Back to Journals » OncoTargets and Therapy » Volume 11
Case report of a primary prostatic urothelial carcinoma patient with sustained fever
Received 13 January 2018
Accepted for publication 17 May 2018
Published 3 August 2018 Volume 2018:11 Pages 4547—4550
DOI https://doi.org/10.2147/OTT.S162441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr XuYu Yang
Songsong Zhang, Zhenxing Guo
Department of Hematology/Oncology, Cancer Center, First Hospital of Tsinghua University, Beijing, 100016, People’s Republic of China
Abstract: A 77-year-old-male patient presented with recurrent gross hematuria for 3 months with a fever for 1 month, and so was admitted to The First Hospital of Tsinghua University. The medical history revealed the patient exhibited no symptoms of night sweat, dysuria, or abdominal pain. The patient experienced sustained fever with maximal temperature of 39°C for 1 month without any infectious symptoms. The biochemical examination revealed that renal and hepatic function tests were normal. Serum prostate-specific antigen and free prostate-specific antigen were at a normal level. Enhanced computed tomography and magnetic resonance imaging was performed and indicated prostatic cancer with bilateral pulmonary metastases. Subsequently, prostatic urothelial carcinoma was confirmed by a histopathology assay of the prostate biopsy. Following 1 course of chemotherapy, the temperature of the patient returned to normal.
Keywords: prostatic urothelial carcinoma, primary, fever, chemotherapy, metastases
Introduction
The prostate gland consists of glandular elements and fibromuscular stroma. Prostatic urothelial carcinoma (PUC) may originate from the mucosa of prostatic transitional urothelium, prostatic ducts, or prostatic stroma. The incidence of primary PUC is extremely rare and accounts for only 1%–4% of prostatic malignant tumors.1
In the present study, we report a case of a 77-year-old-male, who was admitted to the Department of Hematology/Oncology, The First Hospital of Tsinghua University (Beijing, People’s Republic of China) with a history of recurrent visible hematuria for 3 months with fever for 1 month. Subsequently, the patient was diagnosed with primary PUC. Following 1 course of chemotherapy, his temperature returned to normal.
Case report
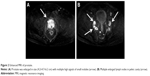
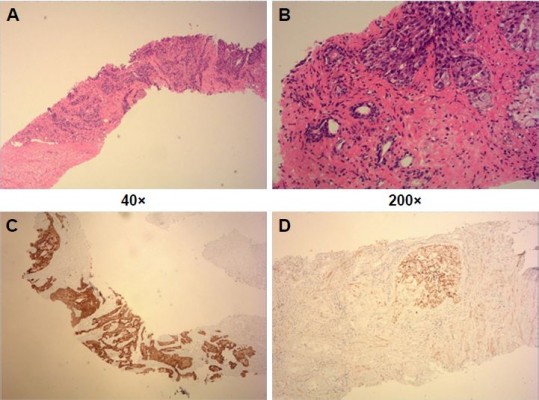
A 77-year-old-male was admitted to our hospital with a history of recurrent visible hematuria for 3 months and fever for 1 month on February 4, 2017. Medical history showed he had no symptoms of night sweat, dysuria, or abdominal pain. He experienced sustained fever with maximal temperature of 39°C for 1 month without any infectious symptoms. Prostate cancer was highly suspected after palpation of the prostate. Biochemical examination showed that renal and hepatic function tests were normal. Serum prostate-specific antigen (PSA) and free PSA were normal. Subsequent pulmonary enhanced computed tomography (CT) scan showed multiple metastatic tumors in both lungs (Figure 1). Moreover, an enhanced magnetic resonance imaging (MRI) of the prostate was performed and revealed the prostate was enlarged in size (4.2×4.7×6.2 cm) with multiple high signals indicating small nodules in the diffusion-weighted imaging image. Also, multiple enlarged lymph nodes in the pelvic cavity were found (Figure 2). Pelvic cavity-enhanced CT scan showed the bladder was well filled with a uniform wall, no abnormal density foci, and macroscopic lesions. Then, prostate needle biopsy was performed by transrectal ultrasonography. Postoperative histopathology revealed poorly differentiated transitional cell carcinoma. Immunohistochemical staining showed the tumor cells were positive for cytokeratin (CK) 20(+), CK7(+), CK8/18(+++), CK5/6(+++), 34βE12(+++), and P504S(+), but negative for CK34, D2-40, uroplakin, and PSA (Figure 3). Therefore, he was diagnosed as having primary prostatic poorly differentiated urothelial carcinoma. Finally, he underwent a chemotherapy regimen consisting of gemcitabine 1,200 mg d1, d8; cis-platinum 40 mg d2–3 q3W. Following 1 course of chemotherapy, normal temperature was achieved by the patient.
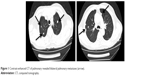  | Figure 1 Contrast-enhanced CT of pulmonary revealed bilateral pulmonary metastases (arrow). |
Discussion
Primary PUC has an extremely low incidence rate. Melicow and Hollowell2 first reported carcinoma in situ of the prostate coexistent with bladder transitional cell carcinoma as Bowen’s disease in 1952. Since then, several studies have reported transitional cell cancer involvement of the prostate with an incidence between 12% and 48%.3 However, the incidence of primary PUC is rare, and it usually occurs in elderly patients and is accompanied by hematuria or dysuria. The underdiagnosis of PUC is common, and most patients are at an advanced stage with distant metastasis at diagnosis. PSA is a highly specific biomarker for the prostatic lineage and almost exclusively synthesized in the prostate ductal and acinar epithelium. It has been confirmed that PSA level measurement plays an important role in the differential diagnosis of bladder cancer from prostate cancer.4 However, PSA is also expressed in some nonprostatic tissues and extraprostatic neoplasms, such as urethral, periurethral, perianal glands, cloacogenic carcinoma, salivary duct carcinoma, salivary gland pleomorphic adenoma, and rare breast carcinomas.5 Previous studies also suggested PSA staining intensity was negatively correlated with Gleason score, and it was also shown that patients with high-grade (Gleason score 8–10) prostate cancer might have low or even normal PSA level.6 Given the limitations of PSA, the clinical differential diagnosis becomes more difficult. Consistent with previous primary PUC results,7 the patient in our study had a normal serum PSA level, which suggested PSA level might be a useful biomarker to differentiate primary PUC from typical adenocarcinoma of prostate. Cystoscopy is described as a valuable tool to diagnose urothelial tumor if there are macroscopic lesions. In our study, we highly suspected prostate cancer after prostate palpation. Because pelvic cavity-enhanced CT and MRI scan excluded bladder macroscopic lesions, we did not choose further cystoscopy. Postoperative histopathology of prostate needle biopsy provided important evidence to support the diagnosis of primary PUC.
Liedberg et al8 showed primary PUC had a unique and separate staging system, which was different from prostatic adenocarcinoma. Radical surgery should be pursued for patients with PUC who are in the early stage of disease. For advanced-stage PUC, gemcitabine combined with cis-platinum is the first-line chemotherapy regimen.9 Cheville et al10 reported primary PUC with a poor prognosis and 5-year survival rate of 52%. Ichihara et al’s11 study suggested that primary PUC patients with stromal invasion or lymph node metastasis have worse prognosis. Also, PUC patients with contiguous malignant involvement had a 7% 5-year survival rate.12 The patient in our study had a much worse prognosis factor, ie, bilateral pulmonary metastases. Although gemcitabine combined with cis-platinum regimen achieved response, further follow-up is required to observe the long-term efficiency.
Conclusion
Primary PUC is a rare malignant tumor that involves the urinary system and is associated with a poor prognosis. Given the extremely low incidence of prostatic malignant tumors, further studies are needed to enlarge the number of cases and perform a multicenter, prospective cohort study to investigate clinical outcomes of primary PUC.
Consent
Written informed consent was obtained from the patient for publication of this case report and the accompanying images published.
Acknowledgment
This work was supported in part by grants of National Natural Science Foundation of China (81000228).
Disclosure
The authors report no conflicts of interest in this work.
References
Shen SS, Lerner SP, Muezzinoglu B, Truong LD, Amiel G, Wheeler TM. Prostatic involvement by transitional cell carcinoma in patients with bladder cancer and its prognostic significance. Hum Pathol. 2006;37(6):726–734. | ||
Melicow MM, Hollowell JW. Intra-urothelial cancer: carcinoma in situ, Bowen’s disease of the urinary system: discussion of thirty cases. J Urol. 1952;68(4):763–772. | ||
Walsh DL, Chang SS. Dilemmas in the treatment of urothelial cancers of the prostate. Urol Oncol. 2009;27(4):352–357. | ||
Dabbs DJ. Diagnostic Imunohistochemistry. 3rd ed. Philadelphia: Saunders-Elsevier; 2010:621–625. | ||
Kamoshida S, Tsutsumi Y. Extraprostatic localization of prostatic acid phosphatase and prostate-specific antigen: distribution in cloacogenic glandular epithelium and sex-dependent expression in human anal gland. Hum Pathol. 1990;21(11):1108–1111. | ||
Mhawech P, Uchida T, Pelte MF. Immunohistochemical profile of high-grade urothelial bladder carcinoma and prostate adenocarcinoma. Hum Pathol. 2002;33(11):1136–1140. | ||
Osunkoya AO, Epstein JI. Primary mucin-producing urothelial-type adenocarcinoma of prostate: report of 15 cases. Am J Surg Pathol. 2007;31(9):1323–1329. | ||
Liedberg F, Chebil G, Månsson W. Urothelial carcinoma in the prostatic urethra and prostate: current controversies. Expert Rev Anticancer Ther. 2007;7(3):383–390. | ||
Palou J, Wood D, Bochner BH, et al; International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder Cancer 2012. ICUD-EAU International Consultation on Bladder Cancer 2012: urothelial carcinoma of the prostate. Eur Urol. 2013;63(1):81–87. | ||
Cheville JC, Dundore PA, Bostwick DG, et al. Transitional cell carcinoma of the prostate: clinicopathologic study of 50 cases. Cancer. 1998;82(4):703–707. | ||
Ichihara K, Masumori N, Kitamura H, Hasegawa T, Tsukamoto T. Clinical outcomes of urothelial carcinoma of the prostate detected in radical cystectomy specimens. Int J Clin Oncol. 2014;19(1):152–156. | ||
Pagano F, Bassi P, Ferrante GL, et al. Is stage pT4a (D1) reliable in assessing transitional cell carcinoma involvement of the prostate in patients with a concurrent bladder cancer? A necessary distinction for contiguous or noncontiguous involvement. J Urol. 1996;155(1):244–247. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.