Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 13
Carvedilol Alters Circulating MiR-1 and MiR-214 in Heart Failure
Authors Shirazi-Tehrani E, Firouzabadi N , Tamaddon G , Bahramali E, Vafadar A
Received 1 June 2020
Accepted for publication 11 August 2020
Published 3 September 2020 Volume 2020:13 Pages 375—383
DOI https://doi.org/10.2147/PGPM.S263740
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Elham Shirazi-Tehrani,1 Negar Firouzabadi,1,2 Gholamhossein Tamaddon,3,4 Ehsan Bahramali,5 Asma Vafadar3,4
1Department of Pharmacology & Toxicology, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran; 2Noncommunicable Diseases Research Center, Fasa University of Medical Sciences, Fasa, Iran; 3Department of Medical Biotechnology, School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran; 4Diagnostic Laboratory Sciences and Technology Research Center, School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran; 5Digestive Disease Research Center, Digestive Disease Research Institute, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
Correspondence: Negar Firouzabadi
Department of Pharmacology & Toxicology, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
Tel +98-917-314-5303
Email [email protected]
Gholamhossein Tamaddon
Department of Medical Biotechnology, School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
Tel +98-915-141-7043
Email [email protected]
Introduction: MicroRNAs (miRNAs) are recognized as major contributors in various cardiovascular diseases, such as heart failure (HF). These small noncoding RNAs that posttranscriptionally control target genes are involved in regulating different pathophysiological processes including cardiac proliferation, ifferentiation, hypertrophy, and fibrosis. Although carvedilol, a β-adrenergic blocker, and a drug of choice in HF produce cytoprotective actions against cardiomyocyte hypertrophy, the mechanisms are poorly understood. Here we proposed that the expression of hypertrophic-specific miRNAs (miR-1, miR-133, miR-208, and miR-214) might be linked to beneficial effects of carvedilol.
Methods: The levels of four hypertrophic-specific miRNAs were measured in the sera of 35 patients with systolic HF receiving carvedilol (treated) and 20 HF patients not receiving any β-blockers (untreated) as well as 17 nonHF individuals (healthy) using quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Systolic HF was defined as left ventricular ejection fraction < 50% by transthoracic echocardiography.
Results: We demonstrated that miR-1 and miR-214 were significantly upregulated in the treated group compared to the untreated group (P=0.014 and 5.3-fold, 0.033 and 4.2-fold, respectively). However, miR-133 and miR-208 did not show significant difference in expression between these two study groups. MiR-1 was significantly downregulated in the untreated group compared with healthy individuals (P=0.019 and 0.14-fold).
Conclusion: In conclusion, it might be postulated that one of the mechanisms by which carvedilol may exert its cardioprotective effects can be through increasing miR-1 and miR-214 expressions which may also serve as a potential therapeutic target in patients with systolic HF in future.
Keywords: microRNA, β-blocker, carvedilol, systolic heart failure, cardiac hypertrophy
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide.1 Systolic heart failure (HF) is becoming the most prevalent form of chronic CVD and is defined as a state of inadequate cardiac output to meet the tissues’ metabolic needs.2 Despite efforts made towards preventing the disease, the prevalence of HF remains over 26 million worldwide3. Not enough evidence regarding the prevalence of HF is provided in the Middle East region; however, it is assumed that it will soon be equal to that of western countries.4 Pathologic remodeling, which is the hallmark of chronic HF is triggered and aggravated by a variety of stimuli including pressure and volume overload in cardiac chambers. Valvular heart diseases, hypertension, dilated and hypertrophic cardiomyopathies induce acute or chronic stress to the heart and culminate in a hypertrophic and/or apoptotic response which is frequently seen in HF.5,6
Among the pharmacologic remedies for systolic HF, β-blockers have a promising role in reducing mortality. Since proposing the beneficial effects of β-blockers in HF in 1975,7 much evidence is in favor of the role of these agents in improving cardiac function and survival rates in patients with systolic HF.8–10 Among the β-blockers, carvedilol is currently the first-line therapy for symptomatic HF in adults.11,12
The discovery of micro ribonucleic acid (miRNA), small noncoding RNAs (~22 nucleotides), in regulating key protein-coding genes, has opened up new paths in understanding the underlying pathogenic conditions of CVDs, which may have implications in diagnosis, treatment and prognosis of the disease.13,14 MiRNAs as detectable intracellular RNAs, are present in serum or plasma in a remarkably stable form15 and can alter cardiac proliferation, differentiation and other pathological remodeling responses. The proposed mechanisms of posttranscriptional regulation of protein-coding genes, explain how these small molecules affect the cardiovascular system and select them as biomarkers of cardiac pathologic remodeling.16 Several studies corroborate the role of miRNAs in processes of myocyte hypertrophy and apoptosis.17–22 MiR‐1 and miR‐133, two miRs belonging to one transcriptional unit, are expressed at low levels in both animal and human models of cardiac hypertrophy23 and are among the key regulators in these processes.24,25 Deletion of these miRNAs which are highly abundant in the heart may be linked to cardiac defects.26 MiR-208 as well as miR-1 and miR-133 are defined as key regulators of the gene network which is involved in cardiomyocyte differentiation.27 There are implications that some miRNAs like miR-208b, which play a functional role in cardiomyocyte remodeling, may be useful targets for therapeutic purposes.28 Another miRNA, miR-214, is proposed to play a regulatory role in cardiac hypertrophy and negative remodeling involved in HF.29 Downregulation of miR-214 has been reported in the myocardium of patients diagnosed with cardiac hypertrophy.30
Although studies have shown that carvedilol inhibits cardiomyocyte hypertrophy, the exact cardioprotective mechanism is still unknown.31 An in vivo study on rat neonatal cardiomyocytes showed that carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by upregulating miR-133.32 Therefore, the protective role of carvedilol in HF might be linked to alterations in levels of different miRNA expression. In this study we investigated, for the first time, the effects of carvedilol on miRNA expressions in a clinical setting using sera of HF patients registered in a regional hospital-based systolic HF disease registry system, Fasa Registry of Systolic HF (FaRSH).
Methods
Sample Collection
This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and Uniform Requirements for manuscripts submitted to biomedical journals and was approved by the local committee for ethics of medical experiments on human subjects of Shiraz University of Medical Sciences. We obtained written consent from all participants prior to the interview and all participants were informed about the purpose of the study.
We used the infrastructure of a local hospital-based disease registry system FaRSH which has enrolled patients admitted with a diagnosis of systolic HF since July 2015. The main inclusion criterion was left ventricular ejection fraction (LVEF) of <50%. Fifty-five patients with diagnosis of systolic HF secondary to ischemic heart disease were recruited in the study. Patients were matched according to age, sex, and body mass index (BMI). Those with severe valvular heart diseases, GFR <30 cc/min and significant comorbidities were excluded in the first place.
Thirty-five patients (treated) received carvedilol for at least three months, the dose of which ranged from 6.25 mg to 12.5 mg in two divided doses. β-blockers were contraindicated in the other 20 patients (untreated) due to severe sinus bradycardia and partial atrioventricular (AV) block.
Seventeen nonHF individuals (healthy) were also enrolled in the study. The exclusion criteria for healthy individuals was the absence of cardiovascular diseases, hyperlipidemia, malignancies, and autoimmune, neurologic and psychiatric diseases.
Serum Isolation and Storage
Five mL of blood was collected from each participant. Whole blood was allowed to stand at room temperature for five minutes before being centrifuged at 4°C at 12000 g for 20 min. The resultant serum was aliquoted into endonuclease-free Eppendorf tubes and stored at – 80°C.
RNA Extraction and cDNA Synthesis
Total RNA was extracted from serum samples using TRIzol LS Reagent (Invitrogen, Carlsbad, CA, USA). Half a mL of serum was incubated at 37°C overnight with 10 μL proteinase K (Cinaclone, Iran). For RNA isolation 0.25 mL of serum was homogenized in 0.75 mL of TRIzol LS. Two hundred μL of chloroform was added to the sample for phase separation, mixed and centrifuged. RNA was left exclusively in the aqueous phase. After the removal of chloroform and precipitation with isopropanol, the pellet was washed by centrifugation with 75% ethanol. The RNA pellet was dried at room temperature for 5– 10 min and dissolved in 20 µL of diethyl pyrocarbonate (DEPC)-treated water. RNA concentrations and quality was calculated using Nanodrop® Hellma, Denmark. Total RNA samples were kept at −80°C for further analysis.
For reverse transcription (RT) reaction of the miRNAs and synthesis of cDNA strands, specific RT primers (Exiqon, Vedbaek, Denmark) and cDNA synthesis kit (Exiqon, Vedbaek, Denmark) were used according to the manufacturer's protocol. Briefly, in order to fabricate the poly A strand, we mixed 2 µL buffer (10⨰), ATP 2 µL, polyA polymerase 0.5 µL, 1.5–2 µL of total RNA was incubated at 37°C for 10 min. cDNA synthesis was performed as follows: two µL 5⨰ reaction buffer, 1 µL dNTP (10 mM), 0.5 µL RT enzyme, 0.5 µL microRNA cDNA synthesis specific primers, 2 µL polyadenylated RNA. The mixture was incubated for 60 min at 44°C, followed by heat-inactivation enzyme of reverse transcriptase (RT-enzyme for one minute at 85°C in a thermal cycler).
Real-time PCR
Aliquots of cDNA were used for quantitative PCR using Real-time PCR Master Mix (Exiqon, Vedbaek, Denmark), specific primers (Exiqon, Vedbaek, Denmark) and ABI 7500 (Applied Bio systems, Foster City, USA). Four µL of synthesized cDNA was mixed with 10 µL of SYBER green, 0.4 µL ROX dye, and mixed with specific miR primers 1 µL (10 mM) as shown in Table 1.
 |
Table 1 MiRNA Specific Primers for cDNA Synthesis Reaction |
As an internal reference gene, 5S rRNA was used in order to normalize miRNA expressions.
In order to check the accuracy of amplifications we included a negative control (NTC) in each run by eliminating the cDNA sample in the tube. RT-PCR was run in duplicate using ABI 7500 REA real-time quantitative PCR system (Applied Bio System, USA) with the following cycling conditions: preliminary denaturation at 95°C for five minutes, followed by 45 cycles of denaturation at 95°C for 5 seconds, annealing at 63°C for 20 seconds, and elongation at 72°C for 30 seconds.
Statistical Analysis
Statistical analysis was performed using SPSS® 21.0 for Windows® (IBM Corporation, Armonk, NY, USA ). Graphs were plotted using GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA, USA). Distribution of all variables was tested for normal distribution using Kolmogorov–Smirnov test. Comparisons between two groups were performed with an independent sample t-test.
A widely used method for presenting relative expression of miRNA is called the 2-ΔΔct method which presents the data of the miRNA of interest (Ct miRNA of interest) relative to internal control gene (Ct internal control miRNA), termed Δct. Results are calculated as Δct± standard error of the mean (SEM). All values of miRNAs are expressed as ±SEM. P-value<0.05 was considered statistically significant.
Results
A total of 72 Individuals comprised of 17 healthy subjects (male/female: 10/7; age: 67.25±11.06; and 35 HF patients treated with carvedilol (male/female: 19/16; age: 64.83±11.03) and 20 HF patients receiving no β-blockers (male/female: 11/9; age: 67.25±11.06) were enrolled in our study. Demographical characteristics of nonHF (healthy) subjects are presented in Table 2. Clinical characteristics and demographics of HF patients receiving carvedilol (treated) and HF patients not treated with β-blockers (untreated) are shown in Table 3. The two groups were matched according to age, sex, and BMI and were similar regarding smoking habits, HNT and diabetes (P>0.05). Two groups were matched according to age, sex, and BMI and were similar regarding smoking habits, history of malignancies and diabetes (P>0.05).
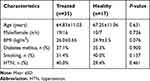 |
Table 2 Characteristics of HF Patients Receiving Carvedilol (Treated) and NonHF Patients (Healthy) |
 |
Table 3 Characteristics of HF Patients Receiving Carvedilol (Treated) and HF Patients Not Receiving Carvedilol (Untreated) |
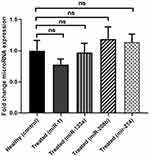
Figure 1 and Supplementary Figure 1 represent fold-change comparison of different miRNA expressions in the treated group compared with healthy individuals. As shown, regarding expression levels of the four miRs, no significant difference was observed between the two groups (P>0.05).
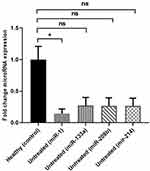
As demonstrated in Figure 2 and Supplementary Figure 2, miR-1 was significantly (P=0.019) and by 0.14-fold downregulated in untreated group compared with healthy subjects.
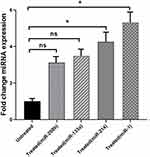
Fold changes in median expression of the study miRNAs of enrolled patients (treated vs untreated) are demonstrated in Figure 3 and Supplementary Figure 3. Real-time polymerase chain reaction (RT-PCR) analysis of miRNA, using precise significance criteria of a two-fold or greater difference in expression level and P value <0.05, revealed that two miRNAs, miR-1 and miR-214 were significantly upregulated in patients with HF who were treated with carvedilol compared to the control group (P=0.014 and 0.033 respectively). Regarding miR-214, its expression was significantly and by 4.2-fold higher in carvedilol receiving patients compared to patients in the control group who did not receive carvedilol (P=0.033). Considering miR-1 expression, a noticeable increase by 5.3-fold was observed in cases compared to the control group (P=0.014). Similarly, the expression level of miR-133 in individuals taking carvedilol showed an increased trend by 3.1-fold compared to the control group; however, this observed difference was not statistically significant (P=0.052). Regarding miR-208, no significant difference was observed with respect to the two study groups (P=0.171).
Discussion
MiRNAs are prominent regulators of cell growth, proliferation, differentiation and apoptosis.33,34 Alteration in miRNAs expressions, apart from their role in the pathophysiology of CVDs, would nominate them as emerging sensitive and noninvasive biomarkers in diagnosis, treatment surveillance and prognosis in many disease states such as systolic HF. In this study, we speculated whether the expression of hypertrophy-related miRNAs is involved in the protective mechanism of carvedilol in systolic HF.
Results of miRNA expression in our enrolled patients with systolic HF who received carvedilol compared to the group who were not treated with carvedilol revealed: (1) significant upregulated miR-214 expression; (2) markedly upregulated miR-1 expression; (3) no significant effect on miRNA-133; and (4) miR-208 expression. Furthermore, comparison of expression of the four studied miRs between untreated and healthy subjects showed that miR-1 was significantly downregulated in untreated patients. No significant difference was observed between expression levels of miRs in healthy individuals compared with treated patients.
Systolic HF is a complex disorder with many possible causes, the most prevalent of which is coronary artery disease leading to myocardial ischemia.3 β-blockers were first advocated for treatment of HF in the late 1970s.7 Results from clinical trials suggest that among β-blockers, carvedilol, metoprolol succinate and bisoprolol reduce mortality rates when added to conventional therapy.35–37 Carvedilol consistently improves survival compared with metoprolol.38 However, molecular mechanisms underlying this favorable effect remain elusive. HF is a pathological condition in which the regulation among specific genes is disrupted. As a consequence, the expression of oncogenes is increased, while the expression of normal and mature genes decreases leading to cardiac hypertrophy and/or remodeling, a pathological status that ultimately causes further damage to the failing cardiomyocytes, thus perpetuating the impaired cardiac function.39 Compelling evidence points at the role of miRNAs in the regulation of cardiac remodeling and advocates the role of these small molecules as potential therapeutic biomarkers in HF.40–42 In agreement with such findings, our results showed that miR-214 and miR-1 expressions were significantly upregulated in sera of patients with systolic HF who were treated with carvedilol. It should be mentioned that no significant difference was observed between expression levels of miRs in healthy individuals compared with treated patients, which advocates the beneficial role of carvedilol therapy by means of miRs. Moreover, miR-1 was significantly downregulated in untreated patients vs healthy subjects as observed in previous studies.43,44 Therefore, overexpression of miR-1 and miR-214 in treated patients might propose a molecular mechanism by which carvedilol benefits HF patients.
Numerous studies have reported that overlap mechanisms exist in ventricular remodeling and HF, but little is known about the role of key regulators in this regard.45 Biological role and clinical importance of miRNAs in different tissues as well as different cardiovascular diseases stand controversial46,47 and these controversies may be secondary to tissue-specific miRNA features and their different functions in different cells and pathophysiologic conditions.
Apoptosis of cardiomyocytes has been identified as a fundamental process in HF and is strongly associated with this illness.48 MiR-214 was first introduced for its role in apoptosis.49 Overexpression of miR‐214 has been shown to inhibit H2O2‐mediated apoptosis in rat cardiomyocytes. Furthermore, H2O2‐mediated apoptosis was exacerbated following downregulation of miR‐214 expression.50 Beside subsiding cellular apoptosis, overexpression of miRNA-214 significantly ameliorates left ventricular remodeling and hemodynamics of the heart in animal models.22 According to many subsequent reports, miR-214 is involved in muscle cell differentiation, proliferation and hypertrophy.51–53 MiR-214 knocked out mice have been shown to exhibit reduced cardiac function and thus be more susceptible to death. Mir-214 protects cardiomyocytes by preventing the release of excessive Ca2+ into the cytoplasm and induces its cardioprotective action by suppressing Ca2+ effector kinase, CaMKII, and cell death mediators. In the absence of miR-214 expression in the heart, higher levels Ca2+ effectors further perpetuate Ca2+ overload and cell death, resulting in greater impairment of cardiac function.46 A report by Tang et al demonstrated that miR-214 exhibits antihypertrophic properties rather than prohypertrophic effect in both an animal model of cardiac hypertrophy and in patients diagnosed with HF. MiR-214 inhibits cardiomyocytes hypertrophy by downregulating MEF2C (myocyte enhancer factor-2C) expression which has been involved in cardiac transcriptional program and is upregulated during cardiac hypertrophy.30 Mef2a, a transcription factor, is a critical factor in the growth process of cardiomyocytes. In line with these observations and considering that this miRNA does not further aggravate the process of cardiac remodeling,25 we measured a significant upregulation of miR-214 in HF patients receiving carvedilol compared to the control group (fold= 4.28, P=0.033). Therefore, we can propose that carvedilol’s cardioprotective effects may be mechanistically linked to regulating the expression of miR-214, which itself exerts favorable effects on cardiac remodeling and ultimately leads to cardiac function stabilization or improvement.
Serum levels of miR-1 in patients receiving carvedilol were significantly elevated compared with the control group (fold=5.33, P=0.01). This alteration in expression is consistent with most of the previous studies regarding the role of miR-1 as one of the key regulating miRNAs in cardiac defects that attenuates cardiac hypertrophy.2,54-56 Downregulation of miR-1 is one of the earliest changes, before any other miRNA expression change, after increase in cardiac overload, and precedes increase in cardiac mass and contractile dysfunction.43 Data from animal models indicate that among the earliest changes due to increase in pressure overload observed in the heart, is the reduction of miR-1, prior to cardiac mass increase.56 In vitro and in vivo data suggest that reduced expression of miR-1 is required for increased cardiac mass. Insulin growth factor (IGF)-1 as one of the main regulators of cardiac myocyte growth and differentiation, is repressed by miR-157 and its mRNA expression is inhibited by carvedilol.58 Studies in neonatal myocyte culture are suggestive of downregulation of some miR-1-related hypertrophic target genes, such as Ras GTPase-activating protein (RasGAP), cyclin-dependent kinase 9 (CDK9), Ras homolog enriched in brain (Rheb) and fibronectin. Moreover, miR-1 inhibits the CaN/NFAT signaling pathway in cardiomyocytes by influencing the expression of Mef2a and GATA binding protein 4 (Gata4). In addition, administration of a miR-1 mimic to rats with left ventricular hypertrophy causes reversion of cardiac hypertrophy and alleviation of fibrosis and apoptosis which are recognized to be a major feature of cardiac remodeling and HF. Although a report by Hu et al is suggestive of downregulation of miR-1 by means of carvedilol in an animal model of myocardial infarction (MI),59 it seems that miRNAs play different roles in different species as well as different physiological and pathological conditions.46,47 Altogether, it can be postulated that miR-1 may reduce cardiac hypertrophy,60 that mediates the protective effects of carvedilol or at least this inference deserves further studies to obtain supporting evidence.
MiR-133 has been identified as a regulatory biomarker associated with cardiac hypertrophy which is expressed at low levels in HF.23 Recent studies show that miR-133 exhibits anti-apoptotic properties by means of suppressing the expression of caspase-3 and caspase-9.61 MiR-133 protection against cardiomyocyte cell death can be viewed as a mechanism of action for β-blockers in HF. Upregulation of miR-133 expression by carvedilol and the resultant anti-apoptotic effect, may contribute to the protective role of carvedilol in many pathological conditions such as MI and HF.32 In our study, a trend toward the upregulation of MiR-133 was observed in carvedilol treated patients, although the difference between the two study groups was not significant (P=0.052) which might be due to the small sample size of patients recruited.
MiR-208 is essential for expression of the genes involved in cardiac fibrosis and hypertrophy and is expressed in the heart.62,63 Overexpression of miR-208 has been shown to reduce the expression of antihypertrophy markers which ultimately leads to cardiomyocyte hypertrophy.54 We observed lack of association between miR-208 expression and treatment with carvedilol in HF. MiR-208 is not advocated as a possible biomarker for favorable effects of carvedilol in HF.
The limitation of our study is the small sample size of enrolled patients. Moreover, the cohort of patients not receiving β-blockers were clinically more ill, which might have interfered with the expression of the studied miRNAs. We have taken into account these baseline differences to a high extent while choosing the controls for them, however, residual confounding factors might remain.
In summary, our study will serve as a base study for future investigations. Taken together, our findings indicated that the differential expressions of circulating miRNAs may relate to protective effects of carvedilol in cardiac remodeling and may be promising in HF treatment and prognosis. MiRNAs could be proposed as potential biomarkers in diagnosis, prognosis and treatment of HF and this study specifically provides evidence of altered miRNA expression in HF patients receiving carvedilol which can be viewed as explanations for mechanism of action of β-blockers in HF. However, to fully understand the mechanistic cardioprotective role of carvedilol in HF by means of these miRNAs investigating posttranscriptional changes are required to determine whether these miRNAs confer possible overlapping/compensatory effects on carvedilol-mediated cardioprotection.
Acknowledgments
This project was a part of MS thesis conducted by Elham Shirazi-Tehrani. The grant number of this project is 95-01-05-13912, Shiraz University of Medical Sciences, Shiraz, Iran.
Disclosure
The authors report no potential conflicts of interest in this work.
References
1. Chiong M, Wang Z, Pedrozo Z, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011;2(12):e244. doi:10.1038/cddis.2011.130
2. Wong LL, Wang J, Liew OW, Richards AM, Chen Y-T. MicroRNA and heart failure. Int J Mol Sci. 2016;17(4):502. doi:10.3390/ijms17040502
3. Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Failure. 2014;1(1):4–25. doi:10.1002/ehf2.12005
4. Al-Shamiri MQ. Heart failure in the Middle East. Curr Cardiol Rev. 2013;9(2):174–178. doi:10.2174/1573403X11309020009
5. Montgomery RL, Hullinger TG, Semus HM, et al. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124(14):1537–1547. doi:10.1161/CIRCULATIONAHA.111.030932
6. Spudich JA. Hypertrophic and dilated cardiomyopathy: four decades of basic research on muscle lead to potential therapeutic approaches to these devastating genetic diseases. Biophys J. 2014;106(6):1236–1249. doi:10.1016/j.bpj.2014.02.011
7. Waagstein F, Hjalmarson Å, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Heart. 1975;37(10):1022–1036. doi:10.1136/hrt.37.10.1022
8. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of β blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55. doi:10.1136/bmj.f55
9. Cadrin-Tourigny J, Shohoudi A, Roy D, et al. Decreased mortality with beta-blockers in patients with heart failure and coexisting atrial fibrillation: an AF-CHF substudy. JACC Heart Fail. 2017;5(2):99–106. doi:10.1016/j.jchf.2016.10.015
10. Kotecha D, Flather MD, Altman DG, et al. Heart rate and rhythm and the benefit of beta-blockers in patients with heart failure. J Am Coll Cardiol. 2017;69(24):2885–2896. doi:10.1016/j.jacc.2017.04.001
11. Chen J, Huang C, Zhang B, Huang Q, Chen J, Xu L. The effects of carvedilol on cardiac structural remodeling: the role of endogenous nitric oxide in the activity of carvedilol. Mol Med Rep. 2013;7(4):1155–1158. doi:10.3892/mmr.2013.1329
12. Ruffolo RR, Feuerstein GZ. Carvedilol case history: the discovery and development of the first β-blocker for the treatment of congestive heart failure. Expert Opin Drug Discov. 2006;1(1):85–89. doi:10.1517/17460441.1.1.85
13. Van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov. 2012;11(11):860. doi:10.1038/nrd3864
14. Duggal B, Gupta M, Naga Prasad S. Potential role of microRNAs in cardiovascular disease: are they up to their hype? Curr Cardiol Rev. 2016;12(4):304–310. doi:10.2174/1573403X12666160301120642
15. Fichtlscherer S, De Rosa S, Fox H, et al. Circulating microRNAs in patients with coronary artery diseasenovelty and significance. Circ Res. 2010;107(5):677–684. doi:10.1161/CIRCRESAHA.109.215566
16. Yan H, Ma F, Zhang Y, et al. miRNAs as biomarkers for diagnosis of heart failure: a systematic review and meta-analysis. Medicine. 2017;96:22. doi:10.1097/MD.0000000000006825
17. Divakaran V, Mann DL. The emerging role of microRNAs in cardiac remodeling and heart failure. Circ Res. 2008;103(10):1072–1083. doi:10.1161/CIRCRESAHA.108.183087
18. Topkara VK, Mann DL. Clinical applications of miRNAs in cardiac remodeling and heart failure. Per Med. 2010;7(5):531–548. doi:10.2217/pme.10.44
19. Ramasamy S, Velmurugan G, Rajan KS, Ramprasath T, Kalpana K. MiRNAs with apoptosis regulating potential are differentially expressed in chronic exercise-induced physiologically hypertrophied hearts. PLoS One. 2015;10(3):e0121401. doi:10.1371/journal.pone.0121401
20. Park K-M, Teoh J-P, Wang Y, et al. Carvedilol-responsive microRNAs, miR-199a-3p and-214 protect cardiomyocytes from simulated ischemia-reperfusion injury. Am J Physiol Heart Circulatory Physiol. 2016;311(2):H371–H383. doi:10.1152/ajpheart.00807.2015
21. Lv L, Li T, Li X, et al. The lncRNA Plscr4 controls cardiac hypertrophy by regulating miR-214. Mol Ther Nucl Acids. 2018;10:387–397. doi:10.1016/j.omtn.2017.12.018
22. Yang X, Qin Y, Shao S, et al. MicroRNA-214 inhibits left ventricular remodeling in an acute myocardial infarction rat model by suppressing cellular apoptosis via the phosphatase and tensin homolog (PTEN). Int Heart J. 2016;57(2):247–250. doi:10.1536/ihj.15-293
23. Care A, Catalucci D, Felicetti F, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13(5):613–618. doi:10.1038/nm1582
24. Xiao J, Chen Y-H. MicroRNAs: novel regulators of the heart. J Thorac Dis. 2010;2(1):43.
25. Wang N, Zhou Z, Liao X, Zhang T. Role of microRNAs in cardiac hypertrophy and heart failure. IUBMB Life. 2009;61(6):566–571. doi:10.1002/iub.204
26. Vegter EL, van der Meer P, de Windt LJ, Pinto YM, Voors AA. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail. 2016;18(5):457–468. doi:10.1002/ejhf.495
27. Babiarz JE, Ravon M, Sridhar S, et al. Determination of the human cardiomyocyte mRNA and miRNA differentiation network by fine-scale profiling. Stem Cells Dev. 2012;21(11):1956–1965. doi:10.1089/scd.2011.0357
28. Zhou Q, Schötterl S, Backes D, et al. Inhibition of miR-208b improves cardiac function in titin-based dilated cardiomyopathy. Int J Cardiol. 2017;230:634–641. doi:10.1016/j.ijcard.2016.12.171
29. Duan Q, Yang L, Gong W, et al. MicroRNA‐214 is upregulated in heart failure patients and suppresses XBP1‐mediated endothelial cells angiogenesis. J Cell Physiol. 2015;230(8):1964–1973. doi:10.1002/jcp.24942
30. Tang C-M, Liu F-Z, Zhu J-N, et al. Myocyte-specific enhancer factor 2C: a novel target gene of miR-214-3p in suppressing angiotensin II-induced cardiomyocyte hypertrophy. Sci Rep. 2016;6:36146. doi:10.1038/srep36146
31. Barone FC, Willette RN, Nelson AH, Ohlstein EH, Brooks DP, Coatney RW. Carvedilol prevents and reverses hypertrophy-induced cardiac dysfunction. Pharmacology. 2007;80(2–3):166–176. doi:10.1159/000103384
32. Xu C, Hu Y, Hou L, et al. β-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J Mol Cell Cardiol. 2014;75:111–121. doi:10.1016/j.yjmcc.2014.07.009
33. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102–114. doi:10.1038/nrg2290
34. Almeida MI, Reis RM, Calin GA. MicroRNA history: discovery, recent applications, and next frontiers. Mut Res Fund Mol Mech Mutagenesis. 2011;717(1–2):1–8. doi:10.1016/j.mrfmmm.2011.03.009
35. Investigators C-I. The cardiac insufficiency bisoprolol study II (CIBIS-II). Lancet. 1999;353:9–13.
36. Group M-HS. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in-congestive heart failure (MERIT-HF). The Lancet. 1999;353(9169):2001–2007. doi:10.1016/S0140-6736(99)04440-2
37. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–1658. doi:10.1056/NEJM200105313442201
38. Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. The Lancet. 2003;362(9377):7–13. doi:10.1016/S0140-6736(03)13800-7
39. Zhu S, Han Z, Luo Y, et al. Molecular mechanisms of heart failure: insights from Drosophila. Heart Fail Rev. 2017;22(1):91–98. doi:10.1007/s10741-016-9590-3
40. Ottaviani L, da Costa Martins PA. Non‐coding RNAs in cardiac hypertrophy. J Physiol. 2017;595(12):4037–4050. doi:10.1113/JP273129
41. Leimena C, Qiu H. Non-Coding RNA in the pathogenesis, progression and treatment of hypertension. Int J Mol Sci. 2018;19(4):927.
42. Topkara VK, Mann DL. Role of microRNAs in cardiac remodeling and heart failure. Cardiovasc Drugs Ther. 2011;25(2):171–182. doi:10.1007/s10557-011-6289-5
43. Da Costa Martins PA, De Windt LJ. MicroRNAs in control of cardiac hypertrophy. Cardiovasc Res. 2012;93(4):563–572. doi:10.1093/cvr/cvs013
44. Seronde M-F, Vausort M, Gayat E, et al. Circulating microRNAs and outcome in patients with acute heart failure. PLoS One. 2015;10(11):e0142237. doi:10.1371/journal.pone.0142237
45. Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128(4):388–400. doi:10.1161/CIRCULATIONAHA.113.001878
46. Aurora AB, Mahmoud AI, Luo X, et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca 2+ overload and cell death. J Clin Invest. 2012;122:4. doi:10.1172/JCI59327
47. Lu H-Q, Liang C, He Z-Q, Fan M, Wu Z-G. Circulating miR-214 is associated with the severity of coronary artery disease. J Geriatric Cardiol. 2013;10(1):34.
48. Abbate A, Biondi-Zoccai GG, Bussani R, et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol. 2003;41(5):753–760. doi:10.1016/S0735-1097(02)02959-5
49. Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33(4):1290–1297. doi:10.1093/nar/gki200
50. Lv G, Shao S, Dong H, Bian X, Yang X, Dong S. MicroRNA‐214 protects cardiac myocytes against H2O2‐induced injury. J Cell Biochem. 2014;115(1):93–101. doi:10.1002/jcb.24636
51. Penna E, Orso F, Taverna D. miR-214 as a key hub that controls cancer networks: small player, multiple functions. J Invest Dermatol. 2015;135(4):960–969. doi:10.1038/jid.2014.479
52. Yang T, Gu H, Chen X, et al. Cardiac hypertrophy and dysfunction induced by overexpression of miR-214 in vivo. J Surg Res. 2014;192(2):317–325. doi:10.1016/j.jss.2014.06.044
53. Wu Y, Li Z, Yang M, et al. MicroRNA-214 regulates smooth muscle cell differentiation from stem cells by targeting RNA-binding protein QKI. Oncotarget. 2017;8(12):19866. doi:10.18632/oncotarget.15189
54. Dong D-L, Yang B-F. Role of microRNAs in cardiac hypertrophy, myocardial fibrosis and heart failure. Acta Pharm Sin B. 2011;1(1):1–7. doi:10.1016/j.apsb.2011.04.010
55. Fu S, Zhuo R, Yao M, Zhang J, Zhou H, Xiao J. MicroRNA basis of physiological hypertrophy. Front Genet. 2013;4:253. doi:10.3389/fgene.2013.00253
56. Sayed D, Hong C, Chen I-Y, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100(3):416–424. doi:10.1161/01.RES.0000257913.42552.23
57. Elia L, Contu R, Quintavalle M, et al. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation. 2009;120(23):2377–2385. doi:10.1161/CIRCULATIONAHA.109.879429
58. YUAN X-Z, ZHANG Q-H, JIANG Z-X, Shah GD-L. Effects of carvedilol on the intimal proliferation and expression of insulin-like growth factor-1 in rats with vascular injury. Shandong Med J. 2010;7:019.
59. Hu Y, Chen X, Li X, et al. MicroRNA‑1 downregulation induced by carvedilol protects cardiomyocytes against apoptosis by targeting heat shock protein 60. Mol Med Rep. 2019;19(5):3527–3536. doi:10.3892/mmr.2019.10034
60. Latronico MV, Condorelli G. microRNAs in hypertrophy and heart failure. Exp Biol Med. 2011;236(2):125–131. doi:10.1258/ebm.2010.010269
61. Zhu H, Fan G-C. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc Res. 2011;94(2):284–292. doi:10.1093/cvr/cvr291
62. Nakao K, Minobe W, Roden R, Bristow MR, Leinwand LA. Myosin heavy chain gene expression in human heart failure. J Clin Invest. 1997;100(9):2362–2370. doi:10.1172/JCI119776
63. Van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117(9):2369–2376. doi:10.1172/JCI33099
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.