Back to Journals » Cancer Management and Research » Volume 14
Case Report: A Rare Case of Hepatoid Adenocarcinoma in Stomach and Duodenum Simultaneously
Authors Zhang Y, Han S, Lv L, Wang X, Zhu Y, Ying L
Received 11 January 2022
Accepted for publication 5 July 2022
Published 13 July 2022 Volume 2022:14 Pages 2185—2191
DOI https://doi.org/10.2147/CMAR.S354869
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Yue Zhang,1,* Shuanglin Han,1,* Li Lv,2 Xiaomei Wang,3 Yu Zhu,1 Li Ying1
1Department of Gastroenterology, The Second Hospital of Dalian Medical University, Dalian, Liaoning Province, 116027, People’s Republic of China; 2Department of Pathology, The Second Hospital of Dalian Medical University, Dalian, Liaoning Province, 116027, People’s Republic of China; 3Department of Nuclear Medicine, The Second Hospital of Dalian Medical University, Dalian, Liaoning Province, 116027, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li Ying, Department of Gastroenterology, The Second Hospital of Dalian Medical University, No. 467 Zhongshan Road, Shahekou Region, Dalian, Liaoning, 116023, People’s Republic of China, Tel +86-17709870657, Email [email protected]
Abstract: HAC is a rare extrahepatic malignancy histologically resembling hepatocellular carcinoma which can occur in many organs. Especially for cases carrying HAC within the stomach or duodenum, we found that typically HAC only happened in either of them and there was no case exhibiting HAC in both sites. Here, we presented a case having HAC in both sites and to our knowledge, this is the first report presenting HAC in these two distinct organs simultaneously. Firstly, a 57-year-old male was tested for increased serum alpha-fetoprotein level. Following PET-CT images showed strengthened FDG uptake in the gastric antrum and proximal duodenum. Esophagogastroduodenoscopy also revealed an irregular lump at the gastric antrum and a deep ulceration at the duodenum bulb. Next, an immunohistochemistry panel confirmed the diagnosis of HAC. Finally, genetic tests were performed on this patient after the clinical diagnosis of HAC to reveal the molecular etiology.
Keywords: HAC, case report, AFP, SALL4, endoscopy, NGS
Introduction
Hepatoid adenocarcinoma (HAC) refers to a scarce extrahepatic malignant neoplasm with the characteristics of hepatocellular differentiation. The higher the percentage of the HAC cell component in a tumor, the more alpha-fetoprotein (AFP) is secreted.1 HAC arises mostly in the stomach2 and also in other sites including esophagus,3 duodenum,4 jejunum,5 colon,6 peritoneum,7 pancreas,8 and ovary.9 Based on the reported cases for HAC, it only happens in a single organ and patients suffering from HAC typically undergo a poor prognosis due to the aggressive phenotypes of HAC containing various primary lesion sites, advanced tumor stages and early metastasis, thus early diagnosis becomes the most urgent issue for HAC patients.
To date, the diagnosis of HAC still heavily depends on the histology and immunohistochemistry (IHC) tests. Histological examination reveals large, polygonal cells with abundant eosinophilic cytoplasm in both trabecular and glandular structures.4 In addition to histological tests, HAC can be confirmed by several biomarkers such as Sal-like protein 4 (SALL4), AFP, hepatocyte and glypican 3 (GPC3)10 in which AFP and SALL4 are the most commonly used ones. AFP, as an oncogenic glycoprotein, is widely used in clinical practice to screen HCC. Elevated serum AFP had also been found in different kinds of cancers and one of which is AFP-positive gastric cancer (AFPGC). From empirical viewpoint, serum AFP level of AFPGC patients is only slightly higher than that in patients with the other common gastric cancers. The other commonly used biomarker, SALL4, is an oncofetal protein with unique expression pattern which is found to be expressed in the fetal liver but silenced in the adult liver. It could be detected in 94.7% of stomach HAC patients,1 but we could not find any article reporting the correlation between the expression level of SALL4 and HAC progression or patients’ prognosis.
In this report, we present the first case report about a patient who was diagnosed as HAC in our hospital and had HAC in both the stomach and duodenum.
Case Presentation
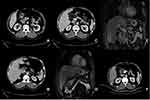
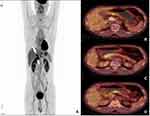
The patient was a 57-year-old male patient with a 30-year medical history of chronic hepatitis B virus (HBV) infection, and he was admitted to our department simply because of a significantly elevated serum level of AFP at 6998 IU/mL detected by routine serum test for tumor markers, but some other commonly used tumor markers including CA125, CA199 and CA724 were tested to be in the normal range. The patients did not have any uncomfortable symptoms and we did a physical examination for this patient where no positive sign could be found. Other laboratory tests showed that most of the clinical indexes related to blood cell count and liver function were still in the normal range. HBV surface antigen (HBsAg) was tested as seropositive whereas serum HBV- DNA was shown to be lower than the prescribed minimum of the test. Based on these results, originally, he was highly suspected to have primary HCC and to confirm our initial diagnosis, he underwent computerized tomography (CT) and magnetic resonance imaging (MRI) examinations which showed that no tumor mass could be found in the liver area. Although we did not find any tumor within the liver, we did discover a diffuse, uneven thickening and abnormal enhancement in the pyloric area which was approximately 22×24 mm in diameter under CT scan (Figure 1A). Cancerous thrombi were detected in the hepatic portal vein and left gastric vein by CT scan (Figure 1B and C). MRI scan also showed the same results in the left gastric vein and hepatic portal vein, respectively (Figure 1D and E). Compared to Figure 1B and C, Figure 1F represented the CT scan results for this patient finishing four months/four cycles of therapy after the initial AFP test which will be discussed in detail later. To get a more comprehensive understanding about the primary sites, PET-CT was performed, and the results illustrated that no more abnormal fluorodeoxyglucose (FDG) uptake could be detected other than the gastric antrum, proximal duodenum, left gastric vein and hepatic portal vein. In detail, maximum intensity projection (MIP)-FDG PET-CT image revealed an strengthened FDG activity at the gastric antrum and proximal duodenum (Figure 2A upper arrow: gastric antrum and proximal duodenum, lower arrow: tumor thrombus of the portal vein) and transverse views of the PET-CT image showed strengthened tracer uptake in gastric antrum (Figure 2B), left gastric vein (Figure 2C) and hepatic portal vein (Figure 2D) accompanying with standardized uptake values (SUV) as 4.1, 5.7, and 6.7, respectively. Meanwhile, esophagogastroduodenoscopy (EGD) showed a large irregular lump at the gastric antrum (Figure 3A) and a deeply ulcerating mass at the duodenum bulb (Figure 3B). Finally, pathological examinations for these two lesions revealed that both belonged to poorly differentiated adenocarcinoma. Figure 3C and D illustrated the hematoxylin and eosin (H&E) staining results for the tumor residing in gastric antrum and duodenum. Immunohistochemically, the tumor cells were demonstrated to be positive for SALL4 (Figure 3E and F: gastric antrum and duodenum), AFP (Figure 3G and H: gastric antrum and duodenum) and hepatocyte (Supplementary Figure 1A and B: gastric antrum and duodenum). Whereas GPC3 was shown to be positive in the tumor from gastric antrum (Supplementary Figure 1C) but barely positive in the tumor from duodenum (Supplementary Figure D). On the contrary, caudal-type homeobox 2 (CDX2) which is thought to be a highly sensitive and specific marker for adenocarcinomas of intestinal origin11 was negative in the tumor from the gastric antrum (Supplementary Figure 1E) but positive in the tumor from the duodenum (Supplementary Figure 1F) which met the diagnostic criteria for HAC precisely. To this stage, all the data suggested that the final diagnosis for this patient should be HAC rather than HCC.
To establish a comprehensive molecular concept of this tumor, we performed next-generation sequencing (NGS) on the 830 genes covering all the exons and parts of introns. The testing provided the reports for all the possible mutation types including point mutations, insertion or deletion mutations, copy number variations (CNV), rearrangement mutations. Besides these mutations, the results also covered tumor mutations burden (TMB) and microsatellite instability (MSI) reports. Surprisingly, common mutations found in TP53, GARD11, GEFR, FGA, and NOTCH3 and ERBB2 during the progression of HAC10 were not detected for our patient, which determined that no targeted drug could be used to promote his prognosis (Table 1). Instead of targeted agents, we utilized an empiric therapy consisting of docetaxel, oxaliplatin, tegafur gimeracil, and oteracil potassium capecitabine and the patients received this therapy for six cycles. As we mentioned before, according to a follow-up inspection for this patient (four months/four cycles of therapy after the initial AFP test), the tumor lesion disappeared in the CT scan and the blood AFP level was demonstrated to be lower than the limitation of detection, which suggested that our empiric therapy was effective, at least partly. After six cycles of therapy, the patient received regular follow-up examinations according to our suggestions and to control the tumor growth, the patient received another three cycles during the year of 2021. To date, the patients is still alive, and quality of life is not affected by this disease severely. All the mutated genes were listed in Table 1 and noticeably, most of these mutations were firstly reported here to the best of our knowledge. Next, we performed the pathway analysis for these 10 genes using DAVID online tool.12 Based on Supplementary Figure 2, the most profound pathway for these 10 genes was shown to be Notch signaling pathway which has been extensively studied and proved to play either an oncogenic or a tumor-suppressive role in different kinds of cancers.13 Supplementary Figure 3 demonstrated the timeline of events including diagnosis of chronic hepatitis B, date of raised AFP, treatment duration and dates of follow-up scans and tests.
 |
Table 1 Mutations Found by NGS for our Patient |
Discussion
In the last few years, an increasing number of patients have been described with AFP-producing malignant tumors of extrahepatic origin14 and referred to as HAC due to morphological features similar to HCC.15 The severity of HAC is highlighted by the fact that they show more aggressive behavior and poorer prognosis than cancers without HCC-like morphology, and 51.2% of patients die within the first 12 months of diagnosis.16 As we emphasized before, HAC typically only happens in a single site, whereas our case is the first case of HAC in which the tumor exists in both the stomach and duodenum. Then a diagnostic issue was introduced here about the primary site for our case. Apparently, there were three possible mechanisms for our case: (1) the original site was the and then it migrated to duodenum; (2) the original site was the duodenum and then it migrated to stomach; (3) the tumors in both sites were originally formed by themselves. Some clues in our results suggested that both the stomach and duodenal bulb might be the primary sites. The most significant difference was presented by EGD examination in which the morphology of the tumor in the gastric antrum was irregular lumps while the appearance of the tumor at the duodenal bulb was an ulcerative mass. But clearly, we could not generate the final conclusion solely depending on gross appearance. As the second most critical method for differential diagnosis, our IHC staining results showed some differences, but unfortunately, it seemed that we could not obtain a firm conclusion depending on them. The tumor cells from both sites were shown to be positive for common biomarkers for HAC including SALL4, AFP and hepatocyte. GPC3 was shown to be highly expressed by the tumor from the gastric antrum but barely positive in the tumor from the duodenum. Nevertheless, GPC3 is not a specific marker for either gastric HAC or duodenal HAC. In addition, CDX2 was positive in the tumor from the duodenum, but negative in the tumor from the gastric antrum. According to some recent studies, CDX2 was demonstrated to be a sensitive and highly, but incompletely specific marker of intestinal adenocarcinomas.11 Based on our results, the tumor located in the gastric antrum did not show any CDX2 expression which proved that the tumor in the antrum should be a primary site since it did not show any intestinal characters. Whereas whether the tumor in the duodenal area originated from the tumor in the gastric area or originated by itself remained unclear due to lacking direct evidence from our IHC results.
Currently, no effective chemotherapy approach has been developed for HAC. Platinum-based chemotherapy is the most efficient first-line treatment with a 75% positive response.17 The combination of chemotherapy regimens with targeted therapy (ie, trastuzumab) in HER2-positive tumors seems to be promising, although there lacks robust evidence.18 More clinical trials are urgently needed for the future treatment for HAC patients. To get a deeper understanding for the molecular mechanism of HAC, numerous NGS related projects were performed, and it turned out that the common mutations were related to TP53, GARD11, GEFR, FGA, and NOTCH3, suggesting that no matching targeted therapeutic agents could be used for patients’ benefits. Our pathway analysis suggested the development and progression of HAC is possibly closely related to Notch signaling pathway and for the future studies, to get a deeper understanding about the role of Notch signaling pathway in HAC might be helpful to reveal the actual pathogenic elements for HAC and provide new therapeutic targets for HAC patients.
In summary, in this report, we presented an extremely rare case of HAC occurring in the stomach and duodenum simultaneously. The diagnosis of HAC was made based on a series of laboratory tests, imaging tests, endoscopic examinations, and pathological confirmation. Moreover, genetic tests provided an overview of the molecular etiology for our case. Although noparticular therapeutic target for our patient existed, based on the genetic tests, the combination of docetaxel, oxaliplatin, tegafur, gimeracil, and oteracil potassium capecitabine eliminated the tumor bulk successfully which was confirmed by CT reexamination and the fact that the serum AFP level was reduced profoundly. From our perspective, the prognosis of HAC patients is dismal since HAC typically exhibits highly aggressive behaviors and early stage lymph node metastases. As a result of insufficient attentions on this disease, unless the patients have some specific clinical manifestations, HAC is prone to delayed diagnosis and management. Hence, at this stage, to increase the clinical awareness of HAC is an outstanding topic for clinicians. To the best of our knowledge the case we reported here is the first case who had HAC in two distinct sites synchronously and we suggest that in the future, it is worthwhile to be aware that HAC might happen in distinct sites within one single patient. Under this circumstance, comprehensive IHC tests of all the relevant markers we mentioned in the introduction for distinct sites are vital for the diagnosis of HAC and also valuable for resolving the matter of their origin. In addition, NGS tests for all the tumor sites are needed to provide genetic clues for distinguishing the primary sites within distinct tumor sites and molecular proofs for selecting effective targeted drugs.
Ethical Statement
The authors have completed the CARE reporting checklist. All data generated and analyzed during the current study are available from the corresponding author on reasonable request. The authors declare they have no conflict of interest. This study complied with the tenets of the Declaration of Helsinki and was approved by the Ethics Board of the Second Hospital of Dalian Medical University. Case report (CARE) guidelines were followed in this case study. A written consent from the patient was obtained prior to collecting the case material, analyzing the sequencing data, writing the final manuscript and publishing the case report.
Acknowledgments
This study was supported by fund from Department of Science and Technology of Liaoning Province named “The biological role of FGF21 in the bidirectional regulation of entero-hepatic axis in fatty hepatitis” (2019-ZD-0913).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Akiyama S, Tamura G, Endoh Y, et al. Histogenesis of hepatoid adenocarcinoma of the stomach: molecular evidence of identical origin with coexistent tubular adenocarcinoma. Int J Cancer. 2003;106:510–515. doi:10.1002/ijc.11246
2. Gao HY, Zhang YP, Yan YW, Shen HF. A case report of hepatoid adenocarcinoma of the stomach with liver and spleen metastasis misdiagnosed as advanced liver cancer. Zhonghua Gan Zang Bing Za Zhi. 2019;27:719–720. Chinese. doi:10.3760/cma.j.issn.1007-3418.2019.09.013
3. Nagai Y, Kato T, Harano M, et al. A case of AFP-producing esophagogastric junction cancer with liver metastases with a good response to chemotherapy. Gan to Kagaku Ryoho. 2014;41:2349–2351. Japanese.
4. Ogbonna OH, Sakruti S, Sulieman M, Ali A, Shokrani B, Oneal P. Hepatoid adenocarcinoma of the duodenum: an unusual location. Case Rep Oncol. 2016;9:182–187. doi:10.1159/000444746
5. Zeng XY, Yin YP, Xiao H, et al. Clinicopathological characteristics and prognosis of hepatoid adenocarcinoma of the stomach: evaluation of a pooled case series. Curr Med Sci. 2018;38:1054–1061. doi:10.1007/s11596-018-1983-1
6. Ogiwara S, Furihata M, Fukami K, Yamashita A, Yao T, Osada T. Hepatoid adenocarcinoma with enteroblastic differentiation in the sigmoid colon: lessons from a rare case. Am J Gastroenterol. 2019;114:684–685. doi:10.14309/ajg.0000000000000176
7. Zou M, Li Y, Dai Y, et al. AFP-producing hepatoid adenocarcinoma (HAC) of peritoneum and omentum: a case report and literature review. Onco Targets Ther. 2019;12:7649–7654. doi:10.2147/OTT.S216501
8. Williams NL, Palmer JD, Bar-Ad V, et al. Hepatoid carcinoma of the pancreas: a case report and review of the literature. Case Rep Pancreat Cancer. 2015;1:3–6. doi:10.1089/crpc.2015.29001.nlw
9. Choi WK, Cho DH, Yim CY, Lee NR. Primary hepatoid carcinoma of the ovary: a case report and review of the literature. Medicine. 2020;99:e20051. doi:10.1097/MD.0000000000020051
10. Xia R, Zhou Y, Wang Y, Yuan J, Ma X. Hepatoid adenocarcinoma of the stomach: current perspectives and new developments. Front Oncol. 2021;11:633916. doi:10.3389/fonc.2021.633916
11. Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303–310. doi:10.1097/00000478-200303000-00003
12. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi:10.1038/nprot.2008.211
13. Aster JC, Pear WS, Blacklow SC. The varied roles of notch in cancer. Annu Rev Pathol. 2017;12:245–275. doi:10.1146/annurev-pathol-052016-100127
14. Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med. 1970;78:1277–1278. Danish.
15. Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer. 1985;56:840–848. doi:10.1002/1097-0142(19850815)56:4<840::AID-CNCR2820560423>3.0.CO;2-E
16. Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol. 2013;19:321–327. doi:10.3748/wjg.v19.i3.321
17. Simmet V, Noblecourt M, Lizee T, et al. Chemotherapy of metastatic hepatoid adenocarcinoma: literature review and two case reports with cisplatin etoposide. Oncol Lett. 2018;15:48–54. doi:10.3892/ol.2017.7263
18. Soreide JA. Therapeutic approaches to gastric hepatoid adenocarcinoma: current perspectives. Ther Clin Risk Manag. 2019;15:1469–1477. doi:10.2147/TCRM.S204303
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.