Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Caveolin-1 Promotes the Imbalance of Th17/Treg in Chronic Obstructive Pulmonary Disease by Regulating Hsp70 Expression
Authors Zhang T, Shang F, Ma Y, Xu Y, Sun W, Song H
Received 23 November 2022
Accepted for publication 27 March 2023
Published 12 April 2023 Volume 2023:18 Pages 565—574
DOI https://doi.org/10.2147/COPD.S398780
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Tongsong Zhang,1,* Fangfang Shang,2,* Yanhui Ma,3 Yanxia Xu,1 Weihong Sun,4 Haiping Song1
1Department of Oncology, Affiliated Qingdao Central Hospital of Qingdao University, Qingdao Cancer Hospital, Qingdao, Shandong, 266042, People’s Republic of China; 2Department of Pathology, No. 971 Hospital of People’s Liberation Army Navy, Qingdao, 266071, People’s Republic of China; 3Department of Clinical Laboratory, Biotherapy Center, Affiliated Qingdao Central Hospital, Qingdao University, Qingdao, 266042, People’s Republic of China; 4Affiliated Qingdao Central Hospital of Qingdao University, Qingdao Cancer Hospital, Qingdao, Shandong, 266042, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haiping Song; Yanxia Xu, Department of Oncology, Affiliated Qingdao Central Hospital of Qingdao University, Qingdao Cancer Hospital, 127 Siliu South Road, Qingdao, 266042, People’s Republic of China, Tel +86 532 8496 2202 ; +86 532 84962203, Fax +86 532-84963506, Email [email protected]; [email protected]
Objective: To investigate whether the expression of Hsp70 is associated with Cav-1 in promoting the imbalance of Th17/Treg cells in COPD.
Methods: The plasma Cav-1, Hsp70 expression were quantified by enzyme-linked immunosorbent assay (ELISA). The frequencies of circulating Th17, Treg cells and Th17/Treg ratio were determined by flow cytometry. Peripheral blood mononuclear cells (PBMCs) from subjects were transfected with Cav-1 or control plasmids and Hsp70 plasmid.
Results: We found that Cav-1 expression was lower but the levels of Hsp70 and Th17 cells were higher in COPD than in healthy control (HC). Hsp70 expressions were positively correlated with Cav-1 levels, Th17 cells, and Th17/Treg ratio in COPD but not in HC. Cav-1 over-expression resulted in an increase in Hsp70 and Th17 levels. Suppressing Hsp70 expressing by small interfering RNA (siRNA), the decline of Th17 frequency was observed in Cav-1-overexpressed PBMCs.
Conclusion: Collectively, our results illuminate that Cav-1 contributes to the imbalance of Th17/Treg through potentially regulating Hsp70 expression.
Keywords: caveolin-1, COPD, Hsp70, IL-17+ Th17 cells, Foxp3+ Treg cells
Introduction
Chronic obstructive pulmonary disease (COPD) is pathologically characterized by chronic and progressive inflammatory process caused by cigarette smoke, harmful particles or gases that are able to activate the cascade of inflammatory reactions.1,2 Although the immunopathogenesis of COPD remains unclear, the current researches indicate multiple immune cells (eg, neutrophils, macrophages, CD4+ and CD8+ T lymphocytes, etc) and inflammatory cytokines (eg, interleukin (IL)-22, IL-17, IL-6 and TGF-β, etc) are implicated in the pathophysiology and development of COPD.3–5
Generally, bacteria and viruses are the most prevalent etiological agents (with 50–70% and 30%, respectively)6 in acutely exacerbated COPD. CD4+ Th17 lymphocytes producing IL-17 are believed to participate in protection against microorganisms. It has been reported that IL-17A induces bronchial fibroblasts, epithelial cells and smooth muscle cells to synthesize proinflammatory cytokines responsible for the recruitment of neutrophils and their local infiltration.7 In addition, it has also been reported that IL-17A recruits neutrophils and influences most cells in the lung parenchyma, including macrophages and dendritic cells(DCs), which express receptors for IL-17A and synthesize proinflammatory cytokines such as IL-6 and TNF-α.8 These data suggest that Th17 cells play a vital role in the inflammatory reactions in COPD.
Caveolin (Cav)-1, a 21–22 kDa integral membrane protein, is considered as a potential important regulatory protein in the pathogenesis of chronic inflammatory diseases of the respiratory tract such as COPD.3 Cav-1 expression is evident in immune c,ells such as monocytes, lymphocytes, macrophages, dendritic cells (DCs).9,10 It has been demonstrated that Cav-1 can regulate the maturation and differentiation of many immune cells by controlling signaling pathways.11
The 70-kDa heat shock protein (Hsp70) is the most conserved and important member in the HSP family and plays a key role in the process of inflammation and innate immunity response under stressful events like bacterial or viral infections.12–14 Njemini et al15,16 demonstrated that the serum levels of Hsp70 are directly related to the inflammatory status of the subjects and also undergo significant increases with increasing degree of inflammation. A recent paper revealed that heat-stressed exosomes originating from tumors (HS-TEXs) containing high levels of Hsp70 contributed to converting Treg cells into Th17 cells via IL-6.17
Previous researches indicated that Cav-1 was linked with Hsp response.18,19 In one study from Bocanegra et al,20 interactions between Cav-1 and HSPA/Hsp70 were observed in the rat kidney under experimental conditions. The exposure of human or mouse skin to sulfur mustard caused elevation of Cav-1, Hsp27, and Hsp70, and these proteins were localized in caveolae, which supports caveolae-mediated regulation of Hsp expression.21 Wang et al22 demonstrated that Cav-1 augmented Hsp70 up-regulation in Cav-1-mediated HIV envelope-induced bystander apoptosis. The study from Sun et al showed that Cav-1 promoted the imbalance of Th17/Treg in patients with COPD.23 Thus, we hypothesize that Cav-1-mediated up-regulation of Hsp70 may be responsible for the imbalance of Th17/Treg in COPD.
In the present study, we examined the plasma levels of Cav-1 and Hsp70 as well as frequencies of circulating Th17 and Treg cells, and assessed the relationship between the expression of Cav-1 and Hsp70, and their association with frequency of Th17 or Treg cells as well as Th17/Treg ratio in COPD.
Materials and Methods
Subjects
Between June 2016 and August 2017, 58 subjects with COPD and 26 smokers with normal lung function as healthy control (HC) from the Affiliated Central Hospital of Qingdao University (Qingdao, China) were enrolled in this study. The age and sex of the control subjects matched with COPD patients. COPD was diagnosed according to the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) guidelines.24 Fifty-eight patients were subdivided into two groups: 28 patients with clinically stable COPD (SCOPD) diagnosed according to GOLD criteria, and 30 patients with acute exacerbation COPD (AECOPD) defined by criteria described previously.25 Inclusion and Exclusion criteria were executed as described in our previous work.23 Simply, patients with either SCOPD or AECOPD treated without steroid for >3 months prior to collection of blood samples and a smoking history of more than 10 pack-years were enrolled in this study. The exclusion criteria included asthma, cardiac arrhythmias, and other relevant lung diseases such as lung cancer, known α1-antitrypsin deficiency, or other immune-related diseases. All subjects signed an informed consent, and reviewed the protocol. This study was approved by the Ethic Committee for Application of Human Samples, Qingdao University Medical College.
Blood Samples
Heparinized peripheral blood samples (10 mL) were collected from healthy subjects and COPD patients. Anti-coagulated whole blood (1 mL) is used for analysis of flow cytometer (FCM) within 4 h. Serum samples were kept frozen at −80°C for enzyme-linked immunosorbent assay (ELISA). Peripheral blood mononuclear cells (PBMCs) were isolated by using a Ficoll gradient (GE Healthcare Life Science, Shanghai, China) as previously described26 for further culture.
Cytokine ELISA
Levels of Cav-1 and Hsp70 were determined using commercially available ELISA kits (Cav-1 kit from USCN life Science, Inc., Wuhan, China; Hsp70 kit from R&D systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Flow Cytometry Analysis
Aliquots of heparinized whole blood (100 μL) and cultured PBMCs were stained with FITC-conjugated, APC-conjugated, PerCP-Cy5.5-labeled, or PE-conjugated mAb against CD4, CD25, FoxP3, and IL-17 (BD Biosciences, San Jose, CA, USA). For intracellular cytokine staining of Foxp3 and IL-17, cells were stimulated with 50 ng/mL PMA (Alexis) and 1 μg/mL Ionomycin (Alexis) for 4 h, and then 100 μg/mL Brefeldin A (eBioscience) was added for the last 2 h; cells were then stained with intracellular cytokines after fixation and permeabilization according to the manufacturer’s instruction. Multicolor flow cytometric analyses were performed using flow cytometry (FCM) (FASCcan or FACS Vantage SE; BD Biosciences). Data analysis was performed using CellQuest software (BD Biosciences).
PBMCs Transfection
PBMCs freshly isolated were nucleofected with Cav-1 or control plasmids (Invitrogen, Carlsbad, CA) and Hsp70 plasmid (pIRES2-Hsp70, which was constructed as previously described27 using Nucleofector (Amaxa Biosystems, Cologne, Germany) as previously described.23 For transient knockdown of Hsp70, siRNA against human Hsp70 (Hsp70-siRNA) and a scrambled siRNA for control (siRNA-Ctrl) were designed as previously described.17 PBMCs were seeded into each well of 24-well plates and incubated in standard supplemented serum-free media (Gibco, Auckland, USA) overnight, and then transfected with siRNA using Nucleofector kit for human primary T cells (Amaxa, Gaithersburg, MD) according to the manufacturer’s instructions.
Statistical Analysis
Statistical analyses were performed using SPSS software (version 21.0; SPSS-IBM, Armonk, NY, USA). The differences between the two groups and differences among multiple groups were evaluated using Student’s t-test and one-way analysis of variance (ANOVA), respectively. Pearson correlation was applied to assess correlation analysis. A two-tailed p < 0.05 was considered as statistically significant.
Results
Patient Characteristics
In general, participant demographics were balanced among the three groups.
The severity of lung function impairment in patients with AECOPD was higher than SCOPD. The demographic and clinical data of the subjects are presented in Table 1.
 |
Table 1 Demographic and Clinical Characteristics of All Subjects |
Increased Hsp70 Expression and Th17 Cells Frequency in COPD Patients
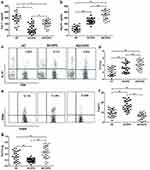
ELISA analysis revealed that Cav-1 expression was higher but Hsp70 was lower in HC when compared with COPD patients. Alongside elevated Cav-1 levels, Hsp70 expression was significantly increased in acutely exacerbated COPD (AECOPD) compared with stable COPD (SCOPD) patients (Figure 1a and b).
FCM analysis showed that the frequency of Th17 cells was significantly increased in COPD compared with HC (Figure 1c and d). Treg frequency was obviously higher in SCOPD as compared to AECOPD and HC (Figure 1e and f).
Th17/Treg ratio is the important indicator for the balance between the suppressive and pro-inflammatory subsets of CD4+ T cells. As shown in Figure 1g, the highest and lowest Th17/Treg imbalance ratio existed in AECOPD patients and SCOPD, respectively, compared with HC subjects (Figure 1g).
The Positive Correlation of Hsp70 with Cav-1 Expression, Th17 Cells Frequency and Th17/Treg Ratio in COPD but Not in HC
In HC, there was not a correlation of Hsp70 with Cav-1, the percentage of Th17, Treg cells or Th17/Treg ratio (Figure 2a). In contrast, the levels of Hsp70 displayed high degree of correlation with Cav-1 levels, frequency of Th17 and Treg and Th17/Treg ratio in COPD patients (Figure 2b and c).
Dysregulation of Cav-1 Affects the Frequencies of Treg and Th17 Cells via Regulating Hsp70 Expression
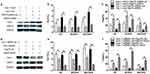
To explore whether Cav-1 affects Hsp70 expression, PBMCs from patients with COPD were transfected with either Cav-1-plasmid or Cav-1-siRNA for 24 h as described in “Materials and Methods”. As shown in Figure 3a, using the Cav-1 specific plasmid or siRNA, Cav-1 protein expression was effectively increased or reduced, respectively. Hsp70-specific ELISA analysis showed that the overexpression of Cav-1 resulted in the up-regulation of Hsp70, whereas inhibition of Cav-1 expression gave rise to the decline of Hsp70 (Figure 3b). These data suggested that Cav-1 potentially regulated Hsp70 expression. To further test this possibility, we analyzed Hsp70 mRNA levels by qRT-PCR in PBMCs transfected with Cav-1-siRNA for various times. We found that mRNA levels of Hsp70 decreased with the inhibition of Cav-1 (Figure 3c).
Cav-1-overexpressed and -suppressed PBMCs were then treated with Hsp70-specific siRNA and Hsp70 expression plasmids, respectively. Western blot analysis revealed that the protein levels of Hsp70 in Cav-1-overexpressed PBMCs were significantly decreased by Hsp70-siRNA compared with matched controls (Figure 4a), and Hsp70 overexpression could rescue the decline of Hsp70 levels in Cav-1-specific siRNA-pretreated PBMCs (Figure 4d). In addition, Hsp70-siRNA resulted in the reduction of Th17 frequency and elevation of Treg frequency in Cav-1-overexpressed PBMCs (Figure 4b and c). Similarly, Hsp70 overexpression increased Th17 frequency and diminished the expression of Treg cells in Cav-1-siRNA-pretreated PBMCs (Figure 4e and f). These results further support Cav-1 disrupting balance between Th17 and Treg cells through modulating Hsp70 expression.
Discussion
Previous studies have reported that Cav-1 and Hsp70 are involved in differentiation of Th17 and Treg cell.17,28 However, the associations of Cav-1 with Hsp70 and Th17 or Treg cells have not been studied in patients with COPD. It has been demonstrated that Cav-1 is able to augment Hsp70 expression in HIV envelope-induced bystander apoptosis.22 We determined that Hsp70 expression was positively correlated with Cav-1 levels and Th17 frequency in patients with COPD but not in HC. In addition, in Cav-1-overexpressed PBMCs, we demonstrated that suppressing Hsp70 expression resulted in the decrement of Th17 frequency. These findings suggest that Cav-1 might promote the increased levels of Hsp70 thereby to affect the proportion of Th17 and Treg cells in COPD.
Cav-1 serves as a potential critical regulatory protein in the pathogenesis of chronic inflammatory diseases such as COPD and asthma.3 Several groups reported that the levels of Cav-1 were obviously declined in asthma and COPD.23,29 We also showed Cav-l levels were lower in COPD than HC. A recent study has shown that the Cav-l peptide increased Hsp70 expression in a dose-dependent manner.22 Moreover, Bocanegra and coworkers20 demonstrated that Hsp70 was present in Cav-1 immunoprecipitates prepared from cortical cytosol and membrane fractions, suggesting the potential association of Cav-1 with Hsp70. In this study, Cav-1 levels displayed high degree of Hsp70 expression in COPD. In addition, inhibiting Cav-1 expression by siRNA resulted in decline of Hsp70 levels in PBMC from subjects. Unfortunately, the mechanisms underlying Hsp70 being uncorrelated with Cav-1 levels in HC are currently unknown.
HSPs were at increased levels in the various infectious diseases caused by bacterial and protozoan pathogens.30,31 Generally, COPD patients tend to experience frequent exacerbations induced by bacteria and viruses, and it has been revealed that Hsp70 was involved in the pathogenesis of COPD.32 Studies by Lim et al33 showed that extracellular Hsp70 can activate the innate immune system in the presence of inflammation or other stress. Recent studies34–36 reported that the serum levels of Hsp70 rose in COPD patients and higher levels of plasma Hsp70 might be correlated with an increased risk of COPD among coal workers. In addition, Dong et al37 displayed that both mRNA and protein levels of Hsp70 in lung tissues were correlated with COPD disease severity. We observed that Hsp70 expressions were higher in COPD patients than in HC, and that the highest levels of Hsp70 existed in AECOPD.
Th17 and Tregs are subsets of T cell population and play a major role in modulating various inflammatory disease conditions. Th17 cells are considered as the critical proinflammatory mediators, with the production of IL-17, IL-22, and IL-21. It has been shown that these cytokines can induce the secretion of CXCL8, CXCL5 and GM-CSF by airway epithelial, thereby contributing to migration of neutrophils to the inflammation region.38 In contrast, Treg cells suppress the immune response and inflammation through synthesizing anti-inflammatory mediators IL-10 and TGF-β.39,40 The recent observations showed increased levels of Th17 cells in the bronchial mucosa and peripheral blood of COPD patients.41–44 In the present study, the percentage of Th17 cells was significantly increased in COPD (especially in AECOPD patients) compared to HC. In addition, we detected significantly higher frequency of Tregs in SCOPD, but lower in AECOPD, as compared with that in the HC. Several recent studies showed an imbalance in Th17 and Treg cells in COPD.45–47 We found Th17 to Tregs ratio was higher in AECOPD but lower in SCOPD than in HC. These findings were consistent with a previous study.43
It has been reported that Cav-1 augments Hsp70 up-regulation22 and Hsp70 contributes to converting Treg cells into Th17 cells via IL-6.17 In addition, Guo and workers17 reported that HS-TEXs (containing high levels of Hsp70) converted Treg into Th17 cells in tumor tissues in a Hsp70-dependent manner. And we also found the positive correlation of Cav-1 levels with Hsp70 expression in COPD. Based on these data, we speculate that Cav-1 may disrupt the imbalance of Th17/Treg cells through modulating Hsp70 expression in COPD. To further verify this idea, we conducted the overexpression of Hsp70 in Cav-1-siRNA-pretreated PBMCs and the down-regulation of Hsp70 in Cav-1-overexpressed PBMCs from patients with COPD, and found that the expression of Hsp70 was directly associated with the percentages of Th17 and Treg cells. These findings further support that Cav-1 promotes the disequilibrium between Th17 and Treg cells via potentially affecting Hsp70 expression.
The main limitation of this study is that specimens only from peripheral blood and not airway are employed. So, more studies are needed to explore the mechanisms of Cav-1 and Hsp70 promoting the imbalance of Th17 and Treg cells in COPD patients.
In conclusion, our findings have demonstrated that Cav-1 contributes to the imbalance Th17/Treg through modulating Hsp70 expression in COPD. Moreover, our results further show that there is an excellent relation of Hsp70 expression with Cav-1 levels and the frequencies of Th17 and Treg in COPD. Further studies are needed to explore the detailed mechanisms of Cav-1 regulating Hsp70 in COPD patients. There are still some shortcomings in our study, for instance, the small sample size.
Compliance with Ethics Guidelines
This study was conducted following the Declaration of Helsinki and all applicable national and international ethical guidelines for medical and health research involving human participants.
Acknowledgments
We acknowledge Dr Chunling Zhang for helpful discussions. We also thank Peng Zhao for her assistance in experimental procedures.
Funding
This work was supported by Qingdao Outstanding Health Professional Development Fund; Qingdao 2017, 2018 medical and health research plan project (2017-WJZD061, 2018-WJZD048); Qingdao Science and Technology Project (for Benefiting the People, 19-6-1-27-nsh) and Qingdao Key Disciplines of Traditional Chinese Medicine. This work is also partially supported by Medical and Health Technology Development Program of Shandong Province (No. 2016WS0324) and Qing Dao Expert Workstation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Mannino DM. The changing definition and perception of COPD. Respir Care. 2022;67(6):750–755. doi:10.4187/respcare.10059
2. Lu Z, Coll P, Maitre B, Epaud R, Lanone S. Air pollution as an early determinant of COPD. Eur Respir Rev. 2022;31:165. doi:10.1183/16000617.0059-2022
3. Royce SG, Le Saux CJ. Role of caveolin-1 in asthma and chronic inflammatory respiratory diseases. Expert Rev Respir Med. 2014;8(3):339–347. doi:10.1586/17476348.2014.905915
4. Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324(1):23–33. doi:10.1124/jpet.107.127209
5. Le Rouzic O, Koné B, Kluza J, et al. Cigarette smoke alters the ability of human dendritic cells to promote anti-Streptococcus pneumoniae Th17 response. Respir Res. 2016;17(1):94. doi:10.1186/s12931-016-0408-6
6. Huang YJ, Charlson ES, Collman RG, Colombini-Hatch S, Martinez FD, Senior RM. The role of the lung microbiome in health and disease. A National Heart, Lung, and Blood Institute workshop report. Am J Respir Crit Care Med. 2013;187(12):1382–1387. doi:10.1164/rccm.201303-0488WS
7. D’Anna SE, Balbi B, Cappello F, Carone M, Di Stefano A. Bacterial-viral load and the immune response in stable and exacerbated COPD: significance and therapeutic prospects. Int J Chron Obstruct Pulmon Dis. 2016;11:445–453. doi:10.2147/COPD.S93398
8. Doe C, Bafadhel M, Siddiqui S, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;138(5):1140–1147. doi:10.1378/chest.09-3058
9. Harris J, Werling D, Koss M, Monaghan P, Taylor G, Howard CJ. Expression of caveolin by bovine lymphocytes and antigen-presenting cells. Immunology. 2002;105(2):190–195. doi:10.1046/j.1365-2567.2002.01362.x
10. Liu P, Li WP, Machleidt T, Anderson RG. Identification of caveolin-1 in lipoprotein particles secreted by exocrine cells. Nat Cell Biol. 1999;1(6):369–375. doi:10.1038/14067
11. Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10(8):554–567. doi:10.1038/nri2808
12. Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi:10.1146/annurev.physiol.61.1.243
13. Sedger L, Ruby J. Heat shock response to vaccinia virus infection. J Virol. 1994;68(7):4685–4689. doi:10.1128/jvi.68.7.4685-4689.1994
14. Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol. 2004;173(10):6319–6326. doi:10.4049/jimmunol.173.10.6319
15. Njemini R, Demanet C, Mets T. Inflammatory status as an important determinant of heat shock protein 70 serum concentrations during aging. Biogerontology. 2004;5(1):31–38. doi:10.1023/B:BGEN.0000017684.15626.29
16. Njemini R, Bautmans I, Onyema OO, Van Puyvelde K, Demanet C, Mets T. Circulating heat shock protein 70 in health, aging and disease. BMC Immunol. 2011;12:24. doi:10.1186/1471-2172-12-24
17. Guo D, Chen Y, Wang S, et al. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology. 2017;154(1):132–143.
18. Fanelli MA, Montt-Guevara M, Diblasi AM, et al. P-cadherin and beta-catenin are useful prognostic markers in breast cancer patients; beta-catenin interacts with heat shock protein Hsp27. Cell Stress Chaperones. 2008;13(2):207–220. doi:10.1007/s12192-007-0007-z
19. Kampinga HH, Hageman J, Vos MJ, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi:10.1007/s12192-008-0068-7
20. Bocanegra V, Manucha W, Peña MR, Cacciamani V, Vallés PG. Caveolin-1 and Hsp70 interaction in microdissected proximal tubules from spontaneously hypertensive rats as an effect of Losartan. J Hypertens. 2010;28(1):143–155. doi:10.1097/HJH.0b013e328332b778
21. Black AT, Hayden PJ, Casillas RP, et al. Regulation of Hsp27 and Hsp70 expression in human and mouse skin construct models by caveolae following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol. 2011;253(2):112–120. doi:10.1016/j.taap.2011.03.015
22. Wang XM, Nadeau PE, Lo YT, Mergia A. Caveolin-1 modulates HIV-1 envelope-induced bystander apoptosis through gp41. J Virol. 2010;84(13):6515–6526. doi:10.1128/JVI.02722-09
23. Sun N, Wei X, Wang J, Cheng Z, Sun W. Caveolin-1 promotes the imbalance of Th17/Treg in patients with chronic obstructive pulmonary disease. Inflammation. 2016;39(6):2008–2015. doi:10.1007/s10753-016-0436-x
24. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
25. Husebo GR, Bakke PS, Gronseth R, et al. Macrophage migration inhibitory factor, a role in COPD. Am J Physiol Lung Cell Mol Physiol. 2016;311(1):L1–7. doi:10.1152/ajplung.00461.2015
26. Wu Y, Wan T, Zhou X, et al. Hsp70-like protein 1 fusion protein enhances induction of carcinoembryonic antigen-specific CD8+ CTL response by dendritic cell vaccine. Cancer Res. 2005;65(11):4947–4954. doi:10.1158/0008-5472.CAN-04-3912
27. Yu WW, Cao SN, Zang CX, et al. Heat shock protein 70 suppresses neuroinflammation induced by alpha-synuclein in astrocytes. Mol Cell Neurosci. 2018;86:58–64. doi:10.1016/j.mcn.2017.11.013
28. Schönle A, Hartl FA, Mentzel J, et al. Caveolin-1 regulates TCR signal strength and regulatory T-cell differentiation into alloreactive T cells. Blood. 2016;127(15):1930–1939. doi:10.1182/blood-2015-09-672428
29. Bains SN, Tourkina E, Atkinson C, et al. Loss of caveolin-1 from bronchial epithelial cells and monocytes in human subjects with asthma. Allergy. 2012;67(12):1601–1604. doi:10.1111/all.12021
30. Dzaman-Serafin S, Telatyńska-Mieszek B, Ciechanowski K. Heat shock proteins and their characteristics. Pol Merkur Lekarski. 2005;19(110):215–219.
31. Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2(3):185–194. Polish. doi:10.1038/nri749
32. Qu B, Jia Y, Liu Y, Wang H, Ren G, Wang H. The detection and role of heat shock protein 70 in various nondisease conditions and disease conditions: a literature review. Cell Stress Chaperones. 2015;20(6):885–892. doi:10.1007/s12192-015-0618-8
33. Lim WK, Kanelakis KC, Neubig RR. Regulation of G protein signaling by the 70kDa heat shock protein. Cell Signal. 2013;25(2):389–396. doi:10.1016/j.cellsig.2012.11.002
34. Hacker S, Lambers C, Hoetzenecker K, et al. Elevated HSP27, HSP70 and HSP90 alpha in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction. Clin Lab. 2009;55(1–2):31–40.
35. Tamási L, Bohács A, Tamási V, et al. Increased circulating heat shock protein 70 levels in pregnant asthmatics. Cell Stress Chaperones. 2010;15(3):295–300. doi:10.1007/s12192-009-0143-8
36. Cui X, Xing J, Liu Y, et al. COPD and levels of Hsp70 (HSPA1A) and Hsp27 (HSPB1) in plasma and lymphocytes among coal workers: a case-control study. Cell Stress Chaperones. 2015;20(3):473–481. doi:10.1007/s12192-015-0572-5
37. Dong J, Guo L, Liao Z, et al. Increased expression of heat shock protein 70 in chronic obstructive pulmonary disease. Int Immunopharmacol. 2013;17(3):885–893. doi:10.1016/j.intimp.2013.09.003
38. Aujla SJ, Dubin PJ, Kolls JK. Interleukin-17 in pulmonary host defense. Exp Lung Res. 2007;33(10):507–518. doi:10.1080/01902140701756604
39. Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011;131(9):1853–1860. doi:10.1038/jid.2011.139
40. Sasaki N, Yamashita T, Takeda M, Hirata K. Regulatory T cells in atherogenesis. J Atheroscler Thromb. 2012;19(6):503–515. doi:10.5551/jat.10934
41. Di Stefano A, Caramori G, Gnemmi I, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157(2):316–324. doi:10.1111/j.1365-2249.2009.03965.x
42. Zhang L, Cheng Z, Liu W, Wu K. Expression of interleukin (IL)-10, IL-17A and IL-22 in serum and sputum of stable chronic obstructive pulmonary disease patients. Copd. 2013;10(4):459–465. doi:10.3109/15412555.2013.770456
43. Profita M, Albano GD, Riccobono L, et al. Increased levels of Th17 cells are associated with non-neuronal acetylcholine in COPD patients. Immunobiology. 2014;219(5):392–401. doi:10.1016/j.imbio.2014.01.004
44. Vargas-Rojas MI, Ramírez-Venegas A, Limón-Camacho L, Ochoa L, Hernández-Zenteno R, Sansores RH. Increase of Th17 cells in peripheral blood of patients with chronic obstructive pulmonary disease. Respir Med. 2011;105(11):1648–1654. doi:10.1016/j.rmed.2011.05.017
45. Wang H, Ying H, Wang S, et al. Imbalance of peripheral blood Th17 and Treg responses in patients with chronic obstructive pulmonary disease. Clin Respir J. 2015;9(3):330–341. doi:10.1111/crj.12147
46. Li H, Liu Q, Jiang Y, Zhang Y, Zhang Y, Xiao W. Disruption of th17/treg balance in the sputum of patients with chronic obstructive pulmonary disease. Am J Med Sci. 2015;349(5):392–397. doi:10.1097/MAJ.0000000000000447
47. Li XN, Pan X, Qiu D. Imbalances of Th17 and Treg cells and their respective cytokines in COPD patients by disease stage. Int J Clin Exp Med. 2014;7(12):5324–5329.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.