Back to Journals » International Medical Case Reports Journal » Volume 10
Case report of a computerized tomography sign in Strongyloides stercoralis infection
Received 17 August 2016
Accepted for publication 11 May 2017
Published 27 June 2017 Volume 2017:10 Pages 219—222
DOI https://doi.org/10.2147/IMCRJ.S119960
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Levent Filik
Sandeep Yerra1 Pradeep Yarra2
1Anesthesiology, University of Virginia, Charlottesville, VA, USA; 2Internal Medicine, Wake Forest Baptist Health, Winston Salem, NC, USA
Abstract: This is a case of strongyloidiasis showing colon enhancement on a computerized tomography (CT) scan. The patient presented with chief complaints of diarrhea and abdominal pain. She gave a history of recent travel to El Salvador and her stool was positive for Strongyloides stercoralis. A CT scan revealed a circumferential enhancement of the sigmoid colon. This CT sign was unusual in strongyloidiasis and when combined with the symptoms, caused us to rule out inflammatory bowel disease and shock bowel. Stool examination revealed ova and parasites of Strongyloides stercoralis. She was then treated with ivermectin which resulted in resolution of her symptoms.
Keywords: strongyloidiasis of colon, inflammatory bowel disease, parasitic infections, ulcerative colitis
Introduction
Strongyloidiasis is caused by Strongyloides stercoralis, a parasite seen commonly in the developing world, whereas in the developed world it is not so common. It is usually seen in travelers with a recent history of travel to tropical countries or in the immigrant population.1 It is a treatable infection but diagnosis is difficult because the larvae are secreted infrequently and low infectious burden of the parasite. Single stool examinations often present false negatives with a sensitivity of only 50%.2 Serial stool examinations using the agar plate method is most sensitive for the detection of S. stercoralis; however enzyme-linked immunosorbent essay (ELISA) (in vitro diagnostic-ELISA and Bordier-ELISA) test can yield results much faster with the sensitivity reaching 97.2%.3 If the patients are immunosuppressed, S. stercoralis can cause hyperinfection which can be rapidly fatal.
The purpose of this case report is to show that a circumferential enhancement of colon on a computerized tomography (CT) scan can occur in strongyloidiasis. Circumferential homogenous enhancement of colon, i.e., white enhancement, particularly in diarrhea, is seen in many diseases like inflammatory bowel disease (IBD), shock bowel, and several infections like Enterobius. This sign on a CT scan can easily be confused with above mentioned diseases, as CT signs of strongyloidiasis are relatively rare in sigmoid colon. Though colonic wall invasion with S. stercoralis is seen in strongyloidiasis hyperinfection, this is not usually shown on CT scans.4 There have been several case reports of an intermediate presentation where the diseased presents were between a normal infection and hyperinfection.5 We believe our patient belongs in this spectrum of the disease, as this patient had no clinically appreciable signs of hyperinfection in the lungs, liver, or brain.
Symptoms of S. stercoralis range from simple urticarial purpura of the feet to pulmonary symptoms like infiltrates, hemorrhage and edema.6,7 The life cycle of strongyloidiasis starts once they penetrate the skin of the feet in their filarial stage.8,9 After penetrating the skin they migrate through blood to the lungs. From the lungs the larvae migrate through the tracheobronchial tree where they cause irritation and dry cough. Finally they may reach the stomach and duodenum when sputum is swallowed. Here they mature into adult worms in the mucosa of the stomach and duodenum. Autoinfection by this parasite in particular can be dangerous and can lead to manifestations in the colon and perianal area. Autoinfection is also the major cause of Strongyloides hyperinfection syndrome, especially in an immunocompromised host. Hallmarks include high prevalence in immigrants, upper abdominal pain mimicking a duodenal ulcer, and the larva currens (a serpiginous, raised, erythematous track that develops at the point of entry of larva due to their migration)10 and eosinophilia occurring in 70% of the patients.
Consent
Written informed consent was obtained from the patient for the publication of this case report.
Case
A 78-year-old underweight Hispanic woman with underlying mitral stenosis presented to the emergency department with complaints of abdominal pain, nausea, dehydration, and diarrhea for the past 2 days. She apparently had similar episodes in the past for which she did not receive any treatment. The last episode was 4 years ago. She also gave a history of travel the previous 4 weeks to El Salvador. Her daughter traveled with her but she did not have any symptoms. The patient was not on any medication before, during, and after her travel. She also did not get any travel vaccinations barring the annual flu vaccine 4 months prior. Review of other systems was negative except as stated previously. The patient also denied blood in stool, symptoms of urinary infection, respiratory infection, headaches, illicit drug use, fever, thyroid symptoms, herbal medications, and any exotic food consumption. Though, she did admit to consuming local food and water in El Salvador. Significant past medical history included mitral stenosis and hypertension. Family history and social history were insignificant.
On examination she was anorexic with a fever of 39.1°C. Vitals were: systolic blood pressure of 116 mm of hg, diastolic blood pressure of 64 mm of hg, pulse rate of 96, respiratory rate of 19, and oxygen saturation was at 93%. Abdominal examination was significant for generalized tenderness to palpation, but without rigidity and guarding suggestive of peritonitis. Bowel sounds were normal and review of other systems revealed a cardiac murmur. Also to be noted was the absence of dermatologic signs and symptoms like larva currens and petechial eruptions.
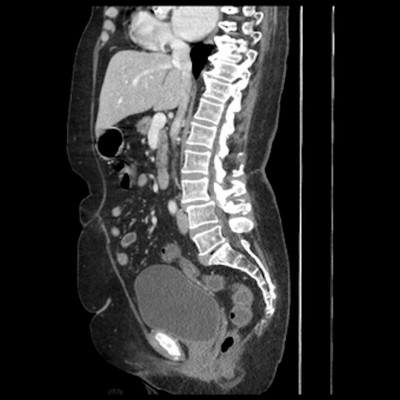
Chest X-ray showed possible pneumonia but there were no physical signs or symptoms. Stool samples were sent for culture and examination. Other pertinent negatives include Helicobacter pylori toxin, sepsis screen, urine toxicology screen, hematochezia, negative work-up of hepatitis, and liver function tests. Based on the travel history, chest X-ray showing possible pneumonia, lack of clinically appreciable respiratory symptoms, presence of diarrhea, and absence of initial work-up for abdominal infections, a preliminary diagnosis of pneumonia or intestinal infection was considered. She was started on metronidazole and ciprofloxacin for IBD and other infections. Fluids were started to rehydrate the patient. She continued to have more than six bowel movements per day on day 2. As the nutritional status of the patient was poor and there was a concern for some other pathology leading to these symptoms, a CT scan of the abdomen was ordered. It showed mild circumferential enhancement and haziness of the wall of the sigmoid colon and the rectum. As per radiology, this finding was non-specific and usually seen in proctocolitis. Figures 1 and 2 show the CT findings in this case. Later, her stool examination showed ova and parasites of S. stercoralis. She was put on ivermectin 200 micrograms/kilogram once a day for 2 consecutive days while continuing her antibiotics. This was a very unusual sign and rarely seen in strongyloidiasis. After eliminating other pathologies through a detailed history ruling out ulcerative colitis and other infections, we went back to confirm our diagnosis with ELISA. We ruled out hyperinfection since the patient was not immunosuppressed and there were no physical signs of involvement of other systems. We attribute her history of weight loss possibly to chronic or sub-acute infection. She recovered well with two doses of oral ivermectin. Follow-up 4 weeks later for her heart condition showed that she had put on 2 kilograms of weight and had no recurrence of her symptoms. Repeat testing of her stool was negative for ova and parasites.
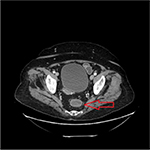  | Figure 1 Axial view of computerized tomography scan of abdomen. Notes: The arrow shows mild circumferential enhancement and haziness of the wall of the distal sigmoid colon and rectum. |
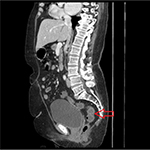  | Figure 2 Sagittal view of computerized tomography scan of abdomen. Notes: The arrow shows mild circumferential enhancement and haziness of the wall of the distal sigmoid colon and rectum. |
Discussion
In this case report, we report an atypical case of S. stercoralis infection, involving an unusual complex of signs and symptoms which include a sigmoid colon abnormality. This is possibly a new spectrum of this disease, lying between an acute infection and hyperinfection.
Radiological signs of strongyloidiasis in stomach, duodenum, and jejunum have been well documented in the literature and are known in current clinical practice. Figures 1 and 2 show colon enhancement due to strongyloidiasis. This finding of colon and rectal enhancement is a very unusual finding in strongyloidiasis. This sign is seen in many diseases in conjugation with diarrhea. This can potentially lead to a misdiagnosis or searching for alternative pathology, particularly since this sign in Strongyloides is relatively unknown in clinical practice. As per the radiology report, the increased enhancement was nonspecific and seen in proctocolitis. We can assume this radiological finding is due to S. stercoralis, as the patient has had past infections with Strongyloides and it is known to cause edema and ulcerations in the colon. This is supported by the fact that Strongyloides infections can recur and last decades, and our patient might very well have had autoinfections for which there is no diagnostic test other than to rely on history. The patient also did not have any other coexisting bacterial or viral infection. This presentation is somewhere in the spectrum between an acute infection and hyperinfection with S. stercoralis. Since hyperinfection with Strongyloides usually leads to colonic pathology, we can safely assume it is the cause of the CT abnormality in our patient, especially in the absence of IBD symptoms and history. In the differential that we presented, there is a possibility of using immune-suppression as a treatment option or mis-diagnosing the symptomatology. Strongyloidiasis may lead to hyper-infection if the patient is immuno-suppressed or misdiagnosed. Strongyloides hyper-infection has a high mortality rate which reaches 90% if left untreated. This was particularly true in our patient since her nutritional status was poor and we had to make the judgment call of using ivermectin. We suspect that this radiological sign is seen in patients who have had multiple S. stercoralis infections or a chronic infection. We also advocate the need for more studies of similar cases and an expanded classification of the disease of a localized colonic infection without signs of systemic infection.
Disclosure
The authors report no conflicts of interest in this work.
References
Posey DL, Blackburn BG, Weinberg M, et al. High prevalence and presumptive treatment of schistosomiasis and strongyloidiasis among African refugees. Clin Infect Dis. 2007;45(10):1310–1315. | ||
Boulware DR, Stauffer WM 3rd, Walker PF. Hypereosinophilic syndrome and mepolizumab. N Engl J Med. 2008;358(26):2839. | ||
Ramanathan R, Nutman TB. Strongyloides stercoralis infection in the immunocompromised host. Curr Infect Dis Rep. 2008;10(2):105–110. | ||
Minematsu H, Hokama A, Makishi T, Arakaki K, Kinjo F, Fujita J. Colonoscopic findings and pathologic characteristics of Strongyloides colitis: a case series. Digestion. 2011;83(3):210–214. | ||
Berry AJ, Long EG, Smith JH, Gourley WK, Fine DP. Chronic relapsing colitis due to Strongyloides stercoralis. Am J Trop Med Hyg.1983;32(6):1289–1293. | ||
Mackey SL, Wagner KF. Dermatologic manifestations of parasitic diseases. Infect Dis Clin North Am. 1994;8(3):713–743. | ||
Meinking TL, Burkhart CN, Burkhart CG. Changing paradigms in parasitic infections: common dermatological helminthic infections and cutaneous myiasis. Clin Dermatol. 2003;21(5):407–416. | ||
Schupf N, Ortiz M, Kapell D, Kiely M, Rudelli RD. Prevalence of intestinal parasite infections among individuals with mental retardation in New York State. Ment Retard. 1995;33(2):84–89. | ||
Lindo JF, Robinson RD, Terry SI, Vogel P, Gam AA, Neva FA, Bundy DA. Age-prevalence and household clustering of Strongyloides stercoralis infection in Jamaica. Parasitology. 1995;110(Pt 1):97–102. | ||
Berkmen YM, Rabinowitz J. Gastrointestinal manifestations of stongyloidiasis. Am J Roentgenol Radium Ther Nucl Med. 1972;115(2):306–311. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
