Back to Journals » Cancer Management and Research » Volume 12
CASC8 lncRNA Promotes the Proliferation of Retinoblastoma Cells Through Downregulating miR34a Methylation
Authors Yang B, Gu B, Zhang J, Xu L, Sun Y
Received 18 June 2020
Accepted for publication 12 October 2020
Published 30 December 2020 Volume 2020:12 Pages 13461—13467
DOI https://doi.org/10.2147/CMAR.S268380
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Bo Yang,* Baoyu Gu,* Jing Zhang, Long Xu, Yong Sun
Department of Ophthalmology, Shenzhen Hospital of Integrated Chinese and Western Medicine, Shenzhen 518101, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong Sun
Department of Ophthalmology, Shenzhen Hospital of Integrated Chinese and Western Medicine, 3 Shajing Street, Bao’an District, Shenzhen 518101, Guangdong Province, People’s Republic of China
Tel +86 755-2772-2241
Email [email protected]
Background: CASC8 lncRNA has been proven to be oncogenic in a variety of cancers, but its role in other types of cancer remains unclear. This study was to investigate the role of CASC8 in retinoblastoma (Rb).
Methods: RT-qPCR was performed to determine the expression of CASC8 and miR34a in paired Rb and nontumor tissue. Overexpression of CASC8 and miR34a in Rb cells was achieved to evaluate the interaction between them. Methylation-specific PCR was used to analyze the effect of CASC8 overexpression on MIR34A gene methylation. CCK8 assays were used to analyze cell proliferation.
Results: The results showed that CASC8 expression was upregulated and miR34a expression downregulated in Rb tissue. Moreover, miR34a expression was negatively correlated with the of CASC8 expression in Rb tissue. Overexpression of CASC8 decreased expression of miR34a and increased methylation of MIR 34A in Rb cells. In addition, overexpression of CASC8 reduced the inhibitory effects of miR34a on Rb-cell proliferation.
Conclusion: CASC8 may promote Rb cell proliferation by downregulating miR34a methylation.
Keywords: retinoblastoma, CASC8, miR34a, methylation, proliferation
Introduction
Retinoblastoma (Rb) is a rare malignant tumor that originates from the photosensitive tissue of the eye, known as the retina.1 Rb is caused mainly by genetic mutation in retinal nerve cells. Cells with certain mutations will grow uncontrollably, leading to the formation of tumors.2 Rb affects mainly patients <5 years old.3 With proper treatment, 95% of Rb patients can be cured, and both eyes can be saved in 70%–80% cases.4,5 However, Rb in some cases can also spread to other parts of the body, such as the spine and brain, leading to poor prognosis.6 Therefore, novel therapeutic approaches are needed to further improve the survival of Rb patients.
Molecular factors play crucial roles in the pathogenesis of Rb.7,8 Functional characterization of molecular pathways, such as p53, RB1, and NFκB, provides novel insights to the development of novel anticancer therapies, such as targeted therapy.9,10 It has been well established that ncRNAs, such as lncRNAs), andmiRNAs are not involved in protein synthesis, but regulate the expression of cancer-related genes to suppress or promote cancer development.11 Therefore, ncRNAs are potential targets for targeted cancer therapy.12 However, the function of most ncRNAs is barely known. lncRNA CASC8 has been proven to be significantly correlated with several types of cancer, such as lung cancer,13 while its involvement in Rb is unknown. Our preliminary RNA-seq analysis revealed altered expression of CASC8 in Rb, and expression of CASC8 in Rb was negatively correlated with miR34a (data not shown), which is a well-established tumor-suppressive miRNA.14 This study was thus carried out to analyze interactions between CASC8 and miR34a in Rb.
Methods
Rb Patients
The Ethics Committee of Shenzhen Hospital of Integrated Chinese and Western Medicine approved this study. A total of 62 Rb patients (39 males and 23 females, 9 months to 3 years, 4 months, 2.0±0.4 years) were enrolled at this hospital between March 2016 and January 2019. All patients were newly diagnosed Rb patients. No recurrent Rb patients were enrolled. Patients with a family history of malignancy were excluded. Before this study, no therapy had been performed on these patients. The International Classification for Intraocular Retinoblastoma was used to stage the 62 Rb patients. Based on these criteria, patients were divided into group A (n=11), B (n=12), C (n=10), D (n=15), and E (n=14). In addition, patients included 28 cases with low-grade Rb and 34 high-grade Rb. All patients provided signed informed consent.
Specimen Collection and Rb Cells
During histopathological biopsy for the diagnosis of Rb, paired Rb and nontumor tissue samples were collected from each patient. A liquid-nitrogen tank was used to store all fresh tissue samples before subsequent experiments. Y79 and C33A (ATCC) human Rb cell lines were used. Cells were cultivated in a 5% CO2 and 95% humidity incubator at 37°C. The cell-culture medium was composed of RPMI 1640 (80%) and FBS (20%).
Cell Transfection
A CASC8-expression vector was constructed using a pcDNA3.1 vector (Invitrogen) as a backbone. Vector construction was provided by Invitrogen. Mimics of miR34a and negative control (NC) miRNA were from Invitrogen. Y79 and C33A cells were cotransfected with 40 nM miRNA mimic or 10 nM vector using Lipofectamine 2000 (Sangon). Control cells were untransfected. NC cells were miRNA- or empty vector–transfected cells. The following experiments were carried out at 48 hours after all transfections.
RNA Preparations
A Trizol Plus RNA-purification kit (Thermo Fisher Scientific) was used to extract total RNA from both paired nontumor and Rb tissue samples, as well as Y79 and C33A cell lines. Genomic DNA removal was performed using DNase I (Invitrogen) at 37°C for 2 hours. RNA integrity was checked using a urea–PAGE gel (6%). Only RNA samples of satisfactory quality were subjected to the following experiments.
RT-qPCR
An SSRT III reverse transcription (RT) system (Thermo Fisher Scientific) was used to perform RT to synthesize cDNA samples with RNA samples as a template. Samples of cDNA were used as a template to perform all qPCR using a Kapa SYBR Fast qPCR kit (Roche). The endogenous control of CASC8 was 18S rRNA. To measure expression levels of mature miR34a, addition of poly(A), miRNA RT, and qPCRwere performed using a GeneCopoeia All-in-One miRNA qRT-PCR detection kit. The internal control of miR34a was U6. Three replicates were included in each experiment. The 2–ΔΔCT method was used to normalize gene-expression levels.
Methylation-Specific PCR
Isolation of genomic DNA from Y79 and C33A cells was performed using a Monarch genomic DNA-purification kit (NEB), with all steps performed following the manufacturer’s instructions. DNA samples were converted using a Methylation-Gold kit (Zymo Research). To analyze methylation of the MIR34A gene, methylation-specific PCR and routine PCR were performed using Taq 2× master mix (NEB). PCR products were sequenced to make sure that the correct PCR products were obtained.
CCK8 Assays
Y79 and C33A cells were collected at 48 hours posttransfection. Cells were digested with 0.25% trypsin, washed with cold PBS, and counted. Cells were then cultivated in a 96-well plate at 37°C with 3,000 cells in 0.1 mL medium per well. To determine cell proliferatione, OD values at 450 nm were measured every 24 hours for 96 hours. At 4 hours before measurement, CCK8 solution (Sigma-Aldrich) was added to each well to reach 10%.
BrdU Assays
After transfection, Y79 and C33A cells were transferred to a 96-well cell-culture plate (3,000 cells in 0.1 mL medium per well). Cell culture was performed for a further 48 hours, and peroxidase-coupled anti-BrdU antibody (Sigma–Aldrich) was used to incubate cells after fixation. Peroxidase substrate was then used to incubate cells for 2 hours after washing, and OD values at 450 nm were measured later.
Statistical Analysis
Data of three biological replicates of each experiment are expressed as means ± SD. Paired t-tests were used to compare paired tissue samples. ANOVA with Tukey’s test was used to compare multiple groups. Correlations were analyzed by linear regression. P<0.05 was statistically significant.
Results
Expression of CASC8 Upregulated in Rb tissue
Expression of CASC8 in paired Rb and nontumor tissue samples was determined by RT-qPCR. Compared with non-tumor tissue, expression of CASC8 was significantly higher in Rb tissue (Figure 1A, p<0.001), indicating the possible involvement of CASC8 in Rb. Expression of CASC8 in Rb tissue was compared among patients at different clinical stages. It was observed that CASC8 expression was not significantly different among groups A–E (Figure 1B).
miR34a Downregulated in Rb and Inversely Correlated with CASC8
Expression of miR34a in paired Rb and nontumor tissue samples from 62 Rb patients was determined by RT-qPCR. It was observed that expression of miR34a was significantly decreased in Rb tissue compared with nontumor tissue (Figure 2A, p<0.001). Linear regression was carried out to analyze the correlation between the expression of miR34a and CASC8 across Rb tissue and nontumor tissue. It was found that the expression of miR34a and CASC8 were inversely and significantly correlated in Rb tissue (Figure 2B). However, the correlation between them was not significant in nontumor tissue (Figure 2C).
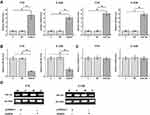
CASC8 Overexpression Downregulated miR34a in Rb Cells Through Methylation
To analyze possible interactions between CASC8 and miR34a, the CASC8-expression vector or miR34a mimic was transfected into Y79 and C33A cells. RT-qPCR was performed at 48 hours posttransfection to confirm overexpression of CASC8 and miR34a (Figure 3A, p<0.05). Compared with controls, downregulated miR34a was observed in cells transfected with the CASC8-expression vector (Figure 3B, p<0.05), while no significant effect on CASC8 expression was observed after miR34a-mimic transfection (Figure 3C, p>0.05). Methylation-specific PCR was performed to analyze the effects of CASC8 overexpression on methylation of miR34a. Compared with cells transfected with the empty vector, cells transfected with CASC8-expression vector showed significantly increased methylation of miR34a (Figure 3D).
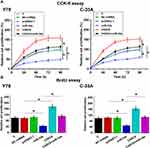
CASC8 Overexpression Promoted Proliferation of Rb Cells Through miR34a
Possible involvement of CASC8 in regulation of the proliferation of Rb cells was analyzed by CCK8 (Figure 4A) and BrdU assays (Figure 4B). Compared with the control group, CASC overexpression significantly increased the proliferation rate of Rb cells, while miR34a overexpression significantly decreased the proliferation rate of Rb cells. In addition, CASC8 overexpression reduced the inhibitory effects of miR34a on Rb-cell proliferation (Figure 4, p<0.05).
Discussion
In this study, we analyzed the interaction between CASC8 and miR34a in Rb, and found that CASC8 expression was upregulated in Rb. Moreover, CASC8 downregulated expression of miR34a by methylation of the MIR34A gene, thus promoting the proliferation of Rb cells.
Studies have investigated correlations between genetic polymorphisms of CASC8 and the risk of cancer.15 It has been observed that compared with the population with the rs10505477 TT genotype, those with TC or CC genotype were at significantly decreased risk of many types of cancer.15 Correlations between CASC8 polymorphisms and risk of cancer have been reported in colorectal cancer,16 hepatocellular carcinoma,17 lung cancer,13 and acute lymphoblastic leukemia.18 However, the expression pattern and function of CASC8 in cancer biology remains unclear. This study is the first to report on the upregulation of CASC8 in Rb. In addition, we found that upregulation of CASC8 increased the proliferation rate of Rb cells. Therefore, CASC8 may play oncogenic roles in Rb by promoting cancer-cell proliferation.
miR34a plays tumor-suppressive roles in many types of cancer, including Rb.19–22 In cancer biology, miR34a targets multiple oncogenic genes, such as CD44 and CMET, to suppress the growth and metastasis of tumors. In Rb, miR34a not only regulates the chemosensitivity of cancer cells to chemotherapy19 but also suppresses cancer development by inhibiting cancer-cell proliferation.20 Therefore, overexpression of miR34a is considered a promising target for the treatment of Rb. However, based on our knowledge, the upstream regulator of miR34a in many cancers, including Rb, is unknown. This study confirmed the inhibitory effects of miR34a on Rb-cell proliferation. miR34a is involved in cancer biology mainly through interaction with cancer-related pathways, such as MAGEA–p53 signaling.19 Our results showed that CASC8 might be an upstream inhibitor of miR34a, and could downregulate the expression of miR34a through a methylation pathway. However, the methylation factors involved in this process remain unclear.
Interestingly, we found that CASC8 and miR34a were inversely correlated in Rb tissue, but not nontumor tissue. Therefore, the interaction between CASC8 and miR34a might be mediated by certain pathological factors. Future studies are needed to identify these factors.
In conclusion, the expression of CASC8 is upregulated in Rb, and CASC8 may upregulate miR34a expression in Rb cells through methylation of the MIR34A gene, thus promoting the proliferation of Rb cells.
Ethics Approval and Consent to Participate
The Ethics Committee of Shenzhen Hospital of Integrated Chinese and Western Medicine approved this study, and informed consent was obtained from all participants. Data collected from participants were kept confidential and accessible only by the researchers.
Acknowledgments
We thank the reviewers for all constructive suggestions.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, agreed to submit to the current journal, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no competing interests associated with the manuscript.
References
1. Dimaras H, Kimani K, Dimba EAO, et al. Retinoblastoma. Lancet. 2012;379(9824):1436–1446. doi:10.1016/S0140-6736(11)61137-9
2. Benavente CA, Dyer MA. Genetics and epigenetics of human retinoblastoma. Annu Rev Pathol. 2015;10:547–562. doi:10.1146/annurev-pathol-012414-040259
3. Little MP, Kleinerman RA, Stiller CA, et al. Analysis of retinoblastoma age incidence data using a fully stochastic cancer model. Int J Cancer. 2012;130(3):631–640. doi:10.1002/ijc.26039
4. Abramson DH, Shields CL, Munier FL, et al. Treatment of retinoblastoma in 2015: agreement and disagreement. JAMA Ophthalmol. 2015;133(11):1341–1347. doi:10.1001/jamaophthalmol.2015.3108
5. Chawla B, Jain A, Azad R. Conservative treatment modalities in retinoblastoma. Indian J Ophthalmol. 2013;61(9):479–485. doi:10.4103/0301-4738.119424
6. Abramson DH, Shields CL, Jabbour P, et al. Metastatic deaths in retinoblastoma patients treated with intraarterial chemotherapy (ophthalmic artery chemosurgery) worldwide. Int J Retina Vitreous. 2017;3(1):40. doi:10.1186/s40942-017-0093-8
7. Kalmodia S, Parameswaran S, Ganapathy K, et al. Characterization and molecular mechanism of peptide-conjugated gold nanoparticle inhibiting p53-HDM2 interaction in retinoblastoma. Mol Ther Nucleic Acids. 2017;9:349–364. doi:10.1016/j.omtn.2017.10.012
8. Yu C, Jing X, Bian Y. Molecular mechanism of NF-κB signal pathway in autophagy of retinoblastoma cell line HXO-RB44. Int J Clin Exp Med. 2019;12(12):13586–13591.
9. Pascual-Pasto G, Bazan-Peregrino M, N G O, et al. Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Sci Transl Med. 2019;11(476):eaat9321. doi:10.1126/scitranslmed.aat9321
10. Zhang J, Benavente CA, McEvoy J, et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature. 2012;481(7381):329–334. doi:10.1038/nature10733
11. Plousiou M, Vannini I. Non-coding RNAs in retinoblastoma. Front Genet. 2019;10:1155. doi:10.3389/fgene.2019.01155
12. Khorkova O, Hsiao J, Wahlestedt C. Basic biology and therapeutic implications of lncRNA. Adv Drug Deliv Rev. 2015;87:15–24. doi:10.1016/j.addr.2015.05.012
13. Hu L, Chen SH, Lv QL, et al. Clinical significance of long non-coding RNA CASC8 rs10505477 polymorphism in lung cancer susceptibility, platinum-based chemotherapy response, and toxicity. Int J Environ Res Public Health. 2016;13(6):545. doi:10.3390/ijerph13060545
14. Slabáková E, Culig Z, Remšík J, et al. Alternative mechanisms of miR-34a regulation in cancer. Cell Death Dis. 2017;8(10):e3100–e3100. doi:10.1038/cddis.2017.495
15. Cui Z, Gao M, Yin Z, et al. Association between lncRNA CASC8 polymorphisms and the risk of cancer: a meta-analysis. Cancer Manag Res. 2018;10:3141–3148. doi:10.2147/CMAR.S170783
16. Yao K, Hua L, Wei L, et al. Correlation between CASC8, SMAD7 polymorphisms and the susceptibility to colorectal cancer: an updated meta-analysis based on GWAS results. Medicine (Baltimore). 2015;94(46):e1884. doi:10.1097/MD.0000000000001884
17. Wu ER, Hsieh MJ, Chiang WL, et al. Association of lncRNA CCAT2 and CASC8 gene polymorphisms with hepatocellular carcinoma. Int J Environ Res Public Health. 2019;16(16):2833. doi:10.3390/ijerph16162833
18. Hashemi M, Bahari G, Naderi M, et al. Association of lnc-LAMC2-1: 1 rs2147578 and CASC8 rs10505477 polymorphisms with risk of childhood acute lymphoblastic leukemia. Asian Pac J Cancer Prev. 2016;17(11):4985–4989.
19. Yang G, Fu Y, Lu X, et al. miR-34a regulates the chemosensitivity of retinoblastoma cells via modulation of MAGE-A/p53 signaling. Int J Oncol. 2019;54(1):177–187.
20. Su Y, Lu S, Li J, et al. Shikonin-mediated up-regulation of miR-34a and miR-202 inhibits retinoblastoma proliferation. Toxicol Res (Camb). 2018;7(5):907–912. doi:10.1039/C8TX00079D
21. Liu C, Kelnar K, Liu B, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17(2):211–215. doi:10.1038/nm.2284
22. Li N, Fu H, Tie Y, et al. miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett. 2009;275(1):44–53. doi:10.1016/j.canlet.2008.09.035
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.