Back to Journals » Infection and Drug Resistance » Volume 13
Case Report of Primary Lung Abscesses Due to Hypervirulent Klebsiella pneumoniae (Serotype K2, Sequence Type 375): an Emerging Isolate in Okinawa, Japan
Authors Hirai J, Sakanashi D, Momose M, Koga T, Kinjo T, Haranaga S, Motonaga E, Fujita J
Received 8 March 2020
Accepted for publication 26 May 2020
Published 10 June 2020 Volume 2020:13 Pages 1691—1695
DOI https://doi.org/10.2147/IDR.S252251
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Jun Hirai,1,2 Daisuke Sakanashi,3 Masashi Momose,1 Tomomi Koga,4 Takeshi Kinjo,2 Shusaku Haranaga,5 Eiji Motonaga,6 Jiro Fujita2
1Department of Internal Medicine, Okinawa Miyako Hospital, Okinawa 906-0012, Japan; 2Department of Infectious, Respiratory and Digestive Medicine, Graduate School of Medicine, University of the Ryukyus, Okinawa 903-0215, Japan; 3Department of Infection Control and Prevention, Aichi Medical University Hospital, Aichi 480-1195, Japan; 4Department of Radiology, Graduate School of Medicine, University of the Ryukyus, Okinawa 903-0215, Japan; 5Comprehensive Health Professions Education Center, University of the Ryukyus Hospital, Okinawa 903-0215, Japan; 6Department of General Medicine, Okinawa Miyako Hospital, Okinawa 906-0012, Japan
Correspondence: Jun Hirai Email [email protected]
Abstract: Hypervirulent Klebsiella pneumoniae (HV-KP) is typically associated with community-acquired liver abscess and bacteremia with metastatic infection; however, primary lung abscess (PLA) caused by HV-KP is rare, with only one such case report to date. A 69-year-old man with a history of diabetes mellitus (DM) was admitted to hospital complaining of slight bloody sputum. Chest imaging showed multiple consolidations with cavities in both lung fields. A culture of bronchoalveolar lavage fluid confirmed the presence of K. pneumoniae. Genetic analyses identified the isolate as serotype K2 and sequence type 375 (K2-ST375), and that it harbored the rmpA gene. The patient was an Asian middle-aged male with DM, all of which are risk factors for HV-KP infection. Although complicating DM and the presence of the rmpA gene are more likely to induce disseminated infection, metastatic infections were not found in this patient. The clinical and microbiological characteristics of our patient were different from those of a previous reported case, although in both cases the patient was from Asia and had DM. Therefore, DM appears to be one of the predisposing factors for HV-KP lung abscesses and physicians should pay attention to emerging HV-KP lung abscess infection, particularly in Asian countries. Previous studies have also revealed that K2-ST375 is one of the major clones causing HV-KP infection, and that it is mainly isolated from patients with liver abscess. Interestingly, including the present case, most of the infectious cases caused by K2-ST375 have been reported from Okinawa Prefecture in Japan. Therefore, the trend of the K2-ST375 strain should be carefully monitored, particularly in Okinawa, Japan. The serotype of HV-KP that causes PLA is still unknown and further study is needed to elucidate the etiology of PLA due to HV-KP and the relationship between the strain K2-ST375 and PLA.
Keywords: hypervirulent Klebsiella pneumoniae, primary lung abscess, serotype K2, sequence type 375, Okinawa, Japan
Introduction
Generally, hypervirulent Klebsiella pneumoniae (HV-KP) strains cause community-acquired liver abscess, and diabetes mellitus (DM) is a significant risk factor for acquiring HV-KP infection.1 HV-KP also tends to cause metastatic infections, with the most common sites being the eyes and central nervous system, occurring in approximately one-third of the patients.1 Lung abscesses have also been found to occur as a result of metastatic or invasive infection from primary infectious sites.2,3 However, primary lung abscess (PLA) due to this strain is rare; there has been only one reported case of PLA thus far, from Taiwan in 2015, caused by sequence type (ST) 23 clone capsule genotype K1 HV-KP.4
Here, we present a case of PLA caused by serotype K2 HV-KP. Although the patient had a risk factor of metastatic infection and the isolated strain possessed the virulence gene rmpA, which is associated with life-threatening infections with diffuse organ system involvement, concomitant bacteremia or metastatic infections were not found. Written informed consent was obtained from the patient, and the patient also provided written informed consent with publishing the case details and all accompanying images. The ethics committee approved the waiver in this case report, based on the Japanese ethical guidelines for clinical research.
Case Report
In April 2018, a 69-year-old man with a history of DM and chronic kidney disease and undergoing dialysis treatment was admitted to Okinawa Miyako Hospital after complaints of slight bloody sputum for the past seven days and weight loss of 5 kg in the past six months.
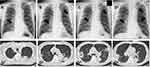
On admission, he was afebrile and did not present with respiratory distress; vital signs were normal. Auscultation revealed clear respiratory sounds, and other physical examinations also yielded unremarkable findings. However, blood tests revealed elevated white blood cell count (12,700 cells/µL) and C-reactive protein levels (14.76 mg/dL). A computed tomography (CT) scan of the chest showed multiple consolidations with cavities in both lung fields (Figure 1). The patient had routine chest X-rays every three months, due to ongoing dialysis treatment, to check his cardio-thoracic ratio and the chest radiographs from one, four, and six months ago also showed multiple interstitial infiltrations. The locations of these infiltrations were consistent with the lung abscesses revealed by the CT on admission; these results indicated that the abscesses had existed for at least six months (Figure 1). He was hospitalized based on the diagnosis of multiple lung abscesses. Additional examinations such as urine culture, cardiac ultrasonography, and CT scans of the abdomen and head were performed to determine whether this patient had other primary source or metastatic infections. Other sources of primary and metastatic infections, such as infective endocarditis, liver abscess, brain abscess, or ocular infection, were all absent.
To determine the causative pathogen, we performed a bronchoscopy. Gram-stain of bronchoalveolar lavage fluid (BAL) showed only capsulated gram-negative rods (Figure 2), and the microorganism was identified as K. pneumoniae by conventional methods. The involvement of other pathogens, such as anaerobes, was not evident from the BAL cultures. Two sets of blood cultures were also negative for other microorganisms. String test of the isolate was positive (the string reached a length of 12 mm), which is characteristic of the hypermucoviscous phenotype. The isolate was susceptible to all routinely tested antibiotics except ampicillin. We initially treated the patient with ampicillin/sulbactam for three weeks. Next, he received oral amoxicillin/clavulanic acid suppression therapy for four weeks, after which he recovered without recurrence.
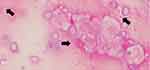 |
Figure 2 Gram-stain of bronchoalveolar lavage. Black arrows indicate capsulated Gram-negative large rod. |
We performed multiplex PCR (Figure 3) and multilocus sequence typing as described previously.5,6 We determined the capsular serotype of the isolate to be K2 and ST 375: K2-ST375; the isolate possessed rmpA (a positive regulator of capsular polysaccharide production), iutA, entB, and mrkD. The isolated strain produced a positive string test and a positive PCR amplification of the rmpA gene; therefore, we identified the isolated strain as hypervirulent.
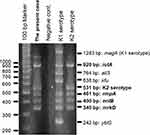 |
Figure 3 Multiplex PCR. The isolated strain possessed iutA, rmpA, entB, and mrkD. |
Discussion
We have described a case of PLA due to K2-ST375 HV-KP. Some of the risk factors for HV-KP infections are (1) male sex, (2) age of 50–60 years old, (3) DM, and (4) Asian origin.1,7-9 Our patient had all these risk factors.
Liver abscesses are frequently caused by HV-KP in the absence of hepatobiliary disease and are almost always from monomicrobial infection.10 Similarly, our patient had a lung abscess without underlying pulmonary disease, and the only bacterium isolated was HV-KP. Lung abscesses usually result from polymicrobial co-infections, mainly caused by anaerobes. Further research is needed to understand whether HV-KP-induced PLA is more likely to have a monomicrobial cause, without the involvement of anaerobes, in patients without underlying pulmonary disease.
An interesting finding is that the patient (with DM) remained clinically asymptomatic (more than six months) without disseminated invasive infections, such as bacteremia and other organ abscesses, although the isolated strain carried the rmpA gene. RmpA-positive strains are highly likely to cause infections in other sites in patients with liver abscess due to HV-KP.11 In addition, DM affects host defense by inhibiting phagocytosis and bactericidal activity, which contributes to the spread of infection.12
The consolidation of different case reports is essential for elucidating the clinical characteristics of HV-KP-induced PLA. There is only one report from Taiwan on PLA due to HV-KP; K1-ST23 was the causal strain.4 The patient was an 80-year-old woman with DM, complaining of fever, hemoptysis, and right chest wall pain for five days.4 Although the clinical and microbiological characteristics, such as age, sex, clinical presentation, serotype, and ST of our patient were different from the reported case, the patient was also from Asia and had DM. Therefore, DM appears to be a predisposing factor for HV-KP lung abscess and clinical physicians should note that HV-KP lung abscess is an emerging infection, particularly in Asian countries. In addition, K1-ST23 has been recognized as the most prevalent HV-KP isolate from liver abscess,4 and all reported cases of K2-ST375 infection showed the presence of liver abscess.15–17 Therefore, these two strains may be more likely to cause lung abscess in addition to liver abscess and should receive attention as causative pathogens of lung abscess, although current data are limited.
One of the possible reasons why PLA caused by HV-KP is rare is that K. pneumoniae, in general, is an uncommon cause of lung abscess, which is caused most frequently by anaerobes.18 Another possible reason is that the distribution of capsular K serotypes among the lung abscess isolates has not been determined adequately thus far. We think it is important, therefore, to investigate which serotype of HV-KP causes PLA in order to clarify the etiology of PLA caused by HV-KP. In addition, a study of the difference between PLA due to classical KP and to HV-KP should be a focus of future research.
In the present case, ST375 was isolated, which is one of the major clones of the K2 serotype of HV-KP.13,14 Importantly, three of the five reported cases of K. pneumoniae K2-ST375 infection (60%), including the case described here, are from Okinawa Prefecture in Japan.15–17 A nationwide molecular epidemiological study that did not include Okinawa Prefecture also reported that K2-ST375 was not isolated in the main islands of Japan.13 Our patient was from Miyako Island, Okinawa, located near Taiwan—380 km west of Taiwan and situated on the same latitude—where HV-KP-induced lung abscess was first reported.4 It is therefore possible that Okinawa has a unique molecular epidemiology of K. pneumoniae infections, as was observed in Taiwan, where HV-KP was first reported in 1986.19
The present study has several strengths. First, we found the causative pathogen using bronchoscopy. Sputum cultures are generally considered not to be useful for the diagnosis of lung abscess because of unavoidable bacterial contamination in the upper respiratory tract. On the other hand, a previous study reported that BAL can provide valuable information for detecting the causative pathogen of lung abscess.18 Second, from radiological findings, we confirmed that the present case had a chronic course without disseminated infection over six months, although the rmpA-positive strains were isolated. Our report has some limitations. First, it is a single case report representing minimal data. However, it is very important to report and accumulate such cases. Second, we did not sequence the isolated strain. However, we performed PCR analysis and showed the presence of virulence genes such as rmpA. Unfortunately, we could not conclusively determine the relationship between the strain K2-ST375 and PLA because reports of invasive infections by this strain are scarce.
In conclusion, we describe an unusual case of PLA caused by HV-KP serotype K2 in a patient with multiple risk factors for HV-KP infection. This report indicates that while PLA due to HV-KP has been rare to date, physicians should investigate the involvement of HV-KP strains when encountering PLA patients with complicating risk factors, such as DM. Importantly, the trend of the K2-ST375 strain, particularly in Okinawa, Japan, needs to be carefully followed.
Further research is required to elucidate the etiology, epidemiology, risk factors, serotype, and ST of HV-KP causing PLA.
Acknowledgments
We would like to thank Editage for English language editing.
Disclosure
The authors declare no conflicts of interest-.
References
1. Choby JE, Howard-Anderson J, Weiss DS. Hypervirulent Klebsiella pneumoniae - clinical and molecular perspectives. J Intern Med. 2020;287(3):283–300.
2. Coutinho RL, Visconde MF, Descio FJ, et al. Community-acquired invasive liver abscess syndrome caused by a K1 serotype Klebsiella pneumoniae isolate in Brazil: a case report of hypervirulent ST23. Mem Inst Oswaldo Cruz. 2014;109:970–971. doi:10.1590/0074-0276140196
3. Hentzien M, Rosman J, Decré D, Brenkle K, Mendes-Martins L, Mateu P. Seven hypervirulent ST380 Klebsiella pneumoniae septic localizations. Med Mal Infect. 2017;47:171–173. doi:10.1016/j.medmal.2016.10.002
4. Cheng KC, Lee MF, Chuang YC, Yu WL. First description of lung abscess caused by ST23 clone capsule genotype K1 Klebsiella pneumoniae. J Formos Med Assoc. 2015;114:379–380. doi:10.1016/j.jfma.2013.08.008
5. Compain F, Babosan A, Brisse S, et al. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J Clin Microbiol. 2014;52:4377–4380. doi:10.1128/JCM.02316-14
6. Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43(8):4178–4182. doi:10.1128/JCM.43.8.4178-4182.2005
7. Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12:881–887. doi:10.1016/S1473-3099(12)70205-0
8. Li J, Ren J, Wang W, et al. Risk factors and clinical outcomes of hypervirulent Klebsiella pneumoniae induced bloodstream infections. Eur J Clin Microbiol Infect Dis. 2018;37:679–689. doi:10.1007/s10096-017-3160-z
9. Zhang Y, Zhao C, Wang Q, et al. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother. 2016;60:6115–6120. doi:10.1128/AAC.01127-16
10. Chan KS, Chen CM, Cheng KC, Hou CC, Lin HJ, Yu WL. Pyogenic liver abscess: a retrospective analysis of 107 patients during a 3-year period. Jpn J Infect Dis. 2005;58:366–368.
11. Yu WL, Ko WC, Cheng KC, et al. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 2006;42:1351–1358. doi:10.1086/503420
12. Lin YT, Wang FD, Wu PF, Fung CP. Klebsiella pneumoniae liver abscess in diabetic patients: association of glycemic control with the clinical characteristics. BMC Infect Dis. 2013;13:56. doi:10.1186/1471-2334-13-56
13. Harada S, Ishii Y, Saga T, Aoki K, Tateda K. Molecular epidemiology of Klebsiella pneumoniae K1 and K2 isolates in Japan. Diagn Microbiol Infect Dis. 2018;91:354–359. doi:10.1016/j.diagmicrobio.2018.03.010
14. Shi Q, Lan P, Huang D, et al. Diversity of virulence level phenotype of hypervirulent Klebsiella pneumoniae from different sequence type lineage. BMC Microbiol. 2018;18:94. doi:10.1186/s12866-018-1236-2
15. Hoashi K, Harada S, Ishii Y, et al. Community-acquired liver abscess caused by capsular genotype K2-ST375 hypervirulent Klebsiella pneumoniae isolates. IDCases. 2019;17:e00577. doi:10.1016/j.idcr.2019.e00577
16. Liao CH, Huang YT, Chang CY, Hsu HS, Hsueh PR. Capsular serotypes and multilocus sequence types of bacteremic Klebsiella pneumoniae isolates associated with different types of infections. Eur J Clin Microbiol Infect Dis. 2014;33:365–369. doi:10.1007/s10096-013-1964-z
17. Sánchez-López J, García-Caballero A, Navarro-San Francisco C, et al. Hypermucoviscous Klebsiella pneumoniae: a challenge in community acquired infection. IDCases. 2019;17:e00547. doi:10.1016/j.idcr.2019.e00547
18. Mukae H, Noguchi S, Naito K, et al. The Importance of Obligate Anaerobes and the Streptococcus anginosus Group in Pulmonary Abscess: a Clone Library Analysis Using Bronchoalveolar Lavage Fluid. Respiration. 2016;92(2):80–89. doi:10.1159/000447976
19. Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146:1913–1916. doi:10.1001/archinte.1986.00360220057011
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.