Back to Journals » Vascular Health and Risk Management » Volume 16
Case Report on a Patient with Steinert Disease Complicated by COVID-19
Authors Chalela R , Caguana O , Zuccarino F, Khilzi K, Rodríguez-Chiaradía DA
Received 6 July 2020
Accepted for publication 29 September 2020
Published 20 November 2020 Volume 2020:16 Pages 463—466
DOI https://doi.org/10.2147/VHRM.S266659
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Professor Magnus Bäck
Roberto Chalela, 1– 4 Oswaldo Caguana, 1, 2 Flavio Zuccarino, 5 Karys Khilzi, 1, 2 Diego A Rodríguez-Chiaradía 1– 4
1Respiratory Medicine Department, Hospital del Mar, Barcelona, Spain; 2Institut Hospital del Mar d’Investigacions Mèdiques (IMIM), Barcelona, Spain; 3Universitat Pompeu Fabra, Barcelona, Spain; 4Centro de Investigación en Red de Enfermedades Respiratorias(CIBERES), Instituto de Salud Carlos III (ISCIII), Madrid, Spain; 5Department of Radiology, Hospital del Mar, Barcelona, Spain
Correspondence: Diego A Rodríguez-Chiaradía
Respiratory Medicine Department, Hospital del Mar, Passeig Maritim, 25, Barcelona, 08003, Spain
Tel +34 932483548
Email [email protected]
Abstract: SARS-CoV-2 infection is predominantly a respiratory disease with a diverse clinical spectrum. Pulmonary thromboembolic complications during COVID-19 pneumonia may be associated with a high mortality rate and post-mortem findings confirm the presence of platelet-fibrin thrombi in arterial vessels of patients together with lung tissue alterations. We present a patient transferred to the emergency department due to a syncope with no other associated symptoms, who was diagnosed with an acute pulmonary embolism (PE) concomitant with SARS-CoV-2 infection without lung infiltrates. Presenting with a PE as the only manifestation of this infection, reinforces our conception of COVID-19 as a heterogeneous disease of which we still know very little. We believe that while the virus is still circulating in our environment, we need to consider ruling out COVID-19 in all thrombotic events, even if the patients have no other risk factors.
Keywords: COVID-19, SARS-CoV-2, pulmonary embolism, syncope, pandemic
Background
SARS-CoV-2 infection has become a critical global issue in 2020 and several reports indicate that pulmonary thromboembolic complications during COVID-19 pneumonia may be associated with a high mortality rate.1,2 Recent reports in Spain suggest that around 26% of patients with SARS-CoV2 infection can be asymptomatic or present with atypical symptoms,3 and post-mortem findings in a large series of COVID-19 patients in Italy confirm the presence of platelet-fibrin thrombi in the small arterial vessels together with lung tissue alterations in all patients.4 We present the case of a patient who was transferred to the emergency department due to syncope with no other associated symptoms, who was diagnosed with an acute pulmonary embolism (PE) concomitant with SARS-CoV-2 infection without lung infiltrates.
Case Report
A 63-year-old man presented a transient loss of consciousness and muscle strength with spontaneous recovery while walking in the street. He was a current smoker and had a past medical history of myotonic dystrophy type 1, also known as Steiner disease, and hiatal hernia. The patient denied fever, cough, sputum production, dyspnea, chills, muscle pain, loss of taste/smell or any other symptoms. He also denied excessive decrease in physical activity during the last week or any other risk factor for venous thromboembolism (VTE). Vital signs at admission were: arterial blood pressure 123/93 mm Hg, temperature 35.6℃, heart rate 115/minute, respiratory rate 23/minute, and oxygen saturation 94% at room air. His physical examination revealed a contusion and hematoma in right supraciliary arch, shallow breathing without respiratory distress. The rest of the physical examination was unremarkable.
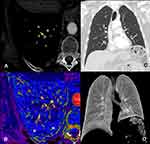
Electrocardiography demonstrated sinus tachycardia and complete right bundle branch block. Chest X-ray showed no relevant alterations. Complete blood count and basic metabolic panel results were within normal ranges. Arterial blood gas analysis results were as follows: pH 7.40, PaCO2 39 mmHg, PaO2 64 mmHg and HCO3- 24 mmol/L. Dimer D was raised (4850 μg/mL). A multidetector computed tomography (CT) pulmonary angiography was performed to exclude pulmonary artery embolism. It demonstrated an obstruction of the posterior-basal and anterior-basal segmental branches of the right lower lobe (RLL) (Figure 1), with no associated areas of consolidation, crazy-paving or ground-glass opacities. High‐sensitivity cardiac troponin, B-type natriuretic peptide (pro-BNP) and echocardiography were normal. Anticoagulation with enoxaparin (1mg/kg/12 hours) was initiated.
Taking into account the current pandemic due to COVID-19 and despite the absence of specific symptoms, we decided to perform a PCR for SARS-CoV-2. The results were positive and COVID-19 related to acute pulmonary embolism was diagnosed. Lactic Acid Dehydrogenase (LDH) was 242 U/L, interleukin 6 (IL-6) 15 pg/mL and ferritin 106 ng/mL. Inpatient hospitalization took an adequate course with no incidents. Hypercoagulability and autoimmune panel ruled out any other associated disease or factor. After seven days, the patient was discharged with home isolation.
Conclusion
COVID-19 is a heterogeneous disease that has a diverse clinical spectrum; however, the great majority of patients requiring hospitalization present with bilateral opacities in CT scan and respiratory failure.7 Previous studies suggest that the absence of pulmonary infiltrates in hospitalized patients allow to reasonably rule out SARS-CoV-2 infection.5,6 In our case, we describe a previously asymptomatic patient with syncope, who was diagnosed with PE. The epidemiological background and the absence of risk factors for thromboembolic disease, beyond his myotonic dystrophy, made us suspect COVID-19 despite the fact that there were no other suggestive clinical findings or symptoms.
In spite of the fact that COVID-19 may lead to arterial and venous thromboembolic events for different reasons (systemic inflammatory response, immobilization, hypoxia, and procoagulant activity), we still do not know whether these hemostatic changes are specific to SARS-CoV-2 or a consequence of the cytokine storm that precipitates VTE, as observed in other viral diseases.8 In our case, there were no laboratory findings to identify an excessive inflammatory response and only d-Dimer was elevated. Bavaro et al recently suggested that higher D-Dimer levels and higher Revised Geneva Score but not the Well’s Score were predictors of PE in COVID-19.9 Our patient had a pre-test intermediate risk by both Geneve Score (5 points) and Well’s Score (4.5 points).
To our knowledge, there are no previous reports of thrombotic complications as the only manifestation of SARS-CoV-2 infection. Steinert’s disease determines a greater risk during life to develop pulmonary thromboembolism and the cumulative incidence of PE in SARS-CoV2 infection is very high even in outpatients.10 Probably the coexistence of both disease conditions even a higher risk of PE. These findings suggest both a great heterogeneity of the disease and how little we currently know about it. These findings also suggest that while the virus is still circulating in our environment, we need to consider ruling out COVID-19 in all the thrombotic events, even if the patients have no other symptoms or risk factors. The use of thromboelastography could be an interesting approach to detect patients at high risk of developing pulmonary embolism since its usefulness in detecting hypercoagulability in COVID-19 has recently been described.11,12
Consent
Written informed consent was obtained from the patient for this case report and any accompanying images to be published. Institutional approval was not required to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi:10.1016/j.thromres.2020.04.013
2. Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi:10.1111/jth.14854
3. Ministerio de Sanidad de España. Estudio ENE-COVID19: primera ronda. Estudio nacional de sero-epidemiología de la infección por SARS-COV-2 en España: informe preliminar. May 13, 2020. Available from: https://www.mscbs.gob.es/gabinetePrensa/notaPrensa/pdf/13.05140520114250669.pdf.
4. Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. Lancet Infect Dis. 2020.
5. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi:10.1148/radiol.2020200642
6. Xiong Y, Sun D, Liu Y, et al. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest Radiol. 2020;55:332–339. doi:10.1097/RLI.0000000000000674
7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi:10.1016/S0140-6736(20)30566-3
8. Ramacciotti E, Agati LB, Aguiar VCR, et al. Zika and Chikungunya virus and risk for venous thromboembolism. Clin Appl Thromb Hemost. 2019;25:1076029618821184. doi:10.1177/1076029618821184
9. Bavaro DF, Poliseno M, Scardapane A, et al. Occurrence of acute pulmonary embolism in COVID-19-A case series. Int J Infect Dis. 2020;98:225. doi:10.1016/j.ijid.2020.06.066
10. Wahbi K, Porcher R, Laforet P, et al. Development and validation of a new scoring system to predict survival in patients with myotonic dystrophy type 1. JAMA Neurol. 2018;75:573–581. doi:10.1001/jamaneurol.2017.4778
11. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi:10.1111/jth.14850
12. Spiezia L, Boscolo A, Poletto F, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi:10.1055/s-0040-1714350
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.