be stated is that since there is a lack of direct evidence between ROS generated by NMs and CNS toxicity, the possible ROS mechanism discussed here is hypothesized by us based on current related studies in other organs.
Interaction of NPs with mitochondria
The mitochondria are the cellular energy house and play a significant role in all physiological and pathological processes via their ability to generate ATP. NPs of various sizes and shapes can accumulate in the mitochondria after interacting with cells. This accumulation can directly damage the mitochondrial membrane and interrupt the respiratory chain function. As the latter is one of the main sites for ROS generation, any perturbation of this electron transport chain can result in increased ROS production.60–62 Oxidative stress and mitochondrial abnormalities have been reported to play a role in numerous neuropsychiatric disorders.63,64 TiO2 NPs were shown to stimulate brain microglia to generate ROS and disturb the mitochondrial energy production.65 Cellular alterations caused by TiO2 NPs, such as changes in the redox state, insufficient defense of antioxidant enzymes, and mitochondrial depolarization, may lead to cell apoptosis by activating apoptotic-signaling pathways.66 Siddiqui et al41 observed a decrease in the mitochondrial membrane potential followed by an increase in the Bax/Bcl-2 ratio, suggesting a mediator role of the mitochondria in ZnO NP-induced apoptosis. Shimizu et al45 found that prenatal exposure to anatase TiO2 NPs altered the expression of genes related to brain development, cell death, response to oxidative stress, and mitochondria in the brain during the prenatal period and the expression of genes related to inflammation and neurotransmitters in the later stage. Furthermore, Huerta-Garcia et al67 found that TiO2 NPs caused intense oxidative stress in both rat and human glial cells (C6 and U373 lines) by regulating changes in the cellular redox state and lipid peroxidation associated with a rise in the expression of glutathione peroxidase, catalase (CAT), and superoxide dismutase 2 (SOD2). TiO2 NPs also induced morphology changes, mitochondrial damage, and increased mitochondrial membrane potential, indicating a cytotoxic effect of TiO2 NPs on glial cells. In a subsequent study, the mitochondrial depolarization observed in C6 and U373 cells led to caspase-3 activation, chromatin condensation, and apoptosis.68
The generation of mitochondrial ROS also plays a key role in several redox-associated signaling processes, such as the aging clock. Many studies have shown that mitochondrial integrity declines with increasing age.69
NP interaction with cytoplasmic enzymes that act in maintaining cellular redox potential
After cellular uptake, NPs can directly interact with the cytoplasmic enzymes responsible for maintaining cellular redox potential, such as antioxidant enzymes and NADPH. Free radicals are constantly generated in living cells and eliminated by antioxidant enzymes, which serve as the front line of defense against ROS in animal and plant cells. These enzymes include SOD, glutathione peroxidase, glutathione reductase, and CATs. Any damage to the antioxidant enzymes will lead to an increased ROS level in cells. Few studies have demonstrated that NPs can reduce the activity of antioxidant enzyme. Freyre-Fonseca et al66 found that cellular changes induced by TiO2 NPs included alteration in the redox state, reduction of the antioxidant enzymatic defense and mitochondrial depolarization, and activation of the apoptotic pathways. In addition, ceria NPs have been shown to be capable of altering brain oxidative stress indicators and antioxidant enzymes after intravenous administration in rats, demonstrating the ability of metal oxide NPs to produce neurotoxicity.70 In their recent study, Hardas et al71 found that cytoprotective Phase II antioxidant activities were inhibited in the rat hippocampus after a single systemic infusion of 30 nm nanoceria. A subsequent study found that both the SOD activity and CAT activity in the liver cells were downregulated after oral administration of purified and functionalized MWCNTs suspended in water.72 Reduced SOD and CAT activity have been observed in the digestive glands of mussels in Mytilus galloprovincialis after exposure to 10 μg/L Cu in the form of CuO NPs and Cu2+ for 15 days.73 Furthermore, nanoiron induces a decrease in the SOD level and increases the malondialdehyde level in a dose-dependent manner in the medaka embryo. In adult medakas, a damaged antioxidative balance occurs during the early exposure period, as indicated by monitoring the hepatic and cerebral SOD and reduced glutathione.74
ROS were once thought to originate nearly entirely from the mitochondrial metabolism. However, increasing evidence has demonstrated that cellular enzymes, such as NADPH oxidase, are also important sources of ROS in humans.75 Wilhelmi et al76 demonstrated that ZnO NPs are able to trigger p47phox NADPH oxidase-regulated ROS formation in macrophages and induce rapid nuclear condensation, DNA fragmentation, and the formation of hypodiploid DNA nuclei and apoptotic bodies in the murine macrophage RAW 264.7 cell line.
Interestingly, in Culcasi et al77 after micromolar doses of nano-CeO2 were applied to human fibroblasts, the membrane NADPH oxidase activation occurred. The cytotoxic effects may be caused by the activation of both the mitochondrial and Nox2- and Nox4-dependent NADPH oxidase complexes. These studies also suggest that the specific inhibition of ROS-producing enzymes may be a new approach promising clinical efficacy in treating ROS-related disease (eg, cardiovascular and neurodegenerative diseases), especially because the current widespread use of antioxidant supplementation has proven largely ineffective in treating diseases caused by a surplus of ROS.57
Although oxidative stress caused by NMs was thought to be the main mechanism for NMs toxicity in different cell models, the exact role of ROS formation and degradation dysfunction in CNS toxicity caused by NMs is still unclear.
Activation of intracellular signaling cascades induces ROS formation
Interactions between NPs and cell surface receptors activate intracellular signaling pathways that induce ROS generation.78 ROS produced by NPs in the cellular environment lead to the activation of stress-dependent signaling pathways, such as mitogen-activated protein kinase (MAPK) or IκB kinase, which ultimately alters the gene expression of the antioxidant response element by activating transcription factors, such as AP-1, NF-kB, or Nrf2, and finally leads to ROS overproduction. Jeong et al79 showed that ROS activated the extracellular signal-regulated kinase (ERK) of MAPK pathways. The upregulation of Egr-1 expression was observed following ZnO NPs stimulation. This upregulation can be inhibited by an ERK inhibitor. In addition, antioxidative N-acetyl-cysteine strongly inhibited the level of Egr-1 and phosphorylated ERK expression in ZnO NP-treated cells. Exposure of primary cultured astrocytes cells to ZnO NPs leads to the phosphorylation of c-Jun N-terminal kinase (JNK), ERK, and p38 MAPK. Moreover, JNK inhibitors (SP600125) significantly reduce ZnO NP-induced cleaved PARP and cleaved caspase-3 expression, whereas ERK inhibitors (U0126) and p38 MAPK inhibitors (SB203580) do not, indicating the involvement of the JNK signaling pathway in ZnO NP-induced apoptosis in primary astrocytes.80
Long-term exposure to TiO2 NPs could lead to the disturbance of both mitotic progression and chromosome segregation via the ERK signaling and production of ROS. The proposed direct action of AgNPs on membrane receptors and subsequent ROS generation and the activation of signaling pathways involving various protein kinases were recently reviewed.81 Although these NPs have different chemical patterns and differentials activities, their ability to activate pathways, nuclear factors, and specific genetic programs are directly or indirectly determined by the level of ROS production outside or inside the cell.82
The surface of NPs and their specific chemical compounds make it easy to adsorb specific biological compounds, especially proteins, resulting in a form of dynamic entities called a protein corona.83 This corona plays a crucial role in the uptake and may also trigger the activation of specific signaling pathways in relation to ROS generation.
NP coating and core degradation in the lysosomal environment
Once taken up by the cell, NPs can be internalized into the lysosome and can disrupt the phospholipid bilayers, resulting in an increased lysosomal membrane permeabilization (LMP). Digestive enzymes (eg, caspases, calpains, and cathepsins) are ultimately released into the cytosol through the highly permeable membrane. Oxidative stress caused by NPs can also damage the lysosome membrane, which further amplifies the stress signal through these digestive enzyme regulators, leading to DNA fragmentation and apoptosis. Furthermore, high intracellular calcium levels caused by NPs may also serve as an alternative mechanism for the activation of these mechanisms.84 Domenech et al85 found that IO MNPs are capable of inducing lysosome LMP in cells. Yang et al86 used both acridine orange staining and transmission electron microscopy (TEM) images to confirm that the accumulation of carbon nanohorns (CNHs) in the lysosomes induced LMP. The immunofluorescence data also indicated that after LMP, the lysosomal components, such as cathepsin D, were released to the cytosol, resulting in further oxidative stress and lysosomal disruption through an amplifying loop process.
The degradation of the NM coating and core in the lysosomal environment can also directly induce ROS production via any by-products created or the presence of a bare (reactive) NP surface in an acidic environment. During the degradation process of iron NPs, cytoplasmic iron levels are increased by the release of iron from late endosomes and lysosomes through the divalent metal transport channel. The reactive species generated by the release of ferric iron can greatly damage cell functionality. Fullerenic NPs often accumulate in the lysosomes after internalization in cells. The acidic environment of lysosomes has a significant but temporary effect on the size distribution of fullerenic NPs, which means that the aggregated C60(C(COOH)2)2 NPs may be easily dispersed into single molecules or smaller aggregates. These single molecules or smaller aggregates may insert themselves into the lysosomal membranes.87
Until now, few studies on degradation of the NM coating and core in the lysosomal environment were carried out on brain cells. More study is needed to test this hypothesis in brain cell models in the future.
Direct ROS-generating NPs
Direct ROS-generating NPs can release redox-active metal ions (eg, Fe2+) that participate in ROS-generating reactions (eg, Fenton reactions). Fenton and Fenton-like reactions comprise reactions between metal ions or metal complexes and hydrogen peroxide. During this process, ROS are believed to be generated when the ROS-generating NPs react with cellular macromolecules and induce oxidative stress.88 The toxicity of metallic NPs, including Zn, Ti, Si, Fe, and Ce, has been characterized by increased ROS generation and oxidative stress and apoptosis.89–91 ROS production mediated via NP–cell interactions involves various mechanisms, including immune cell activation, mitochondrial respiration, and the NADPH oxidase system. In addition to ROS, NPs also arbitrate reactive nitrogen species-mediated injury. Given their chemical reactivity, metal-based NPs induce the oxidative disruption of cellular macromolecules, such as proteins, lipids, and DNA, via Fenton-type and Haber Weiss-type reactions.92
The most important effect of NPs on mammalian cells is oxidative stress. The enhanced generation of ROS affects the mitochondrial respiratory chain and increases the amount of unfolded and misfolded proteins in the endoplasmic reticulum, inducing endoplasmic reticulum stress and unfolded protein response. Both these types of cellular damage lead to further ROS generation, DNA damage, and activation of signaling, resulting in the activation of various cell type-specific pathways for inflammation, apoptosis, or necrotic death.93 As most of the manufactured NPs have ability to generate ROS in cells, it is critical to maintain a balance between their favorable functions and adverse effects.
Immune mechanism
Once NPs enter the CNS, they immediately encounter a complex environment of resident microglial immune cells and neurons. Microglia, accounting for ~20% of the glial cells in the brain, are a type of glial cells, which are the resident innate immune cells in the brain and the predominant regulators of neuroinflammation.94 As macrophages located in the CNS, the microglial cells are the most likely to respond when NPs enter the brain. Once activated, they are able to change in form and function. Choi et al95 observed the microglial uptake of silica-based NPs (SiNPs) using TEM and fluorescence confocal microscopy. The results demonstrated that low levels of SiNPs are able to alter microglial function by changing the expression of proinflammatory genes and cytokine release. The surrounding neurons were also affected. Nerve pathology and clinical studies have shown that the activation of microglia plays a critical role in neurodegenerative diseases, such as PD, multiple sclerosis, and AD. The appropriate response of microglia to various stimuli is thought to be necessary to maintain normal brain function; however, excessive and chronic activation can lead to neurotoxicity, such as the initiation and/or amplification of neuronal disruption. Excessively activated or uncontrollable microglia can cause nerve toxicity by inducing proinflammatory factors, such as interleukin-1β, tumor necrosis factor (TNF)-α, prostaglandin E2, and interferon-γ. Gualtieri et al96 found that various nanosized organic carbon particles induced cytotoxic and proinflammatory effects on the in vitro systems with A549 (epithelial cells) and BEAS-2B (bronchial cells) cells by releasing proinflammatory interleukins 8 and 6. Both TiO2 NPs and hydroxyapatite (HAP) NPs can induce marked inducible nitric oxide synthase expression, resulting in nitric oxide release from the microglia after direct exposure to NPs.97 The expression levels of monocyte chemotactic protein 1 (MCP-1) and monocyte chemotactic protein 1α (MCP-1α) are also upregulated by TiO2 NPs and HAP NPs, suggesting that the NPs stimulate microglial activation and subsequently lead to the release of proinflammatory factors, which ultimately cause PC12 cell dysfunction and cytotoxicity.97
Apoptosis and autophagy dysfunction mechanism
Autophagy, a highly regulated cellular process for eliminating long-lived proteins and damaged organelle components via the lysosomal mechanism, has received considerable attention. Increasing evidence shows that autophagy alterations may lie at the root of neurodegenerative diseases, such as AD, PD, and HD.98,99 Moreover, neurodegeneration and an elevated level of protein aggregation have been detected in mice with neuron-specific knockdown of key autophagy proteins (ie, Atg5, Atg7, and beclin-1).100 The cell autophagy process can be induced by various forms of stress, such as interactions with various types of NPs. Thus, NPs are considered a novel class of autophagy inducers.101 A growing number of reports have confirmed that NPs can induce and modulate autophagy in different cells. The autophagy process is induced by uncoated and oleic acid-coated IO NPs, SiNPs, TiO2 NPs, and polymeric NPs in human brain-derived endothelial cells. This process has been found to be correlated with an increase in DNA strand breaks and defensive mechanisms.102 In addition, Roy et al103 found that ZnO NP exposure induced oxidative stress in macrophages and initiated autophagy and apoptosis simultaneously, suggesting that autophagosomes may be a cellular defense mechanism against oxidative stress. Moreover, autophagy induced by ZnO NPs followed a PI3K/mTOR/Akt signaling cascade.
According to the current literature, NPs induce autophagy in two ways: NP-mediated ROS-dependent autophagy and NP-mediated lysosome-dependent autophagy. Among these mechanisms, ROS-modulated autophagy is thought to play a key role in NP-induced autophagy.
As noted above, the interactions between NPs and intracellular molecules can lead to the overgeneration of ROS, which can damage the entire cytoplasmic environment, including organelles, proteins, and lipids. These alterations in the cytoplasm activate the process of autophagy in an attempt to manage this stressful situation by the removal of the corresponding components, typically the mitochondria. As mitochondria are the major source of ROS and are also sensitive to ROS-induced damage, they are seen as the main regulators of ROS-induced autophagy.104 Interruption of the autophagy process by genetic knockdown results in the accumulation of dysfunctional mitochondria as well as the accumulation of ROS. Lee et al62 provided a potential relationship between NM-induced autophagy blockade and oxidative stress. NM-induced autophagy blockade may also be involved in NM-associated inflammation, as there is evidence that autophagy plays a key role in negatively regulating the NOD-like receptor containing pyrin domain 3 (NLRP3) inflammasome.105
Because the majority of NPs enter cells through endocytosis, lysosomes are also frequently a target for their toxicity. NPs can cause lysosomal dysfunction by alkalization of the lysosomal lumen, NP overload, or cytoskeleton disruption.106,107 This dysfunction can indirectly upregulate autophagy as a mechanism for the cell to compensate for insufficient degradative capacity.108 The signaling link between the lysosomal sensing of stress and autophagy is affected by the transcription factor EB (TFEB), a main regulator of the coordinated lysosomal expression and regulation network. TFEB is a molecule proven to play an important role in cellular degradative processes. TFEB is able to reduce neurofibrillary tangle pathology, rescue behavioral and synaptic deficits, and effectively compensate for neurodegeneration in the rTg4510 mouse model of autopathy.109 Based on these studies, Stern et al107 proposed that autophagy and lysosomal dysfunction may be emerging mechanisms of NM toxicity (ie, NP-mediated lysosome-dependent autophagy). Autophagy can play dual roles in cell survival after interaction with NPs: autophagy can play a cytoprotective role or a cytotoxic role under different conditions. It has gradually been revealed that autophagy and apoptosis are interconnected at several levels. Dysfunctions of autophagy, including both overstimulation and understimulation, lead to cell apoptosis or necrosis.110 Cell death is a dynamic phenomenon, and multiple cell death types are often observed within the same cell.111 Autophagy and apoptosis are often simultaneously detected upon treatment with NMs (Figure 3 shows the mechanistic steps of NP-induced autophagy). Freyre-Fonseca et al66 reported that cellular changes induced by TiO2 NPs, including alteration in the redox state, reduced antioxidant enzymatic defense ability, and mitochondrial depolarization, can activate apoptotic pathways. Márquez-Ramírez et al112 found that glial cells exposed to TiO2 NPs exhibited apoptotic death. Furthermore, TiO2 NPs at a concentration above 1 μg/mL have been shown to cause cell death in human astrocytes-like astrocytoma U87 cells.113 Different concentrations of TiO2 NPs decreased the cell viability of PC12 cells after different exposure times, leading to the intracellular accumulation of ROS and apoptosis. Similar toxic effects have also been found in brain microglia cells (BV2).114,115
Autophagy is the only cell process able to degrade large components; therefore, NPs may be processed by cells using autophagy (Figure 4 shows ZnO NPs triggering autophagy). NP-mediated autophagy may be an adaptive cellular response facilitating the degradation and clearance of NPs, but may also cause harmful cellular dysfunction. The physicochemical and biochemical properties of the NPs, such as their surface properties, charge, size, and adsorption of biological components, are important factors mediating their interactions with cells, including autophagy.58,116 We will review how the properties of NPs affect their interaction with different cells in “Key factors influencing the neurotoxicity of NMs”.
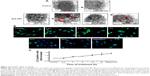  | Figure 4 ZnO NPs trigger autophagy. |
Activated cell-signaling pathways
NPs can destroy or travel through the cellular membrane into the cell. Concomitant with ROS, NPs can change the configuration and conformation of biological macromolecules (including membrane lipid, protein, and nucleus), resulting in their corresponding dysfunction. Certain cell-signaling pathways can be activated upon NP interactions, such as the proinflammatory cytokines referred to as MAPK and nuclear factor-κB cascades. The proposed direct action of AgNPs on membrane receptors and the subsequent ROS generation and activation of signaling pathways involving various protein kinases were recently reviewed by Marano et al.81 Wang et al80 demonstrated that ZnO NP-induced oxidative stress activates the JNK signaling pathway, compromising the integrity of cellular membranes and leading to the apoptosis of astrocytes. Furthermore, exposure to TiO2 NPs through intranasal administration has been shown to lead to the activation of HO-1 through the p38-Nrf-2 signaling pathway in mice, subsequently leading to ROS accumulation; the oxidation of lipids, proteins, and DNA; and brain injury.117
Key factors influencing the neurotoxicity of NMs
The physicochemical and biochemical properties of NPs are important factors mediating their interactions with cells, including cell-stress reactions and the biological characteristics of particular cells. Moreover, dosage, mode of administration, and exposure time are key factors that affect the severity of toxicity induced by any given NP.118 Many data show that cellular uptake pathways, subcellular processing mechanisms, and cytotoxicity are determined by particle surface properties. Here, we will review the key factors affecting the toxicity of NPs in the CNS.
Size
The toxic behaviors of NPs differ from those of their bulk counterparts.119 NP-induced cytotoxicity against tissue cells is strongly influenced by the size of the particle; thus, the particle size should be taken into careful consideration in the design of NPs for biomedical uses. Smaller AgNPs (10 nm size) have been found to be more toxic than larger AgNPs (50 and 100 nm) in inducing necrotic cell death in PC12 cells.120 Sharma et al121 showed that small NPs induced more pronounced BBB breakdown, brain edema formation and neuronal injuries, glial fibrillary acidic protein upregulation, and myelin vesiculation in young animals compared to controls. These studies suggest that the effect of NPs on neurotoxicity is inversely related to the size of the NPs. Serious attention should be paid to the physicochemical characteristics of NMs when assessing their neurological effects.
Shape
Compared to the numerous studies addressing the effect of various types of NPs on cell functions, very few reports address the effect of NP shape on cellular function. Different shapes of the same NM can potentially induce different cellular responses by nonspecific uptake into cells.122 Using spherical and rod-shaped mesoporous silica NPs (MSNPs), Meng et al123 showed that the aspect ratio differences in the MSNPs could be sensed by HeLa and A549 cells through an active sensing mechanism. The rate and abundance of MSNP uptake by the macropinocytosis process were determined by the aspect ratio of the rod-shaped MSNPs. Particle morphology has been thought to play a crucial role in both cellular interactions with NPs and the systemic distribution of NPs.
HAP NPs are widely used as a bone-filling material in the maxilla and mandible. Needle-shaped and short rod-like HAP NPs have been found to induce greater cellular injury in primary cultured rat osteoblasts than spherical and long rod-like HAP NPs by inhibiting growth and activating apoptosis.124 Thus, the shape of the HAP NPs may have a significant effect on the apoptotic level, which may be attributed to the mechanical stresses on the cell surface caused by the particle–cell interactions.116,125
Mesoporous materials are beginning to be utilized in biomedical applications due to their high Brunauer, Emmett and Teller surface area and large pore volume. Long rod-shaped MSNPs induce greater cell apoptosis than short rod-shaped and spherical MSNPs with similar diameters, suggesting that the shape of NPs influences the uptake of NPs by cells via cell endocytosis.126
Charge
Recent studies have shown that the charge of NPs greatly affects their cellular uptake in vitro.127 Because of the electrostatic attraction between the cell membrane and positively charged NPs, particles with positively charged surfaces are more easily adsorbed onto the cell surface and accumulated inside the cells than their negatively charged or neutral counterparts.128 For small NPs (2 nm), for example, a positive charge can interfere with the cell membrane potentiality, leading to Ca2+ influx into cells and inhibiting cell proliferation.129
Relative to anionic and neutral NPs, cationic NPs have been shown to have higher ROS-associated toxicity.130 Negatively charged NPs do not easily adhere to cell surface because of the repulsion with the negatively charged cell membrane and thus were once considered to be nontoxic. However, a recent study showed that even negative NPs can induce a small amount of toxicity in their interactions with membrane proteins or lipid raft structuring transmembrane proteins or channels.131 Membrane potential and membrane integrity are considered to play crucial roles in proper signaling in neuronal cells. A disturbance in membrane potential and membrane integrity could be induced by charged NPs, which may have a remarkable effect on the normal function of the CNS and thus seems to be one of the causes of neurotoxicity.
Surface modification
For clinical applications, NPs must have the ability to disperse in water and other hydrophilic media. Surface modification can be used to prevent the aggregation of NPs and improve the compatibility of NPs with solid matrices or biological environments. Surface modification is considered one of the most effective ways of controlling and modulating cellular interactions with NPs and thus their biological consequences. Surface modification can rescue or even reverse the biological consequences of NPs. For example, sulfur atom affinity is widely used in the surface modification of noble metal NPs. Thiol-capped noble metal NPs are synthesized by reducing noble metal ions in the presence of thiol. While the reduced noble metal atoms aggregate to form NPs, thiol molecules attach on the surface of NPs to passivate the growing surface and minimize aggregation.132 Compared to unmodified fullerene NPs, which can generate ROS to damage cells, surface-modified fullerene NPs can selectively enter oxidized cerebral microvessel endothelial cells and protect these cells by attenuating ROS-induced cellular damage, such as F-actin depolymerization.133,134 Nanocrystalline TiO2 is widely applied in biomedical ceramics and implant-related biomaterials that are translocated into the murine brain, induce pathological lesions to the hippocampus, and alter neurochemical levels in the brain after intranasal instillation.135,136 However, the neurotoxicity of nanocrystalline TiO2 could be affected by surface modification. For example, compared with hydrophobic NPs, hydrophilic nanocrystalline TiO2 NPs exhibit a greater influence on the 5-HT and 5-HIAA monoamine levels in the subbrain regions.137 Sousa et al138 modified the 15 nm gold NP surface by coating with functional polyelectrolyte layers and human serum albumin and found that the modified gold NPs were able to pass the BBB and accumulate in neuronal or glial cells in specific regions close to the brain stem.
These studies show that the physicochemical modification of NMs can influence or even reverse their mammalian neurotoxicity. Other important factors include the “protein corona”, chemical characteristics, metal impurities, and degradation properties.
Aggregation and dispersion
The aggregation and formation of a protein corona in the extracellular environment alters the size, shape, and surface properties of particles, giving them a “biological identity” that is different from their initial “synthetic identity”. Interestingly, the subsequent interaction between NPs and cells is not determined by their “synthetic identity” but by their “biological identity”.139 Relative to organic NPs, inorganic NPs are more susceptible to aggregation in biological environments. Sadhukha et al140 examined the effect of aggregation on NP-induced magnetic hyperthermia. Well-dispersed SPIONPs were found to induce less apoptosis than submicron size aggregates, with the latter causing rapid membrane damage and acute cell death. Their results suggested that the aggregation state of SPIONPs largely determined the extent and mechanism of hyperthermia-induced cell death.
In a complex nonlinear paradox, lower doses of NPs can sometimes increase, rather than decrease, the toxicity of a given source material as a function of the surface properties of the particles themselves. That is, higher concentrations or doses can favor NP agglomeration in the absence of surface modifications to prevent spontaneous agglomeration due to close physical interactions of highly reactive NPs in concentrated colloidal liquids.141 The resultant nanoaggregates as a whole can then hide or quench the originally hyperreactive surfaces of their smaller NP “parts”.142 Consequently, the specific larger agglomerated NP form is less toxic at a higher concentration than a lower dose of smaller but well-dispersed NPs, eg, NPs of PbS or copper.143
Limitations of the current research and future prospects
The mechanism of NM nanotoxicity is still not clear; for example, many studies attribute it to oxidative stress or ROS, but some NMs, such as CNT fullerene, are very good free radical scavengers and antioxidants. Therefore, the mechanism of nanotoxicity is still an important topic for future research.
A lack of common standards is one of the limitations of the current studies of NP cytotoxicity. Different cell lines, exposure times, and colorimetric assays are used in different studies, which make it difficult or even impossible to compare cytotoxic effect among these results. No single method meets all conditions for obtaining information on NP cytotoxicity. Because different NPs exhibit different biological responses, a combination of assays is often suggested to study the mechanisms underlying cytotoxicity. A set of sensitive cell lines would also be required to accurately identify NM cytotoxicity.144
Another limitation is that the NP dose used in in vitro studies often exceeds a realistic relevant dose. Moreover, these in vitro models cannot accurately reproduce various cellular interactions present in the body. Therefore, it is difficult to predict the toxicological behavior of NPs in living organisms by in vitro models alone.145,146
There are still many unanswered questions concerning nanoneurotoxicity. For instance, after bypassing the BBB, where do NPs go? How do they leave the brain? The degradation of NP coatings and NP cores inside the cell environment is an important issue that deserves serious consideration when designing safe and functional NMs. No results have been reported on this issue to date.
When NPs enter the body, the surface properties of NPs may change by adsorbing proteins from biological fluids (such as blood, plasma, or interstitial fluid), leading to a distinct new epitope, for example, protein corona exposure in the biological microenvironment. Furthermore, serum protein binding to the NPs can alter the surface charge and accelerate the cellular uptake of NPs through receptor-regulated endocytosis. However, so far, studies addressing the cell surface protein corona interactions with NPs remain limited.
Data regarding the distribution of metal-based NPs in the brain parenchyma are scarce, including data regarding the disruption of the BBB and adverse brain alterations caused by metal-based NPs. The effects of the persistence of poorly soluble metal-based NPs are of particular concern, and few studies have considered the effect of NPs on the CNS.147
Summary
The rapid pace of breakthroughs in nanotechnology promises great improvements in the diagnosis and treatment of brain disease. However, the risk of danger from these breakthroughs increases with their potential benefit. The scientists and engineers who conduct nanoscale research have the responsibility of considering the public safety aspects of their research and acting to protect society when necessary. When considering the clinical use of a nano-based product, doctors should evaluate the risk/benefit ratio and minimize the dose prescribed.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31070857, 50973045, 81400557) and the Project on the Integration of Industry, Education and Research of Guangdong Province, People’s Republic of China (2012B091000147).
Disclosure
The authors report no conflicts of interest in this work.
References
Buzea C, Pacheco Blandino II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):17–71. | ||
Bleeker EAJ, de Jong WH, Geertsma RE, et al. Considerations on the EU definition of a nanomaterial: science to support policy making. Regul Toxicol Pharmacol. 2013;65(1):119–125. | ||
Kettiger H, Schipanski A, Wick P, Huwyler J. Engineered nanomaterial uptake and tissue distribution: from cell to organism. Int J Nanomedicine. 2013;8:3255–3269. | ||
Kao YY, Cheng TJ, Yang DM, Wang CT, Chiung YM, Liu PS. Demonstration of an olfactory bulb-brain translocation pathway for ZnO nanoparticles in rodent cells in vitro and in vivo. J Mol Neurosci. 2012;48(2):464–471. | ||
Wiley DT, Webster P, Gale A, Davis ME. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc Natl Acad Sci U S A. 2013;110(21):8662–8667. | ||
Raghnaill MN, Bramini M, Ye D, et al. Paracrine signalling of inflammatory cytokines from an in vitro blood brain barrier model upon exposure to polymeric nanoparticles. Analyst. 2014;139(5):923–930. | ||
Wu J, Ding TT, Sun J. Neurotoxic potential of iron oxide nanoparticles in the rat brain striatum and hippocampus. Neurotoxicology. 2013;34: 243–253. | ||
Han DD, Tian YT, Zhang T, Ren GG, Yang Z. Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats. Int J Nanomedicine. 2011;6:1453–1461. | ||
Chen JY, Dong X, Xin YY, Zhao MR. Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (Danio rerio) after a long-term exposure. Aquat Toxicol. 2011;101(3–4):493–499. | ||
Medina C, Santos-Martinez MJ, Radomski A, Corrigan OI, Radomski MW. Nanoparticles: pharmacological and toxicological significance. Br J Pharmacol. 2007;150(5):552–558. | ||
Hu RP, Zheng L, Zhang T, et al. Molecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticles. J Hazard Mater. 2011;191(1–3):32–40. | ||
Tsai MC, Chen YH, Chiang LY. Polyhydroxylated C60, fullerenol, a novel free-radical trapper, prevented hydrogen peroxide- and cumene hydroperoxide-elicited changes in rat hippocampus in-vitro. J Pharm Pharmacol. 1997;49(4):438–445. | ||
Dugan LL, Lovett EG, Quick KL, Lotharius J, Lin TT, O’Malley KL. Fullerene-based antioxidants and neurodegenerative disorders. Parkinsonism Relat Disord. 2001;7(3):243–246. | ||
Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine. 2012;7:49–60. | ||
Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol. 2012;85(1010):101–113. | ||
Boyce VS, Mendell LM. Neurotrophic factors in spinal cord injury. Handb Exp Pharmacol. 2014;220:443–460. | ||
Williamson A, Singh S, Fernekorn U, Schober A. The future of the patient-specific Body-on-a-chip. Lab Chip. 2013;13(18):3471–3480. | ||
Bokara KK, Kim JY, Lee YI, Yun K, Webster TJ, Lee JE. Biocompatability of carbon nanotubes with stem cells to treat CNS injuries. Anat Cell Biol. 2013;46(2):85–92. | ||
Fabbro A, Cellot G, Prato M, Ballerini L. Interfacing neurons with carbon nanotubes: (re)engineering neuronal signaling. Prog Brain Res. 2011;194:241–252. | ||
Ellis-Behnke RG, Liang YX, You SW, et al. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc Natl Acad Sci U S A. 2006;103(13): 5054–5059. | ||
Yu QZ, Xu SL, Zhang KH, Shan YM. Multi-porous electroactive poly(L-lactic acid)/polypyrrole composite micro/nano fibrous scaffolds promote neurite outgrowth in PC12 cells. Neural Regen Res. 2013; 8(1):31–38. | ||
Ekinci KL, Yakhot V, Rajauria S, Colosqui C, Karabacak DM. High-frequency nanofluidics: a universal formulation of the fluid dynamics of MEMS and NEMS. Lab Chip. 2010;10(22):3013–3025. | ||
Lee SA, Vasquez DJ, Bergsneider M, Judy JW. Magnetic microactuators for MEMS-enabled ventricular catheters for hydrocephalus. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2494–2497. | ||
Wang H, Mararenko A, Cao GX, et al. Multifunctional 1D magnetic and fluorescent nanoparticle chains for enhanced MRI, fluorescent cell imaging, and combined photothermal/chemotherapy. ACS Appl Mater Interfaces. 2014;6(17):15309–15317. | ||
Carvalho A, Martins MB, Corvo ML, Feio G. Enhanced contrast efficiency in MRI by PEGylated magnetoliposomes loaded with PEGylated SPION: effect of SPION coating and micro-environment. Mater Sci Eng C Mater Biol Appl. 2014;43:521–526. | ||
Murgia S, Falchi AM, Mano M, et al. Nanoparticles from lipid-based liquid crystals: emulsifier influence on morphology and cytotoxicity. J Phys Chem B. 2010;114(10):3518–3525. | ||
Stockhammer F, Misch M, Horn P, Koch A, Fonyuy N, Plotkin M. Association of F18-fluoro-ethyl-tyrosin uptake and 5-aminolevulinic acid-induced fluorescence in gliomas. Acta Neurochir (Wien). 2009;151(11):1377–1383. | ||
Geraets L, Oomen AG, Schroeter JD, Coleman VA, Cassee FR. Tissue distribution of inhaled micro- and nano-sized cerium oxide particles in rats: results from a 28-day exposure study. Toxicol Sci. 2012;127(2): 463–473. | ||
Kozlovskaya L, Abou-Kaoud M, Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. J Control Release. 2014;189(10):133–140. | ||
Shah L, Yadav S, Amiji M. Nanotechnology for CNS delivery of bio-therapeutic agents. Drug Deliv Transl Res. 2013;3(4):336–351. | ||
Wolak DJ, Thorne RG. Diffusion of macromolecules in the brain: implications for drug delivery. Mol Pharm. 2013;10(5):1492–1504. | ||
von Bohlen und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329(3):409–420. | ||
Hirst SM, Karakoti A, Singh S, et al. Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ Toxicol. 2013;28(2):107–118. | ||
Tang JL, Xiong L, Wang S, et al. Distribution, translocation and accumulation of silver nanoparticles in rats. J Nanosci Nanotechnol. 2009;9(8):4924–4932. | ||
Ma LL, Liu J, Li N, et al. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity. Biomaterials. 2010;31(1):99–105. | ||
Wang JX, Zhou GQ, Chen CY, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168(2):176–185. | ||
Boyes WK, Chen R, Chen CY, Yokel RA. The neurotoxic potential of engineered nanomaterials. Neurotoxicology. 2012;33(4):902–910. | ||
Win-Shwe TT, Fujimaki H. Nanoparticles and neurotoxicity. Int J Mol Sci. 2011;12(9):6267–6280. | ||
Mirsattari SM, Hammond RR, Sharpe MD, Leung FY, Young GB. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62(8):1408–1410. | ||
Hu RP, Gong XL, Duan YM, et al. Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials. 2010;31(31):8043–8050. | ||
Siddiqui MA, Alhadlaq HA, Ahmad J, Al-Khedhairy AA, Musarrat J, Ahamed M. Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS One. 2013;8(8):e69534. | ||
Hougaard KS, Jackson P, Jensen KA, et al. Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Part Fibre Toxicol. 2010;7:16. | ||
Takeda K, Suzuki KI, Ishihara A, et al. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J Health Sci. 2009;55(1):95–102. | ||
Takahashi Y, Mizuo K, Shinkai Y, Oshio S, Takeda K. Prenatal exposure to titanium dioxide nanoparticles increases dopamine levels in the prefrontal cortex and neostriatum of mice. J Toxicol Sci. 2010; 35(5):749–756. | ||
Shimizu M, Tainaka H, Oba T, Mizuo K, Umezawa M, Takeda K. Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Part Fibre Toxicol. 2009;6:20. | ||
Gao X, Yin S, Tang M, et al. Effects of developmental exposure to TiO2 nanoparticles on synaptic plasticity in hippocampal dentate gyrus area: an in vivo study in anesthetized rats. Biol Trace Elem Res. 2011; 143(3):1616–1628. | ||
Mohammadipour A, Fazel A, Haghir H, et al. Maternal exposure to titanium dioxide nanoparticles during pregnancy; impaired memory and decreased hippocampal cell proliferation in rat offspring. Environ Toxicol Pharmacol. 2014;37(2):617–625. | ||
Asharani PV, Hande MP, Valiyaveettil S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009;10:65. | ||
Xia T, Li N, Nel AE. Potential health impact of nanoparticles. Annu Rev Publ Health. 2009;30:137–150. | ||
Rang FJ, Boonstra J. Causes and consequences of age-related changes in DNA methylation: a role for ROS? Biology. 2014;3(2):403–425. | ||
Li J, O W, Li W, Jiang ZG, Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int J Mol Sci. 2013;14(12):24438–24475. | ||
Smith JA, Park S, Krause JS, Banik NL. Oxidative stress, DNA damage, and the telomeric complex as therapeutic targets in acute neurodegeneration. Neurochem Int. 2013;62(5):764–775. | ||
Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11(10):2481–2504. | ||
Thomas MP, Chartrand K, Reynolds A, Vitvitsky V, Banerjee R, Gendelman HE. Ion channel blockade attenuates aggregated alpha synuclein induction of microglial reactive oxygen species: relevance for the pathogenesis of Parkinson’s disease. J Neurochem. 2007;100(2):503–519. | ||
Schappi MG, Jaquet V, Belli DC, Krause KH. Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase. Semin Immunopathol. 2008;30(3):255–271. | ||
Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011;14(10):2013–2054. | ||
Brieger K, Schiavone S, Miller FJ, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659. | ||
Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. | ||
Hanley C, Thurber A, Hanna C, Punnoose A, Zhang JH, Wingett DG. The influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Res Lett. 2009;4(12): 1409–1420. | ||
Luo YH, Wu SB, Wei YH, et al. Cadmium-based quantum dot induced autophagy formation for cell survival via oxidative stress. Chem Res Toxicol. 2013;26(5):662–673. | ||
Yu KN, Yoon TJ, Minai-Tehrani A, et al. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol In Vitro. 2013;27(4):1187–1195. | ||
Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441(2): 523–540. | ||
Tobe EH. Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr Dis Treat. 2013;9:567–573. | ||
Marazziti D, Baroni S, Picchetti M, et al. Psychiatric disorders and mitochondrial dysfunctions. Eur Rev Med Pharmacol. 2012;16(2):270–275. | ||
Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40(14):4346–4352. | ||
Freyre-Fonseca V, Delgado-Buenrostro NL, Gutierrez-Cirlos EB, et al. Titanium dioxide nanoparticles impair lung mitochondrial function. Toxicol Lett. 2011;202(2):111–119. | ||
Huerta-Garcia E, Perez-Arizti JA, Marquez-Ramirez SG, et al. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radical Biol Med. 2014;73:84–94. | ||
Zanotto A, Braganhol E, Battastini AM, Moreira JC. Proteasome inhibitor MG132 induces selective apoptosis in glioblastoma cells through inhibition of PI3K/Akt and NFκB pathways, mitochondrial dysfunction, and activation of p38-JNK1/2 signaling. Invest New Drugs. 2012;30(6):2252–2262. | ||
Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. | ||
Hardas SS, Butterfield DA, Sultana R, et al. Brain distribution and toxicological evaluation of a systemically delivered engineered nanoscale ceria. Toxicol Sci. 2010;116(2):562–576. | ||
Hardas SS, Sultana R, Warrier G, et al. Rat hippocampal responses up to 90 days after a single nanoceria dose extends a hierarchical oxidative stress model for nanoparticle toxicity. Nanotoxicology. 2014;8: 155–166. | ||
Awasthi KK, John PJ, Awasthi A, Awasthi K. Multi walled carbon nano tubes induced hepatotoxicity in Swiss albino mice. Micron. 2013;44:359–364. | ||
Gomes T, Pereira CG, Cardoso C, Pinheiro JP, Cancio I, Bebianno MJ. Accumulation and toxicity of copper oxide nanoparticles in the digestive gland of Mytilus galloprovincialis. Aquat Toxicol. 2012;118:72–79. | ||
Li HC, Zhou QF, Wu Y, Fu JJ, Wang T, Jiang GB. Effects of waterborne nano-iron on medaka (Oryzias latipes): antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicol Environ Safe. 2009;72(3):684–692. | ||
Krause KH. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol. 2007;42(4):256–262. | ||
Wilhelmi V, Fischer U, Weighardt H, et al. Zinc oxide nanoparticles induce necrosis and apoptosis in macrophages in a p47phox-and Nrf2-independent manner. PLoS One. 2013;8(6):e65704. | ||
Culcasi M, Benameur L, Mercier A, et al. EPR spin trapping evaluation of ROS production in human fibroblasts exposed to cerium oxide nanoparticles: evidence for NADPH oxidase and mitochondrial stimulation. Chem Biol Interact. 2012;199(3):161–176. | ||
Soenen SJ, Rivera-Gil P, Montenegro JM, Parak WJ, De Smedt SC, Braeckmans K. Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today. 2011;6(5):446–465. | ||
Jeong SH, Kim HJ, Ryu HJ, et al. ZnO nanoparticles induce TNF-α expression via ROS-ERK-Egr-1 pathway in human keratinocytes. J Dermatol Sci. 2013;72(3):263–273. | ||
Wang J, Deng X, Zhang F, Chen D, Ding W. ZnO nanoparticle-induced oxidative stress triggers apoptosis by activating JNK signaling pathway in cultured primary astrocytes. Nanoscale Res Lett. 2014;9(1):117. | ||
Marano F, Hussain S, Rodrigues-Lima F, Baeza-Squiban A, Boland S. Nanoparticles: molecular targets and cell signalling. Arch Toxicol. 2011;85(7):733–741. | ||
Nel AE, Madler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–557. | ||
Mudunkotuwa IA, Grassian VH. Histidine adsorption on TiO2 nanoparticles: an integrated spectroscopic, thermodynamic, and molecular-based approach toward understanding nano-bio interactions. Langmuir. 2014;30(29):8751–8760. | ||
Wang ZY, von dem Bussche A, Kabadi PK, Kane AB, Hurt RH. Biological and environmental transformations of copper-based nanomaterials. ACS Nano. 2013;7(10):8715–8727. | ||
Domenech M, Marrero-Berrios I, Torres-Lugo M, Rinaldi C. Lysosomal membrane permeabilization by targeted magnetic nanoparticles in alternating magnetic fields. ACS Nano. 2013;7(6):5091–5101. | ||
Yang M, Zhang M, Tahara Y, et al. Lysosomal membrane permeabilization: carbon nanohorn-induced reactive oxygen species generation and toxicity by this neglected mechanism. Toxicol Appl Pharmacol. 2014;280(1):117–126. | ||
Li W, Zhao LN, Wei TT, Zhao YL, Chen CY. The inhibition of death receptor mediated apoptosis through lysosome stabilization following internalization of carboxyfullerene nanoparticles. Biomaterials. 2011;32(16):4030–4041. | ||
Nouri-Nigjeh E, Permentier HP, Bischoff R, Bruins AP. Lidocaine oxidation by electrogenerated reactive oxygen species in the light of oxidative drug metabolism. Anal Chem. 2010;82(18):7625–7633. | ||
Naqvi S, Samim M, Abdin MZ, et al. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomedicine. 2010;5:983–989. | ||
Park EJ, Choi J, Park YK, Park K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology. 2008; 245(1–2):90–100. | ||
Kim IS, Baek M, Choi SJ. Comparative cytotoxicity of Al2O3, CeO2, TiO2 and ZnO nanoparticles to human lung cells. J Nanosci Nanotechnol. 2010;10(5):3453–3458. | ||
Manke A, Wang LY, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. 2013;2013:942916. | ||
Wu YN, Chen DH, Shi XY, et al. Cancer-cell-specific cytotoxicity of non-oxidized iron elements in iron core-gold shell NPs. Nanomed Nanotechnol. 2011;7(4):420–427. | ||
Block ML, Elder A, Auten RL, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33(5):972–984. | ||
Choi J, Zheng QD, Katz HE, Guilarte TR. Silica-based nanoparticle uptake and cellular response by primary microglia. Environ Health Perspect. 2010;118(5):589–595. | ||
Gualtieri M, Capasso L, D’Anna A, Camatini M. Organic nanoparticles from different fuel blends: in vitro toxicity and inflammatory potential. J Appl Toxicol. 2014;34(11):1247–1255. | ||
Xue Y, Wu J, Sun J. Four types of inorganic nanoparticles stimulate the inflammatory reaction in brain microglia and damage neurons in vitro. Toxicol Lett. 2012;214(2):91–98. | ||
Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19(8):983–997. | ||
Cheung ZH, Ip NY. Autophagy deregulation in neurodegenerative diseases – recent advances and future perspectives. J Neurochem. 2011;118(3):317–325. | ||
Podila R, Brown JM. Toxicity of engineered nanomaterials: a physicochemical perspective. J Biochem Mol Toxicol. 2013;27(1):50–55. | ||
Zhang Y, Yu CG, Huang GY, Wang CL, Wen LP. Nano rare-earth oxides induced size-dependent vacuolization: an independent pathway from autophagy. Int J Nanomedicine. 2010;5:601–609. | ||
Kenzaoui BH, Bernasconi CC, Guney-Ayra S, Juillerat-Jeanneret L. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem J. 2012;441:813–821. | ||
Roy R, Singh SK, Chauhan KS, Das M, Tripathi A, Dwivedi PD. Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol Lett. 2014;227(1):29–40. | ||
Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36(1):30–38. | ||
Shi CS, Shenderov K, Huang NN, et al. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13(3): 255–263. | ||
Wang F, Bexiga MG, Anguissola S, et al. Time resolved study of cell death mechanisms induced by amine-modified polystyrene nanoparticles. Nanoscale. 2013;5(22):10868–10876. | ||
Stern ST, Adiseshaiah PP, Crist RM. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol. 2012;9:20. | ||
Neibert KD, Maysinger D. Mechanisms of cellular adaptation to quantum dots – the role of glutathione and transcription factor EB. Nanotoxicology. 2012;6(3):249–262. | ||
Polito VA, Li HM, Martini-Stoica H, et al. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol Med. 2014;6(9):1142–1160. | ||
Loos B, Engelbrecht AM, Lockshin RA, Klionsky DJ, Zakeri Z. The variability of autophagy and cell death susceptibility unanswered questions. Autophagy. 2013;9(9):1270–1285. | ||
Loos B, Engelbrecht AM. Cell death: a dynamic response concept. Autophagy. 2009;5(5):590–603. | ||
Marquez-Ramirez SG, Delgado-Buenrostro NL, Chirino YI, Iglesias GG, Lopez-Marure R. Titanium dioxide nanoparticles inhibit proliferation and induce morphological changes and apoptosis in glial cells. Toxicology. 2012;302(2–3):146–156. | ||
Lai JC, Lai MB, Jandhyam S, et al. Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts. Int J Nanomedicine. 2008;3(4):533–545. | ||
Liu SC, Xu LJ, Zhang T, Ren GG, Yang Z. Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells. Toxicology. 2010;267(1–3):172–177. | ||
Long TC, Tajuba J, Sama P, et al. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Perspect. 2007;115(11):1631–1637. | ||
Verma A, Stellacci F. Effect of surface properties on nanoparticle-cell interactions. Small. 2010;6(1):12–21. | ||
Ze Y, Zheng L, Zhao X, et al. Molecular mechanism of titanium dioxide nanoparticles-induced oxidative injury in the brain of mice. Chemosphere. 2013;92(9):1183–1189. | ||
Yokel RA, MacPhail RC. Engineered nanomaterials: exposures, hazards, and risk prevention. J Occup Med Toxicol. 2011;6:7. | ||
Kumar V, Kumari A, Guleria P, Yadav SK. Evaluating the toxicity of selected types of nanochemicals. Rev Environ Contam Toxicol. 2012;215:39–121. | ||
Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW. Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res A. 2012;100A(4):1033–1043. | ||
Sharma A, Muresanu DF, Patnaik R, Sharma HS. Size- and age-dependent neurotoxicity of engineered metal nanoparticles in rats. Mol Neurobiol. 2013;48(2):386–396. | ||
Gratton SE, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105(33):11613–11618. | ||
Meng H, Yang S, Li ZX, et al. Aspect ratio determines the quantity of mesoporous silica nanoparticle uptake by a small GTPase-dependent macropinocytosis mechanism. ACS Nano. 2011;5(6):4434–4447. | ||
Xu ZL, Liu CS, Wei J, Sun J. Effects of four types of hydroxyapatite nanoparticles with different nanocrystal morphologies and sizes on apoptosis in rat osteoblasts. J Appl Toxicol. 2012;32(6):429–435. | ||
Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm. 2008;5(2):316–327. | ||
Huang XL, Teng X, Chen D, Tang FQ, He JQ. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials. 2010;31(3):438–448. | ||
Asefa T, Tao ZM. Biocompatibility of mesoporous silica nanoparticles. Chem Res Toxicol. 2012;25(11):2265–2284. | ||
Thorek DL, Tsourkas A. Size, charge and concentration dependent uptake of iron oxide particles by non-phagocytic cells. Biomaterials. 2008;29(26):3583–3590. | ||
Arvizo RR, Miranda OR, Thompson MA, et al. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett. 2010;10(7):2543–2548. | ||
Tarn D, Ashley CE, Xue M, Carnes EC, Zink JI, Brinker CJ. Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Acc Chem Res. 2013;46(3):792–801. | ||
Lin JQ, Zhang HW, Chen Z, Zheng YG. Penetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano. 2010;4(9):5421–5429. | ||
Templeton AC, Wuelfing WP, Murray RW. Monolayer-protected cluster molecules. Acc Chem Res. 2000;33(1):27–36. | ||
Lao F, Chen L, Li W, et al. Fullerene nanoparticles selectively enter oxidation-damaged cerebral microvessel endothelial cells and inhibit JNK-related apoptosis. ACS Nano. 2009;3(11):3358–3368. | ||
Zhao F, Zhao Y, Liu Y, Chang XL, Chen CY, Zhao YL. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011; 7(10):1322–1337. | ||
Wang JX, Liu Y, Jiao F, et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. Toxicology. 2008;254(1–2):82–90. | ||
Wang JX, Chen CY, Liu Y, et al. Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol Lett. 2008;183(1–3):72–80. | ||
Zhang LL, Bai R, Li B, et al. Rutile TiO2 particles exert size and surface coating dependent retention and lesions on the murine brain. Toxicol Lett. 2011;207(1):73–81. | ||
Sousa F, Mandal S, Garrovo C, et al. Functionalized gold nanoparticles: a detailed in vivo multimodal microscopic brain distribution study. Nanoscale. 2010;2(12):2826–2834. | ||
Albanese A, Walkey CD, Olsen JB, Guo HB, Emili A, Chan WC. Secreted biomolecules alter the biological identity and cellular interactions of nanoparticles. ACS Nano. 2014;8(6):5515–5526. | ||
Sadhukha T, Wiedmann TS, Panyam J. Enhancing therapeutic efficacy through designed aggregation of nanoparticles. Biomaterials. 2014; 35(27):7860–7869. | ||
Sur I, Altunbek M, Kahraman M, Culha M. The influence of the surface chemistry of silver nanoparticles on cell death. Nanotechnology. 2012;23(37):375102. | ||
Mudunkotuwa IA, Grassian VH. The devil is in the details (or the surface): impact of surface structure and surface energetics on understanding the behavior of nanomaterials in the environment. J Environ Monit. 2011;13(5):1135–1144. | ||
Bell IR, Ives JA, Jonas WB. Nonlinear effects of nanoparticles: biological variability from hormetic doses, small particle sizes, and dynamic adaptive interactions. Dose Response. 2014;12(2):202–232. | ||
Kroll A, Dierker C, Rommel C, et al. Cytotoxicity screening of 23 engineered nanomaterials using a test matrix of ten cell lines and three different assays. Part Fibre Toxicol. 2011;8:9. | ||
Kim JS, Peters TM, O’Shaughnessy PT, Adamcakova-Dodd A, Thorne PS. Validation of an in vitro exposure system for toxicity assessment of air-delivered nanomaterials. Toxicol In Vitro. 2013;27(1):164–173. | ||
Adamcakova-Dodd A, Stebounova LV, Kim JS, et al. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol. 2014;11:15. | ||
Yokel R, Grulke E, MacPhail R. Metal-based nanoparticle interactions with the nervous system: the challenge of brain entry and the risk of retention in the organism. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(4):346–373. | ||
Ji QM, Guo CY, Yu XY, et al. Flake-shell capsules: adjustable inorganic structures. Small. 2012;8(15):2345–2349. | ||
Maes H, Rubio N, Garg AD, Agostinis P. Autophagy: shaping the tumor microenvironment and therapeutic response. Trends Mol Med. 2013;19(7):428–446. | ||
Lyon DY, Adams LK, Falkner JC, Alvarezt PJ. Antibacterial activity of fullerene water suspensions: effects of preparation method and particle size. Environ Sci Technol. 2006;40(14):4360–4366. | ||
Marchesan S, Da Ros T, Spalluto G, Balzarini J, Prato M. Anti-HIV properties of cationic fullerene derivatives. Bioorg Med Chem Lett. 2005;15(15):3615–3618. | ||
Ma HL, Liang XJ. Fullerenes as unique nanopharmaceuticals for disease treatment. Sci China Chem. 2010;53(11):2233–2340. | ||
Meng H, Xing GM, Blanco E, et al. Gadolinium metallofullerenol nanoparticles inhibit cancer metastasis through matrix metalloproteinase inhibition: imprisoning instead of poisoning cancer cells. Nanomedicine. 2012;8(2):136–146. | ||
Jin H, Chen WQ, Tang XW, et al. Polyhydroxylated C(60), fullerenols, as glutamate receptor antagonists and neuroprotective agents. J Neurosci Res. 2000;62(4):600–607. | ||
Huang SS, Tsai SK, Chih CL, et al. Neuroprotective effect of hexasulfobutylated C60 on rats subjected to focal cerebral ischemia. Free Radic Biol Med. 2001;30(6):643–649. | ||
Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev. 2014;71:2–14. | ||
Tosi G, Bortot B, Ruozi B, et al. Potential use of polymeric nanoparticles for drug delivery across the blood–brain barrier. Curr Med Chem. 2013;20(17):2212–2225. | ||
Kulkarni SA, Feng SS. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm Res. 2013;30(10):2512–2522. | ||
Gref R, Luck M, Quellec P, et al. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18(3–4):301–313. | ||
Zhang Y, Boado RJ, Pardridge WM. Marked enhancement in gene expression by targeting the human insulin receptor. J Gene Med. 2003;5(2):157–163. | ||
Chang J, Jallouli Y, Kroubi M, et al. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood–brain barrier. Int J Pharma. 2009;379(2):285–292. | ||
Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 1999;16(10):1564–1569. | ||
Olivier JC, Fenart L, Chauvet R, Pariat C, Cecchelli R, Couet W. Indirect evidence that drug brain targeting using polysorbate 80-coated polybutylcyanoacrylate nanoparticles is related to toxicity. Pharm Res. 1999;16(12):1836–1842. | ||
Alyautdin RN, Petrov VE, Langer K, Berthold A, Kharkevich DA, Kreuter J. Delivery of loperamide across the blood–brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Pharm Res. 1997;14(3):325–328. | ||
Lockman PR, Oyewumi MO, Koziara JM, Roder KE, Mumper RJ, Allen DD. Brain uptake of thiamine-coated nanoparticles. J Control Release. 2003;93(3):271–282. | ||
Salman HH, Gamazo C, Agueros M, Irache JM. Bioadhesive capacity and immunoadjuvant properties of thiamine-coated nanoparticles. Vaccine. 2007;25(48):8123–8132. | ||
Oyewumi MO, Liu SQ, Moscow JA, Mumper RJ. Specific association of thiamine-coated gadolinium nanoparticles with human breast cancer cells expressing thiamine transporters. Bioconjugate Chem. 2003;14(2):404–411. | ||
Grover A, Hirani A, Pathak Y, Sutariya V. Brain-targeted delivery of docetaxel by glutathione-coated nanoparticles for brain cancer. AAPS Pharm Sci Tech. 2014;15(6):1562–1568. | ||
Wei X, Zhan C, Chen X, Hou J, Xie C, Lu W. Retro-inverso isomer of angiopep-2: a stable d-peptide ligand inspires brain-targeted drug delivery. Mol Pharm. 2014;11(10):3261–3268. | ||
Ying X, Wang Y, Liang J, et al. Angiopep-conjugated electro-responsive hydrogel nanoparticles: therapeutic potential for epilepsy. Angew Chem Int Ed Engl. 2014;53(46):12436–12440. | ||
Dreis S, Rothweller F, Michaelis A, Cinatl J, Kreuter J, Langer K. Preparation, characterisation and maintenance of drug efficacy of doxorubicin-loaded human serum albumin (HSA) nanoparticles. Int J Pharm. 2007;341(1–2):207–214. | ||
Damascelli B, Cantu G, Mattavelli F, et al. Intraarterial chemotherapy with polyoxyethylated castor oil free paclitaxel, incorporated in albumin nanoparticles (ABI-007) – phase I study of patients with squamous cell carcinoma of the head and neck and anal canal: preliminary evidence of clinical activity. Cancer. 2001;92(10):2592–2602. | ||
Dadparvar M, Wagner S, Wien S, et al. HI 6 human serum albumin nanoparticles – development and transport over an in vitro blood–brain barrier model. Toxicol Lett. 2011;206(1):60–66. | ||
Chassepot A, Ball V. Human serum albumin and other proteins as templating agents for the synthesis of nanosized dopamine-eumelanin. J Colloid Interf Sci. 2014;414:97–102. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.