Back to Journals » Infection and Drug Resistance » Volume 15
Case Report and Literature Review: Disseminated Histoplasmosis Infection Diagnosed by Metagenomic Next-Generation Sequencing
Authors Wang N, Zhao C, Tang C , Wang L
Received 27 April 2022
Accepted for publication 22 July 2022
Published 12 August 2022 Volume 2022:15 Pages 4507—4514
DOI https://doi.org/10.2147/IDR.S371740
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Neng Wang,* Conglin Zhao,* Congchen Tang, Lichun Wang
Center of Infectious Diseases, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lichun Wang, Center of Infectious Diseases, West China Hospital of Sichuan University, 37 Guoxue Lane, Chengdu, 610041, People’s Republic of China, Tel +86 288 542 2859, Fax +86 288 542 3052, Email [email protected]
Background: Histoplasmosis is a deep fungal infection caused by Histoplasma capsulatum and can be classified as pulmonary, disseminated or central. Disseminated histoplasmosis is the most dangerous of all clinical types and is characterized by rapid onset, rapid progression, high mortality, and difficulty in diagnosis and treatment.
Case Presentation: This report describes a 31-year-old female who presented with fever, with a maximum temperature of 39.8 °C. There were no concomitant symptoms, such as cough, sputum, abdominal pain and diarrhoea, before the onset of fever, and the illness lasted for more than 20 days. On examination, the liver and spleen were enlarged, and laboratory tests showed a significant decrease in CD4 cell count, suggesting immune deficiency. Broad-spectrum antibiotic treatment was ineffective, and specific infectious diseases and haematological neoplasms were considered likely. She was finally diagnosed with disseminated histoplasmosis after undergoing bone marrow aspiration and metagenomic next-generation sequencing (mNGS) and was treated with amphotericin B, fluorouracil and itraconazole, with good results.
Conclusion: This case demonstrates that disseminated histoplasmosis infection can present with unexplained fever and that mNGS can be an important complement to bone marrow aspiration for the diagnosis of this disease.
Keywords: disseminated histoplasmosis, immune deficiency, metagenomic next-generation sequencing, amphotericin B, case report
Background
Histoplasmosis is a deep fungal infection caused by Histoplasma capsulatum and is divided into Histoplasma capsulatum and Histoplasma capsulatum var. duboisii, which have different epidemic regions and clinical manifestations, with the former found mostly in North and South America and the latter mainly in Africa.1 In recent years, the incidence of histoplasmosis has been increasing, with millions of tourists travelling in highly endemic areas and an increase in immunosuppressed populations, such as organ transplant patients on immunosuppressive drugs and diabetic patients.2 However, disseminated Histoplasma capsulatum infection is easily confused with Leishmania and Talaromyces marneffei in diagnosis. These infections have similar clinical manifestations and similarities in bone marrow microscopy, as they are located in mononuclear macrophages and have a similar shape and size. The gold standard for the diagnosis of these diseases remains as direct microscopic examination and culture. Macrogenomic next-generation sequencing (mNGS) provides a good method for the definitive diagnosis of this disease.3,4
mNGS, also known as high-throughput or massively parallel sequencing, can simultaneously sequence hundreds to billions of DNA or RNA fragments independently. It can detect pathogenic microorganisms in a wide range of body fluids and is valuable for diagnosing infectious diseases.5,6 mNGS can overcome the limitations of current diagnostic tests by directly detecting all pathogens in clinical samples, regardless of microbial type. It can even be used to discover new pathogens. Compared with traditional methods, such as microbial culture, nucleic acid amplification, serum pathogen antibody testing and pathogen-specific polymerase chain reaction (PCR), mNGS offers many advantages in pathogen detection. Traditional PCR assays require predesigned primers based on sequence information of known pathogens, while mNGS allows unbiased sequencing of all nucleic acid sequences in clinical samples. When a patient is infected with multiple pathogens, mNGS can detect all of them, thus providing physicians with time to make a definitive diagnosis. In addition, mNGS can detect differences between animal- and human-derived pathogens and elucidate the origins of zoonotic diseases.7
Based on the diagnosis and treatment background of this case, we analysed the epidemiological status, clinical manifestations, diagnostic criteria, and treatment guidelines of disseminated histoplasmosis to provide more data on the diagnosis and treatment of this disease.
Case Presentation
The patient was a 31-year-old female admitted to the hospital with fever. More than 20 days prior to admission, she developed a fever with no apparent cause, with a maximum temperature of 39.8 °C. The fever was accompanied by chills but no sore throat or cough. She received anti-infective treatment, such as imipenem/cilastatin sodium, at other hospitals, but her fever symptoms did not improve significantly; therefore, she was transferred to our hospital for further treatment. She was previously diagnosed with chronic hepatitis B for more than 10 years and took oral tenofovir antiviral therapy regularly. She had been diagnosed with polycystic ovary syndrome 5 years previously and had been treated with prednisone in the past. In addition, she denied recent travel, surgery or trauma, and she reported no history of infectious diseases, diabetes mellitus or use of alcohol or drugs.
On admission, she had a high body temperature (38.5 °C), an increased respiratory rate (23 breaths/min), normal blood pressure (117/61 mmHg), increased heart rate (124 beats/min), and normal oxygen saturation in ambient air (97%). Physical examination showed slightly harsh breath sounds in both lungs, and no rales were heard. The liver and spleen could be palpated by one finger under the rib.
Laboratory investigations showed decreased haemoglobin 97 g/L, platelets [68×109/L; normal range, (100–300)×109/L], CD4 cell count [135 cell/µL; normal range, 471–1220 cell/µL], and albumin (34 g/L; normal range, 40–55 g/L). The patient’s procalcitonin level (0.45 g/L; normal range, <0.046 ng/L), C-reactive protein (28.20 normal range, <5.00 ng/L), and interleukin-2 receptor (4450 μg/L; normal range, 223–710 μg/L) levels were elevated. The patient’s red blood cells [3.92×1012/L; normal range, (3.8–5.1)×1012/L], white blood cells [5.46×109/L; normal range, (3.5–9.5)×109/L], globulin (32.0 g/L; normal 20–35 g/L), triacylglycerol (triglycerides: 1.82 mmol/L), fibrinogen (2.75 g/L; normal 2–4 g/L), ferritin (217 ng/L; normal 24–336 ng/L) and aspergillus-antigen test for serum galactomannan (0.27 μg/L; normal 0–0.85 μg/L) were in the normal ranges. Tests for HBV-DNA, HCV-RNA, CMV-DNA, EB-DNA, anti-dsDNA, ANA profile, HIV-1 antibody, anti-tuberculosis antibody, and 1,3-β-D-glucan were negative. Peripheral blood cultures drawn during repeated fever episodes and SARS-CoV-2 based on PCR testing of a nasopharyngeal swab were both negative. Abdominal CT examination showed enlargement of the liver and spleen and multiple cystic lesions in the bilateral adnexa. (Figure 1A) We also performed head CT, chest CT, urological ultrasonography, thyroid ultrasonography, routine echocardiography and other related tests, all of which yielded negative results. (Figure 1B) After admission, we continued to administer anti-infective treatment with imipenem/cilastatin sodium (500 mg every 8 h), but the patient still had recurrent fevers. Due to the patient’s low CD4 cell count, we still considered the cause of her fever as infection rather than tumour or immunogenic. The patient remained hyperthermic and did not respond to multiple medications, and we encountered difficulties in the diagnosis and treatment of the disease. Then, we performed a bone marrow smear and found some phagosomes in the cytoplasm that were suspected to be Histoplasma capsulatum. (Figure 2) The bone marrow fluid was subjected to mNGS analysis on Day 4 of hospitalization. mNGS was performed according to the standard protocol of Illumina sequencing on the NextSeq550 platform. A total of 363 sequence readings of histoplasmosis were detected in bone marrow within 24 h, accounting for 2.614% of the genome coverage. The clinical diagnosis was disseminated Histoplasma capsulatum infection. On the fifth day of hospitalization, we changed imipenem/cilastatin sodium to amphotericin B deoxycholate (40 mg once a day) intravenous infusion and fluorouracil tablet (1 g once a day) oral anti-infection therapy after searching for evidence of the pathogen. Considering the side effects of amphotericin B deoxycholate, the patient received an acid suppressant for gastric protection, reduced glutathione (GSH) for liver protection, and oral potassium chloride for potassium supplementation. During the course of treatment, the patient’s creatinine level was found to be elevated, and amphotericin B deoxycholate was gradually adjusted to 25 mg once a day intravenously. After active treatment, the patient’s general condition improved, and her body temperature gradually returned to normal. (Figure 3) Prior to discharge, the patient’s interleukin 2 receptor and procalcitonin returned to normal levels. (Table 1) A bone marrow smear did not reveal Histoplasma capsulatum. The patient was discharged from the hospital on oral itraconazole 200 mg twice daily antifungal therapy, and the bone marrow smear was normal after 6 months of follow-up.
 |
Table 1 Blood Test Results |
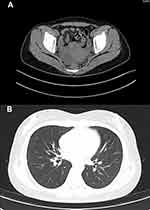 |
Figure 1 Abdominal CT revealed multiple cystic foci in the adnexa bilaterally (A). Chest CT showed scattered striated foci in both lungs with thickening of some lobular septa (B). |
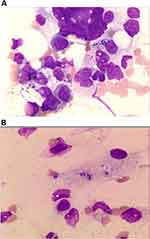 |
Figure 2 Wright’s staining (1000×), some phagocytes phagocytized suspected Histoplasma capsulatum in the cytoplasm (A). These fungi are surrounded by a transparent halo (B). |
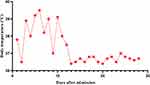 |
Figure 3 The curve of body temperature during hospitalization. Antifungal therapy with amphotericin B and v fluorouracil tablets was administered from day 5 after admission. |
All procedures performed in studies involving human participants were in accordance with institutional and/or National Research Council ethical standards and the Declaration of Helsinki (revised 2013). Written informed consent was obtained from patients for publication of this study and any accompanying images.
Literature Review
Pathogenesis
Histoplasma capsulatum is a dimorphic fungus that mainly invades the reticuloendothelial system or the lungs. It is distributed worldwide and is mainly endemic in the Americas, Africa, Asia and other regions.2 In China, the first case was found in 1955 in Guangzhou.8 It is an endemic disease, and the sources of infection are birds in nature, such as chickens, bats, and pigeons; their faeces-contaminated soil and dust; and infected animals, such as cats, dogs, cattle and horses. The respiratory tract is the main route of infection, but it can also be transmitted through the skin or mucous membranes. It has already been proven that in women undergoing antitumour necrosis factor therapy, it is possible to infect the foetus through the placenta and umbilical cord. Operators of and travellers to caves with bats or large mountain tunnels and mines are susceptible to infection and outbreaks.9,10 Although no clear epidemiological exposure was found in this patient, a careful epidemiological history study of an immunocompromised population would help to better clarify the diagnosis.
Clinical Manifestations
According to the clinical manifestations, the disease can be divided into pulmonary, disseminated and central nervous system infections. The disseminated form is commonly seen in immunodeficient patients and presents with chills, fever, dyspnoea, hepatosplenomegaly, skin and mucous membrane damage, and complete blood cytopenia, as well as symptoms of hyperalgesia, such as hypotension, hyponatremia, and hyperkalaemia. Diffuse infiltrative manifestations are seen on CT of the chest. Central nervous system infections include encephalomyelitis, cerebrovascular accidents and disseminated encephalitis, which manifest as fever, headache, psychoneurological symptoms, and seizures. Disseminated histoplasmosis has diverse clinical manifestations, lacks specificity, and is easily misdiagnosed; from 1990 to 2011, more than 300 cases of histoplasmosis have been reported in China,11 with 75.2% of the relevant literature mentioning misdiagnosis.12 Therefore, the presence of this disease should be considered in those with fever with multiorgan involvement and high-risk factors, such as immunocompromise.
Diagnosis
Histoplasmosis was defined according to the consensus13 of the European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC ⁄ MSG) in 2008. It refers to a disease in which Histoplasma capsulatum is found positive in any culture or confirmed by histopathology of blood, bone marrow or other infected sites. If a patient demonstrates involvement at a single site and lacks symptoms or signs of systemic involvement, the disease is defined as focal histoplasmosis. Disseminated histoplasmosis is considered when Histoplasma capsulatum is confirmed from blood and bone marrow or the fungus is confirmed from multiple nonpolluting sites in the organism. In disseminated histoplasmosis, early diagnosis is the key to saving the patient. The diagnosis of histoplasmosis is based on the corresponding clinical picture and positive culture results or typical histopathological examination. However, the culture process is tedious and complicated, requiring dimorphic culture, with a typical cycle time of 2–6 weeks and a low positivity rate, which is not conducive to early diagnosis. Microscopic examination has early diagnostic value, and the yeast phase generally appears under the microscope as round or ovoid, narrow, germinating yeast-like fungi, approximately 2–4 µm in size, mostly seen in the macrophage cytoplasm. General conventional staining is difficult to detect, and special staining, such as Richter’s stain or hexamine silver stain, can improve the detection rate. The microscopic morphology of Histoplasma capsulatum is similar to that of pathogens such as Talaromyces marneffei, and the atypical histopathological presentation and low detection rate in culture render accurate diagnosis challenging.14 As a result, there are still many cases that are misdiagnosed or missed, especially in individuals from nonendemic areas. mNGS, as a next-generation sequencing technology, does not depend on microbial culture and can identify pathogens quickly and precisely. Hu et al5 found through a large-sample study that mNGS is more sensitive than conventional culture, has obvious diagnostic advantages for fungi, tuberculosis, viruses, and anaerobes and is unaffected by antibiotic application, making it a promising test. An expert consensus in China also noted that mNGS has a high clinical value because of its diagnostic accuracy and the short time needed, which is especially suitable for the pathogenic diagnosis of critical and difficult infections.15 Recently, Zhang et al16 performed simultaneous myeloscopy and mNGS in five patients infected with Histoplasma capsulatum, Leishmania, or Talaromyces marneffei but with similar clinical symptoms and found that the diagnostic accuracy of mNGS was 100%, which was significantly higher than that of conventional tests. In nonendemic areas of China, clinicians are not sufficiently aware of histoplasmosis, and histoplasmosis is often underdiagnosed or misdiagnosed. At this time, mNGS can be used as a supplement to classical diagnostic methods to improve the accuracy of the diagnosis of this disease. However, mNGS also has some limitations. For example, the approach does not clearly prove the relationship between pathogens and disease progression and has a relatively low detection efficiency for intracellular bacteria and fungi with cell walls. In addition, the human host background must be sequenced before mNGS can be used, and not all genomes are available. Specimens are susceptible to contamination by environmental species, which can lead to erroneous results. Inability to determine infection and colonization status is also a concern. The cost of testing is another disadvantage.4 Currently, mNGS cannot replace traditional microbiological testing methods due to limitations in sampling standards, testing costs and testing methods.
Treatment
Disseminated histoplasmosis is aggressive, progresses rapidly, and requires early and effective treatment. According to the guidelines of the Infectious Diseases Society of America,3 itraconazole 200 mg twice daily for at least 12 months is recommended for mild to moderate cases, and amphotericin B liposomes 3–5 mg (kg*d) for 1 to 2 weeks followed by sequential administration of itraconazole 200 mg twice daily for at least 12 months are recommended for severe cases.2 Itraconazole can be used as the oral agent of choice for mild and moderate patients, or it can be tapered after the patient has developed an initial response to amphotericin B. In this case, our patient was treated with amphotericin B deoxycholate antifungal infection after the course of the disease was determined, and the patient’s general condition improved after one week. The indices of interleukin-2 receptor, procalcitonin and IL-6 gradually decreased. After 6 months, no Histoplasma capsulatum was found in the bone marrow examination, and the treatment was considered effective. In addition to amphotericin B and itraconazole, other triazoles, such as fluconazole and voriconazole, are also effective in the treatment of disseminated histoplasmosis, but fluconazole is still less effective than itraconazole at a large dose (800 mg/d); voriconazole is effective, but the dearth of relevant studies has limited its clinical application.17 Currently, amphotericin B and itraconazole are still the first-line drugs for the treatment of disseminated histoplasmosis. Posaconazole can also be used as rescue treatment for histoplasmosis in cases with a poor response.18
Conclusion
Although Histoplasma capsulatum is mainly transmitted via dust and aerosols, we can learn from this case that chest imaging in disseminated histoplasmosis can appear normal. In immunodeficient patients with unexplained fever, the possibility of Histoplasma capsulatum infection needs to be considered. Treatment remains based on classical amphotericin B and itraconazole, and posaconazole may be used as a salvage treatment option. In addition, the rapid development of mNGS provides accurate and timely diagnostic tools for some rare and complex pathogens, which can improve the diagnosis of pathogens such as Histoplasma capsulatum.
Data Sharing Statement
The datasets supporting the conclusions of this article are included in the article.
Consent for Publication
Written informed consent was provided by the patient for the publication of the case details and images. Details of the case can be published without institutional approval.
Acknowledgments
We are grateful for the useful comments and suggestions from the anonymous referees.
Funding
No extramural funding was received for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gascón J, Torres JM, Luburich P, Ayuso JR, Xaubet A, Corachán M. Imported histoplasmosis in Spain. J Travel Med. 2000;7(2):89–91. PubMed PMID: 10759576. doi:10.2310/7060.2000.00028
2. Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807–825. PubMed PMID: 17806045. doi:10.1086/521259
3. Wang C, Li A, Shi Q, Yu Z. Metagenomic next-generation sequencing clinches diagnosis of leishmaniasis. Lancet. 2021;397(10280):1213. PubMed PMID: 33773632. doi:10.1016/s0140-6736(21)00352-4
4. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14:319–338. PubMed PMID: 30355154; PubMed Central PMCID: PMCPMC6345613. doi:10.1146/annurev-pathmechdis-012418-012751
5. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. PubMed PMID: 30423048. doi:10.1093/cid/ciy693
6. Wang S, Chen Y, Wang D, et al. The feasibility of metagenomic next-generation sequencing to identify pathogens causing tuberculous meningitis in cerebrospinal fluid. Front Microbiol. 2019;10:1993. PubMed PMID: 31551954; PubMed Central PMCID: PMCPMC6733977. doi:10.3389/fmicb.2019.01993
7. Harrison EM, Paterson GK, Holden MT, et al. Whole genome sequencing identifies zoonotic transmission of MRSA isolates with the novel mecA homologue mecC. EMBO Mol Med. 2013;5(4):509–515. PubMed PMID: 23526809; PubMed Central PMCID: PMCPMC3628104. doi:10.1002/emmm.201202413
8. Li YCB. A case of histoplasmosis. Natl Med J China. 1958;3:301–302.
9. Ashford DA, Hajjeh RA, Kelley MF, Kaufman L, Hutwagner L, McNeil MM. Outbreak of histoplasmosis among cavers attending the National Speleological Society Annual Convention, Texas, 1994. Am J Trop Med Hyg. 1999;60(6):899–903. PubMed PMID: 10403317. doi:10.4269/ajtmh.1999.60.899
10. Lyon GM, Bravo AV, Espino A, et al. Histoplasmosis associated with exploring a bat-inhabited cave in Costa Rica, 1998–1999. Am J Trop Med Hyg. 2004;70(4):438–442. PubMed PMID: 15100461. doi:10.4269/ajtmh.2004.70.438
11. Pan B, Chen M, Pan W, Liao W. Histoplasmosis: a new endemic fungal infection in China? Review and analysis of cases. Mycoses. 2013;56(3):212–221. PubMed PMID: 23216676. doi:10.1111/myc.12029
12. Yu J, Chen M, Huang Y, Zhu L, Zhang J. A report of seven cases of histoplasmosis and literature review. Chin J Infect Chemother. 2014;6:408–414.
13. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. PubMed PMID: 18462102; PubMed Central PMCID: PMCPMC2671227. doi:10.1086/588660
14. Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20(1):115–132. PubMed PMID: 17223625; PubMed Central PMCID: PMCPMC1797635. doi:10.1128/cmr.00027-06
15. Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the leap from research laboratory to clinic: challenges and opportunities for next-generation sequencing in infectious disease diagnostics. mBio. 2015;6(6):e01888–e01915. PubMed PMID: 26646014; PubMed Central PMCID: PMCPMC4669390. doi:10.1128/mBio.01888-15
16. Zhang HC, Zhang QR, Ai JW, et al. The role of bone marrow metagenomics next-generation sequencing to differential diagnosis among visceral leishmaniasis, histoplasmosis, and talaromycosis marneffei. Int J Lab Hematol. 2020;42(2):e52–e54. PubMed PMID: 31592568. doi:10.1111/ijlh.13103
17. Wheat LJ, Connolly P, Smedema M, et al. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. J Antimicrob Chemother. 2006;57(6):1235–1239. PubMed PMID: 16627592. doi:10.1093/jac/dkl133
18. Restrepo A, Tobón A, Clark B, et al. Salvage treatment of histoplasmosis with posaconazole. J Infect. 2007;54(4):319–327. PubMed PMID: 16824608. doi:10.1016/j.jinf.2006.05.006
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
