Back to Journals » Infection and Drug Resistance » Volume 15
Case Report and Literature Review of Impetigo-Like Tinea Faciei
Authors Zhang F , Feng Y, Wang S, Li D, Shi D
Received 22 January 2022
Accepted for publication 29 April 2022
Published 12 May 2022 Volume 2022:15 Pages 2513—2521
DOI https://doi.org/10.2147/IDR.S359500
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Fangfang Zhang,1,2 Yahui Feng,1 Sisi Wang,3 Dongmei Li,4 Dongmei Shi3,5
1College of Clinical Medicine, Jining Medical University, Jining, 272000, People’s Republic of China; 2Department of Dermatology, Jining Dermatosis Prevention and Treatment Hospital, Jining, 272000, People’s Republic of China; 3Laboratory of Medical Mycology, Jining No. 1 People’s Hospital, Jining, 272000, People’s Republic of China; 4Department of Microbiology & Immunology, Georgetown University Medical Center, Washington, DC, 20057, USA; 5Department of Dermatology, Jining No. 1 People’s Hospital, Jining, 272000, People’s Republic of China
Correspondence: Dongmei Shi, Laboratory of Medical Mycology, Jining No. 1 People’s Hospital, Jining, 272000, People’s Republic of China, Tel +86 537-6051008, Email [email protected]
Abstract: Impetigo, commonly caused by bacteria, is characterized by lesions of pustules, bullae or golden yellow crusts; it is seldom caused by fungi. Here, we report one case of a 17-year-old female patient with a 1-month history of erythematous pustules on her left cheek. She was clinically diagnosed with “impetigo”, but did not respond to 1 week of treatment with topical mupirocin cream (antibacterial agent). We then saw that a fungal colony grew on the culture, which was identified as T. mentagrophytes based on the morphological and molecular characteristics. The patient was then diagnosed with tinea faciei and was topically treated with 0.2% ketoconazole cream twice per day for 7 days. Through a literature review, we found another 18 cases of impetigo-like tinea faciei with similar clinical manifestations and pathogenic characteristics. Among these, the most common causative agent was T. mentagrophytes complex, which frequently occurs in children and adolescents and exhibits no gender preferences. Systemic and topical antifungals such as terbinafine or itraconazole are effective for impetigo-like tinea faciei caused by T. mentagrophytes complex. However, prolonged course of impetigo in more than 50% cases highlights the importance of mycological examination when dealing with apparent antibiotic-resistant impetigo cases in clinical settings.
Keywords: tinea faciei, impetigo, Trichophyton mentagrophytes
Introduction
Tinea faciei is a relatively uncommon superficial dermatophyte infection that occurs on the smooth regions of the face and as reported, affects both sexes and all age groups.1,2 But it has two peaks of incidence: the first peak occurs in children at 2 to 14 years of age with a predominance among males, often after contact with domestic animals, with rare cases described in babies younger than 12 months.3,4 The second peak is seen in adults aged 40 and older, with a prevalence among women resulting from occupational exposure or leisure activities.5 Indeed, the female predominance may not even be accurate, since dermatophyte infections on the bearded areas of males are often diagnosed as tinea barbae. With regard to aetiology, the causative agents of the infection are mainly M. canis, T. mentagrophytes and T. rubrum, respectively.6,7
At present, methods for identification of dermatophytes comprise both molecular methods and phenotypic approaches. Although microscopic examination and culture (of dermatophytes) can be used as a diagnostic tool in the hands of an experienced mycologist, species identification from fungal culture still take a relatively long time for this group of fungi. Molecular techniques such as real-time PCR and gene sequencing have been greatly appreciated for the accurate diagnosis of fungal infection. They still serve as the gold standard for differential diagnosis of fungal infections caused by morphologically similar species, but molecular methods are costly.8
In terms of clinical manifestations, typical lesions of tinea faciei are characterized by one or more erythematous macules and circular erythematous-scaling patches with central clearing. However, the occasional absence of these lesions frequently leads to misdiagnosis as eczema, contact dermatitis, seborrheic dermatitis, folliculitis, cutaneous lupus erythematosus, rosacea, psoriasis, purpura, or pemphigus.9–17 It has even been reported that up to 70% of patients with tinea faciei were initially misdiagnosed.1 As a result of misdiagnosis, lesions are often treated with topical steroids or calcineurin inhibitors, which in turn exacerbate the inflammatory symptoms, leading to more severe erythema and scaling, newly scattered pustules or papules. These inflammatory symptoms deviate from the characteristic features of dermatophytosis and often lead to delays in diagnosis and appropriate antifungal therapy.18–20
Tinea faciei rarely mimic impetigo in clinical manifestations.9,21,22 Impetigo is a contagious, superficial bacterial infection of the skin commonly caused by Staphylococcus aureus and most frequently occurs in children 2–5 years of age.23–25 It is characterized by discrete, thin-walled vesicles that rapidly become pustular and then rupture.23,26 Here, we report a case of tinea faciei caused by T. mentagrophytes which mimics bacterial impetigo. We include 18 published tinea faciei cases whose lesions mimic bacterial impetigo (Table 1) and their clinical and mycological features are reviewed.4,13,27–42
 |
Table 1 Cases of Impetigo-Like Tinea Faciei |
Case Presentation
A 17-year-old female presented with a 1-month history of progressive erythematous and pustules involving the left cheek on her first visit to our hospital on Jan. 5, 2021. One month prior, initial lesions appeared on her left cheek as tiny erythematous macules and pustules. The patient complained of itching. Gradually, most of the erythematous macules came to be covered with loosely stratified golden-yellow crusts; the patient was clinically diagnosed with “impetigo”. The patient was topically treated with 2% mupirocin cream for 7 days, but the lesions gradually enlarged and spread on both cheeks and the chin. The patient had a history of 4 weeks of close contact with a cat, but no minor trauma or other medical events.
Physical examination showed several erythematous macules ranging from 0.5 cm to 1 cm in diameter on both cheeks and chin. Most of erythematous lesions were eventually covered with golden-yellow scales or crusts which were readily removed as shown in Figure 1A–C. Regional lymph nodes were not palpable.
 |
Figure 1 Images of the lesions before treatment (A–C). Image of the lesions on her right cheek (A). Image of the lesions on her chin (B). Image of the lesions on her left cheek (C). |
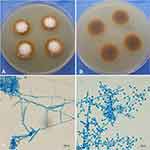
The scales taken from the lesions were stained with calcofluor white and septate hyphae were observed under fluorescent microscopy. Meanwhile, the scales were inoculated and cultured at 28°C on Sabouraud-glucose Agar with Chloramphenicol (SDA) (Hope Bio-Technology Co., Ltd., Qingdao, Shandong, China) for fungal growth and Columbia Blood Agar (CBA) (Hope Bio-Technology Co., Ltd., Qingdao, Shandong, China) for bacterial growth, respectively. A white fungal colony was evident after 1 week, which was peripherally radiating but centrally raised with powdery margins (Figure 2A) and a yellow to brown color on the reverse side of the plate (Figure 2B). Under microscopy, a grape-like arrangement of the microconidia was observed laterally and terminally distributed along filamentous and spiral hyphae (Figure 2C and D). Conversely, there was no growth of bacteria on the CBA.
Subsequently, the isolated fungal species were identified by sequencing the internal transcribed spacer (ITS) and β-tubulin gene using the fungal primers as follows: ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′), ITS4 (5′-TCCTCCGCTTATTGATATGC-3), β-tubulin 2a (5′-AATTGGTGCCGCTTTCTGG-3′) and β-tubulin 2b (5′-AGTTGTCGGGACGGAATAG-3′).43 The sequencing data were aligned with reference sequences in GenBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Our target sequence obtained a 99% coverage and 99.27% homology with T. mentagrophytes No. WCH-AV003, and a 99% coverage and 99.27% homology with T. mentagrophytes No. DSM-108626, which can be accessed in GenBank with registration number MZ712214.1. The strain (No. CCJNMM-1678) was stored in the laboratory of medical mycology, Jining No. 1 People’s Hospital. A phylogenetic tree in Figure 3 was constructed based on the results of BLAST searches through GenBank with ITS and β-tubulin sequences using the maximum-likelihood method in MEGA 7.0.44 Phylogenetic evaluation used the neighbor-joining method with 1000 bootstrap simulations; bootstrap values greater than 70% were regarded as significant. The type strain of Microsporum gypseum (actually Nannizzia gypsea) CBS 146.66 was used as an outgroup. The antifungal sensitivity test was followed using the broth dilution method of the Laboratory Standards Institute (CLSI) M38-A3 protocol with antifungal agents, terbinafine, amphotericin B, ketoconazole, itraconazole, posaconazole, voriconazole, or micafungin.45 The minimum inhibitory concentrations (MIC) for terbinafine, amphotericin B, ketoconazole, itraconazole, posaconazole, voriconazole and micafungin were 0.015, 0.125, 0.03, ≤0.03, ≤0.03, ≤0.03 and 8 μg/mL, respectively.
Our patient was then diagnosed with impetigo-like tinea faciei caused by T. mentagrophytes. The patient was given 0.2% ketoconazole cream twice a day. The lesions completely ceased to be visible after 1 week (Figure 4A–C). Direct and fluorescence mycological examination in a 2-week follow-up showed negative for fungal growth.
Discussion and Conclusion
Tinea faciei is a relatively uncommon superficial dermatophyte infection accounting for 3% to 4% of all cases of tinea corporis.1 The infection, when limited to the glabrous skin of the face, can present with many atypical lesions, including the impetigo-like symptoms we report here.9,21,46,47 Because impetigo-like tinea faciei is rare and easily overlooked in clinical settings, we believe the misdiagnosis rate is high. Indeed, in our literary collection of 18 impetigo-like tinea faciei cases, 11 of the 18 cases were misdiagnosed before pathogen determination. As a result of misdiagnosis, these cases were typically treated with topical steroids or calcineurin inhibitors for their anti-inflammatory effects, which in turn accelerated dermatophyte growth and made the diagnosis more difficult to defend, even without the resulting delays in proper antifungal therapy.29,39,42
We include our case with those 18 cases to summarize the clinical features and fungal distribution of impetigo-like tinea faciei with the aim of helping diagnosis in future. Epidemiologically, tinea faciei is widespread globally but prefers humid tropical climates. Among the 19 cases, 5 were reported in China, 3 in Spain and the remaining 11 appeared to be scattered throughout the rest of the world. Furthermore, the sexes were represented roughly equally: 10 cases of females (53%) and 9 cases of males (47%). Impetigo-like tinea faciei affects all age groups, which differs from “true” impetigo commonly caused by bacteria in children from 2 to 5 years of age.23 Impetigo-like tinea faciei in children and adolescents accounted for 79% of the 19 cases, of which 7 occurred before the age of 5, 8 occurred at ages 5–18, and 4 cases appeared in adults over 18. We have no complete explanation for the predilection of impetigo-like tinea faciei for younger patients, but we note that 17 patients reported a history of close contact with domestic animals, and the remaining two cases were unable to categorically deny such contact. This evidence is bolstered by the fact that the 17 cases were infected by zoophilic species, specifically 12 cases of T. mentagrophytes complex (63.2%), 1 case of T. verrucosum, 2 cases of Nannizzia gypsea, and 2 cases of M. canis-in contrast to the 2 cases of anthropophilic species (T. rubrum and T. tonsurans). As others have observed, infections caused by zoophilic species tend to be self-healing, and the resultant inflammation is more severe when accompanied by papules, vesicles and pustules.48,49 The dominant T. mentagrophytes strain in these patients is a well-known zoophilic fungus that generally only infests the stratum corneum of the epidermis and induces a higher inflammatory reaction than anthropophilic fungi,50 which could easily mimic impetigo in these patients.51,52 We therefore emphasize the importance of obtaining a detailed history of any animal contact for impetigo patients in particular with severe inflammatory lesions. To date, the increase in the keeping of exotic animals as pets has resulted in the emergence of several zoonotic diseases that could potentially be transmitted to humans as well. Although the person-to-person transmission route is still more common for dermatophytosis, the roles of environmental reservoirs such as clothes, bed linen, mattresses and infected pets should not be ignored in order to control fungal infection.53
With regard to the identification of a species within a complex, phylogenetic tree based on the sequences of the ITS regions could be useful to describe the evolutionary relationship among these species. However, even though the use of more genetic markers beyond ITS regions has been remarkably successful in delineating most species, the discrimination of species in Trichophyton complex clade remains difficult.54 T. mentagrophytes complex comprises five species as follows: T. mentagrophytes, T. interdigitale, T. erinacei, T. quinckeanum, and T. benhamie, as well as nine other morphologically similar genotypes of T. mentagrophytes/T. interdigitale.55 In addition to the genetic method, the matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry (MS)-based strategy (an emerging microbial protein profile identification system) has also been explored for facilitating identification of clinical dermatophyte isolates.19 One study even used MALDI-TOF MS strategy combined with the traditional methods to identify T. benhamiae.40 However, lack of complete databases of filamentous fungal species and standardized procedures limit the practicality of MALDI-TOF MS in dermatophyte detection.
Clinically, the lesions of impetigo-like tinea faciei are easily misdiagnosed as bacterial impetigo. The delayed treatment then prolongs the course of the disease. Lesions often persist more than one month and even up to 2 years before the diagnosis are correct. The durations of cases listed in Table 1 range from 4 days to 7 months with 10 cases lasting more than 1 month. This prolonged course again highlights the importance of mycological examination when dealing with apparent antibiotic-resistant impetigo cases.56
Treatment of tinea faciei is the same as for other superficial fungal infections. Most tinea faciei cases are curable with topical antifungal treatments.9,57 Systemic antifungal treatment is recommended for severe or prolonged cases.57 Since zoophilic fungi could induce a high inflammatory response, an anti-inflammatory steroid can be given simultaneously, but never without the antifungals.58 Dermatophytes vary in their susceptibility to the available antifungal agents, but most dermatophytes are susceptible to antifungals although drug resistant strains have been reported recently.59,60 Among these impetigo-like tinea faciei cases, 7 of 19 were cured with topical antifungal treatments alone and 12 cases were cured with oral antifungal treatments alone or in combination with topical drugs. The oral antifungals used in those 12 cured patients were terbinafine or itraconazole (11 patients) or griseofulvin (1 case). In other studies, terbinafine has been shown to be an effective antifungal against dermatophyte isolates.61 However, dermatophytes resistant to terbinafine have emerged recently.7,20,59,60,62 Therefore, it is vitally important to perform in vitro antifungal susceptibility tests (AFST) to guide therapeutic measures.20
In summary, impetigo-like tinea faciei is a superficial dermatophyte infection of the skin commonly caused by T. mentagrophytes complex; it most frequently occurs in children and adolescents and seldom occurs in adults. One limitation of this study is the small number of representative cases which somewhat hamper our conclusion. When children or adolescents with impetigo-like lesions present with severe inflammation but do not respond to antibiotics, mycological examination should be done in order to facilitate a correct diagnosis and provide guidance on treatment.
Abbreviations
A, anthropophilic; AFST, antifungal susceptibility tests; CBA, Columbia blood agar; CMZ, Clotrimazole; D, day; F, female; GRF, griseofulvin; ITC, itraconazole; ITS, internal transcribed spacer; KCZ, Ketoconazole; MALDI-TOF MS, matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry; M, male (in sex); M, month (in age); NTF, Naftifine; O, oral; Refs, references; SDA, Sabouraud-glucose Agar with Chloramphenicol; T, Topical; TRB, Terbinafine; Y, year; Z, zoophilic.
Data Sharing Statement
All the data are fully available without restriction. All data generated or analyzed during this study are included in this published article. The sequence data have been deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/Genbank/index.html) with the accession number MZ712214.1.
Ethics Approval and Informed Consent
This study was approved by the Institutional Research and Ethics Committee of Jining No. 1 People’s Hospital to publish the case details (Ethical approval no. 2021-057). The patient provided written informed consent for publication of this case report and any accompanying images. The study was carried out in accordance with the principles of the Declaration of Helsinki. The first author vouches for the completeness and accuracy of the data and for the adherence of the study to the protocol.
Consent for Publication
Written informed consent was obtained from the patient’s parents described in this report. A copy of the written consent is available by request.
Acknowledgments
We thank the patient for granting permission to publish this information.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (NM 81773337), the Key Research and Development Plan of Shandong Province (NM 2019GSF108191), and the Key Research and Development Plan of Jining (NM 2021YXNS121), Shandong, China.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43(6):437–440.
2. Ebrahimi M, Zarrinfar H, Naseri A, et al. Epidemiology of dermatophytosis in northeastern Iran; a subtropical region. Curr Med Mycol. 2019;5(2):16–21.
3. Stanes EK, Strunk T. Tinea faciei in a very preterm infant. Arch Dis Child Fetal Neonatal Ed. 2018;103(4):F376.
4. Malhotra S, Malhotra SK, Aggarwal Y. Tinea faciei caused by Trichophyton mentagrophytes in a 20-day-old neonate. Indian Dermatol Online J. 2015;6(Suppl 1):S43–S46.
5. Borges A, Brasileiro A, Galhardas C, Apetato M. Tinea faciei in a central Portuguese hospital: a 9-year survey. Mycoses. 2018;61(4):283–285.
6. Takenaka M, Murota H, Nishimoto K. Epidemiological survey of 42 403 dermatophytosis cases examined at Nagasaki University Hospital from 1966 to 2015. J Dermatol. 2020;47(6):615–621.
7. Ansari S, Ahmadi B, Tabatabaeifar SN, et al. Familial cases of Trichophyton benhamiae infection transmitted from a guinea pig in Iran. Mycopathologia. 2021;186(1):119–125.
8. Meena S, Mohanty A, Kaistha N, Sasirekha U, Meena J. Comparative assessment of Matrix-assisted Laser Desorption Ionization-time of Flight Mass Spectrometry (MALDI-TOF-MS) and conventional methods in the identification of clinically relevant yeasts. Cureus. 2021;13(6):e15607.
9. Nicola A, Laura A, Natalia A, Monica P. A 20-year survey of tinea faciei. Mycoses. 2010;53(6):504–508.
10. Shapiro L, Cohen HJ. Tinea faciei simulating other dermatoses. JAMA. 1971;215(13):2106–2107.
11. Meymandi S, Wiseman MC, Crawford RI. Tinea faciei mimicking cutaneous lupus erythematosus: a histopathologic case report. J Am Acad Dermatol. 2003;48(2 Suppl):S7–S8.
12. Nenoff P, Schetschorke C. Images in clinical medicine. Tinea faciei. N Engl J Med. 2014;370(20):e31.
13. Kang DX, Ran YP, Li CH, Dai YL, Lama J. Impetigo-like tinea faciei around the nostrils caused by Arthroderma vanbreuseghemii identified using polymerase chain reaction-based sequencing of crusts. Pediatr Dermatol. 2013;30(6):e136–e137.
14. Arenas R, Moreno-Coutiño G, Vera L, Welsh O. Tinea incognito. Clin Dermatol. 2010;28(2):137–139.
15. Rallis E, Koumantaki-Mathioudaki E. Pimecrolimus induced tinea incognito masquerading as intertriginous psoriasis. Mycoses. 2008;51(1):71–73.
16. Serarslan G. Pustular psoriasis-like tinea incognito due to Trichophyton rubrum. Mycoses. 2007;50(6):523–524.
17. Singh R, Bharu K, Ghazali W, Bharu K, Nor M, Kerian K. Tinea faciei mimicking lupus erythematosus. Cutis. 1994;53(6):297–298.
18. Dolenc-Voljč M, Gasparič J. Human infections with Microsporum gypseum complex (Nannizzia gypsea) in Slovenia. Mycopathologia. 2017;182(11–12):1069–1075.
19. Hedayati MT, Ansari S, Ahmadi B, et al. Identification of clinical dermatophyte isolates obtained from Iran by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Curr Med Mycol. 2019;5(2):22–26.
20. Ansari S, Ahmadi B, Hedayati MT, et al. Investigation of in vitro antifungal susceptibility testing and genetic diversity of clinical isolates of Trichophyton benhamiae and Trichophyton eriotrephon in Iran. Mycoses. 2021;64(3):316–323.
21. Farokhipor S, Ghiasian SA, Nazeri H, Kord M, Didehdar M. Characterizing the clinical isolates of dermatophytes in Hamadan city, Central west of Iran, using PCR-RLFP method. J Mycol Med. 2018;28(1):101–105.
22. Kromer C, Celis D, Hipler UC, Zampeli VA, Mößner R, Lippert U. Dermatophyte infections in children compared to adults in Germany: a retrospective multicenter study in Germany. J Dtsch Dermatol Ges. 2021;19(7):993–1001.
23. Hartman-Adams H, Banvard C, Juckett G. Impetigo: diagnosis and treatment. Am Fam Physician. 2014;90(4):229–235.
24. Johnson MK. Impetigo. Adv Emerg Nurs J. 2020;42(4):262–269.
25. Pereira LB. Impetigo - review. An Bras Dermatol. 2014;89(2):293–299.
26. VanRavenstein K, Durham CO, Williams TH, Smith W. Diagnosis and management of impetigo. Nurse Pract. 2017;42(3):40–44.
27. Kimura U, Yokoyama K, Hiruma M, Kano R, Takamori K, Suga Y. Tinea faciei caused by Trichophyton mentagrophytes (molecular type Arthroderma benhamiae) mimics impetigo: a case report and literature review of cases in Japan. Med Mycol J. 2015;56(1):E1–E5.
28. Criado PR, Costa AR, Vasconcellos C, Ramos RO, Silva CS, Souza S. Tinea faciei in an infant caused by Microsporum gypseum simulating a dry impetigo. Pediatr Dermatol. 2005;22(6):536–538.
29. Amigo M, Milani-Nejad N, Mosser-Goldfarb J. Periocular Tinea Faciei. J Pediatr. 2020;221:255–256.
30. Zeng MH, Chen J, Kong QT, et al. Facial tinea due to Microsporum gypseum: a case report and review of the literature. Chin J Mycol. 2012;7(03):163–165.
31. Chen KL, Chien MM, Lu CY, Chiu HC. Zoophilic Tinea Faciei. J Pediatr. 2017;182:395–395.e1.
32. Rivaya B, Fernández-Rivas G, Cabañes FJ, et al. Trichophyton erinacei: an emergent pathogen of pediatric dermatophytosis. Rev Iberoam Micol. 2020;37(3–4):94–96.
33. Huang SY, Kong QT, Du X, Yang R, Sang H. A case of misdiagnosed tinea faciei caused by Arthroderma vanbreuseghemii. Chin J Mycol. 2016;11(4):
34. Amina A, Sana M. Tinea Faciei Incognito. Indian Pediatr. 2019;56(5):433.
35. Zhuang KW, Dai YL, Ran YP, Lama J, Fan YM. Tinea faciei on the right eyebrow caused by Trichophyton interdigitale. An Bras Dermatol. 2016;91(6):829–831.
36. Concha M, Nicklas C, Balcells E, et al. The first case of tinea faciei caused by Trichophyton mentagrophytes var. erinacei isolated in Chile. Int J Dermatol. 2012;51(3):283–285.
37. Lee DW, Yang JH, Choi SJ, et al. An unusual clinical presentation of tinea faciei caused by Trichophyton mentagrophytes var. erinacei. Pediatr Dermatol. 2011;28(2):210–212.
38. Patel G, Mills C. Tinea faciei due to Microsporum canis abscess formation. Clin Exp Dermatol. 2000;25(8):608–610.
39. Turra N, Navarrete J, Magliano J, Bazzano C. Follicular tinea faciei incognito: the perfect simulator. An Bras Dermatol. 2019;94(3):372–374.
40. Lozano-Masdemont B, Carrasco-Fernández B, Polimón-Olabarrieta I, Durán-Valle MT. Arthroderma benhamiae, an emerging dermatophyte cause of tinea. Actas Dermosifiliogr. 2020;111(2):167–168.
41. Zisova LG, Dobrev HP, Tchernev G, et al. Tinea atypica: report of nine cases. Wien Med Wochenschr. 2013;163(23–24):549–555.
42. Berry A, Abramovici G, Chamlin SL. A 21-day-old boy with an annular eruption. Tinea faciei/Tinea capitis. Pediatr Ann. 2014;43(1):e16–e18.
43. Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61(4):1323–1330.
44. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874.
45. Barbara D, Gary W, Philippe D, et al. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. CLSI Document. M38-A3. Clinical and Laboratory Standards Institute; 2017.
46. Atzori L, Aste N, Aste N, Pau M. Tinea faciei due to microsporum canis in children: a survey of 46 cases in the District of Cagliari (Italy). Pediatr Dermatol. 2012;29(4):409–413.
47. Dev T, Saginatham H, Sethuraman G. Tinea faciei: challenges in the diagnosis. J Pediatr. 2017;187:331.
48. Klinger M, Theiler M, Bosshard PP. Epidemiological and clinical aspects of Trichophyton mentagrophytes/Trichophyton interdigitale infections in the Zurich area: a retrospective study using genotyping. J Eur Acad Dermatol Venereol. 2021;35(4):1017–1025.
49. Shiraki Y, Ishibashi Y, Hiruma M, Nishikawa A, Ikeda S. Cytokine secretion profiles of human keratinocytes during Trichophyton tonsurans and Arthroderma benhamiae infections. J Med Microbiol. 2006;55(Pt 9):1175–1185.
50. Mahmoudabadi AZ. A study of dermatophytosis in South West of Iran (Ahwaz). Mycopathologia. 2005;160(1):21–24.
51. Del Boz J, Crespo V, Rivas-Ruiz F, de Troya M. Tinea incognito in children: 54 cases. Mycoses. 2011;54(3):254–258.
52. Rist TE, Abele DC, Caves JM. Tinea faciale: an often misdiagnosed clinical entity. South Med J. 1974;67(3):331–334.
53. Fijan S, Turk SŠ. Hospital textiles, are they a possible vehicle for healthcare-associated infections. Int J Environ Res Public Health. 2012;9(9):3330–3343.
54. Baert F, Lefevere P, D’hooge E, Stubbe D, Packeu A. A polyphasic approach to classification and identification of species within the Trichophyton benhamiae complex. J Fungi. 2021;7(8):87.
55. Frías-de-león MG, Martínez-Herrera E, Atoche-Diéguez CE, et al. Molecular identification of isolates of the Trichophyton mentagrophytes complex. Int J Med Sci. 2020;17(1):45–52.
56. Tan J, Liu X, Gao Z, Yang H, Yang L, Wen H. A case of tinea faciei caused by Trichophyton benhamiae: first report in China. BMC Infect Dis. 2020;20(1):171.
57. Khaled A, Chtourou O, Zeglaoui F, Fazaa B, Jones M, Kamoun MR. Tinea faciei: a report on four cases. Acta Dermatovenerol Alp Pannonica Adriat. 2007;16(4):170–173.
58. Czaika VA, Lam PA. Trichophyton mentagrophytes cause underestimated contagious zoophilic fungal infection. Mycoses. 2013;56(Suppl 1):33–37.
59. Yamada T, Maeda M, Alshahni MM, et al. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob Agents Chemother. 2017;61(7):8789.
60. Taghipour S, Shamsizadeh F, Pchelin IM, et al. Emergence of terbinafine resistant Trichophyton mentagrophytes in Iran, harboring mutations in the Squalene Epoxidase (SQLE) gene. Infect Drug Resist. 2020;13:845–850.
61. Behnam M, Zarrinfar H, Najafzadeh MJ, Naseri A, Jarahi L, Babič MN. Low in vitro activity of sertaconazole against clinical isolates of dermatophyte. Curr Med Mycol. 2020;6(1):36–41.
62. Rudramurthy SM, Shankarnarayan SA, Dogra S, et al. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62(5):949.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.