Back to Journals » OncoTargets and Therapy » Volume 11
Cardiotoxic effects of the novel approved anti-ErbB2 agents and reverse cardioprotective effects of ranolazine
Authors De Lorenzo C , Paciello R , Riccio G , Rea D, Barbieri A, Coppola C, Maurea N
Received 16 November 2017
Accepted for publication 12 December 2017
Published 19 April 2018 Volume 2018:11 Pages 2241—2250
DOI https://doi.org/10.2147/OTT.S157294
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Supplementary video S3 Cor.4U-TDM1: effects of TDM1 on cardiac cell beating phenotype of Cor.4U cells treated for 72 hours.
Views: 117
Claudia De Lorenzo,1,2,* Rolando Paciello,1,2,* Gennaro Riccio,3 Domenica Rea,4 Antonio Barbieri,4 Carmela Coppola,4 Nicola Maurea4
1Department of Molecular Medicine and Medical Biotechnology, University of Naples “Federico II”, Naples, Italy; 2Ceinge, Biotecnologie Avanzate s.c.a.r.l., Naples, Italy; 3Department of Pharmacy, Federico II University, Naples, Italy; 4Division of Cardiology, Istituto Nazionale Tumori – Irccs Fondazione G. Pascale, Naples, Italy
*These authors contributed equally to this work
Purpose: Pertuzumab, a novel anti-epidermal growth factor receptor 2 humanized monoclonal antibody, and trastuzumab-emtansine (TDM1), a novel antibody–drug conjugate made up of trastuzumab covalently linked to the highly potent microtubule inhibitory agent DM1, have been recently approved by the US Food and Drug Administration for increasing the efficiency and safety of breast cancer therapy with trastuzumab. We investigated for the first time the potential cardiotoxic effects of pertuzumab and TDM1, which are not yet fully elucidated, and we tested whether ranolazine could blunt their cardiotoxicity.
Methods: The cardiotoxic effects were tested in vitro on rat cardiomyoblasts, human fetal cardiomyocytes, adult-like cardiomyocytes, and in vivo on a mouse model.
Results: All the treated cardiac cell lines were significantly affected by treatment with the tested drugs. Surprisingly, TDM1 showed stronger inhibitory effects on cardiac cells with respect to trastuzumab and pertuzumab by more significantly reducing the cell viability and by changing the morphology of these cells. TDM1 also affected the beating phenotype of adult-like cardiomyocytes in vitro and reduced fractional shortening and ejection fraction in vivo in a mouse model. We also found that ranolazine attenuated not only the cardiotoxic side effects of trastuzumab but also those of pertuzumab and TDM1, when used in combinatorial treatments both in vitro and in vivo, as demonstrated by the recovery of fractional shortening and ejection fraction values in mice pretreated with TDM1.
Conclusion: We demonstrated that it is possible to predict the eventual cardiotoxic effects of novel approved anticancer drugs early by using in vitro and in vivo approaches, which can also be useful to screen in advance the cardioprotective agents, so as to avoid the onset of unwanted cardiotoxic side effects.
Keywords: breast cancer, immunotherapy, pertuzumab, trastuzumab-DM1, ranolazine
Introduction
Immunotherapy is currently revolutionizing the treatment of malignant diseases by demonstrating efficacy in a large variety of human cancers1 that represent the leading causes of morbidity and mortality worldwide, with ~14 million new cases and 8.2 million cancer-related deaths annually.2
The tyrosine kinase receptor epidermal growth factor receptor 2 (ErbB2; or HER2) is overexpressed in 25%–30% of breast cancer and is associated with a poor prognosis;3 thus, it has been considered as a promising target for immunotherapy.
Trastuzumab, the first humanized monoclonal antibody (mAb) specific for ErbB2 approved by the US Food and Drug Administration (FDA) for the treatment of breast cancer, is the most widely used drug for targeted therapy of breast cancer.4 Unfortunately, large-scale clinical trials of adjuvant therapy with trastuzumab and recently published retrospective studies in breast cancer patients indicate that it can cause cardiac dysfunction and heart failure in a significant fraction of treated patients.5–7
Trastuzumab-associated cardiotoxicity is likely due to the inhibition of ErbB2, which plays a critical role in heart development and function due to its involvement in intracellular pathways that mediate cell survival and heart regeneration.8–10 Indeed, in cardiac tissues, ErbB2 works as a coreceptor for another ErbB family member, ErbB4, and its peptide neuregulin 1 (NRG1), which promotes the heterodimerization of ErbB4/ErbB2, which in turn triggers the activation of ERK-MAPK and PI3K-Akt pathways, thus promoting cardiomyocyte survival and contractile function.11 For this reason, the blocking ErbB2 could lead to cardiac dysfunction or cardiomyocyte death. Furthermore, NRG1 protects the cardiomyocytes from sarcoendoplasmic reticulum calcium ATPase downregulation triggered by oxidative stress.12 Alterations in Ca2+ homeostasis are involved in several cardiac diseases, including cardiac hypertrophy and heart failure.13
Ranolazine, a drug used to treat chronic angina,14 acts as an inhibitor of the late Na+ ion current in cardiac cells, thus intervening in transmembrane cardiac action potential. The resultant reduction in intracellular Na+ concentration partially inhibits the Na+/Ca2+ exchange current, thus preventing the deleterious effect of intracellular Ca2+ overload under the trigger of ischemia.15 This indirect decrease in intracellular Ca2+ concentration is responsible for the well-documented antianginal effect of ranolazine14,16 and for the attenuation of toxicity due to sarcoendoplasmic reticulum calcium ATPase downregulation.17
As previously described, ranolazine is able to protect the cardiac function from both doxorubicin- and trastuzumab-related cardiotoxicity by reducing the effects of oxidative stress.18,19
To overcome the problems related to cardiac toxicity of trastuzumab, new therapeutic agents specific for ErbB2 receptor, such as pertuzumab and trastuzumab-DM1, have been considered as alternative drugs and recently approved by the FDA.20,21
In particular, pertuzumab, a humanized mAb, which prevents the dimerization of ErbB2 with other ErbB receptors, is currently in clinical use for metastatic breast cancer therapy in combination with trastuzumab and docetaxel.22 Indeed, the addition of pertuzumab to neoadjuvant trastuzumab-based chemotherapy improves the response rates in ErbB2-positive breast cancer; however, it also increases trastuzumab-associated toxicity.23
Trastuzumab-DM1 (TDM1), a novel anti-ErbB2 antibody–drug conjugate approved by the FDA for the therapy of breast cancer patients resistant to trastuzumab,24 is made up of trastuzumab and emtansine (DM1), a highly potent antimicrotubule agent, which binds to tubulin and inhibits microtubule assembly with greater potency than vincristine or vinblastine.25–27
In this study, we investigated for the first time the effects of pertuzumab and trastuzumab-DM1 in vitro on three different cardiac cell lines, that is, rat cardiomyoblasts (H9C2), human fetal cardiomyocytes (HFCs) and human adult-like induced pluripotent stem cells (iPSCs)-derived cardiomyocytes (Cor.4U) and in vivo on a mouse model. Furthermore, we investigated the potential cardioprotective effects of ranolazine in combinatorial treatment with pertuzumab and trastuzumab-DM1 on both the models mentioned above.
Materials and methods
Cell lines
Rat H9C2 cardiomyoblasts were cultured in DMEM containing sodium pyruvate (1.0 mM). The SKBR3 cell line from human breast cancer and the A431 cell line from human epidermoid carcinoma were cultured in Roswell Park Memorial Institute 1640 (Thermo Fisher Scientific, Waltham, MA, USA).
The media were supplemented with 10% heat-inactivated fetal bovine serum, 2.0 nM L-glutamine, 50 U/mL penicillin and 50 μg/mL streptomycin (all from Sigma-Aldrich, St Louis, MO, USA).
The HFC (Innoprot, Derio, Spain) and the iPSC-derived cardiomyocytes (Cor.4U) (Axiogenesis, Cologne, Germany) were cultured according to the manufacturer’s recommendations.
Antibodies
The following antibodies were used: anti-Her2/ErbB2 rabbit mAb (Cell Signaling Technology, Danvers, MA, USA), anti-actin rabbit polyclonal antibody (Sigma-Aldrich), trastuzumab (Herceptin; Genentech, South San Francisco, CA, USA), pertuzumab (Perjeta®; Roche Diagnostic, Mannheim, Germany) and trastuzumab-DM1 (TDM1) (Kadcyla®; Roche).
Western blotting and enzyme-linked immunosorbent assays (ELISA) for ErbB2 detection on Cor.4U cell line
The iPSC-derived cardiomyocytes (Cor.4U), SKBR3 and A431 tumor cells were collected and lysed as previously described.28 Aliquots of 20 μg of lysates were run on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electroblotted onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The ErbB2 protein was detected by using a mouse-anti ErbB2 antibody, followed by a rabbit anti-mouse horseradish peroxidase-conjugated antibody. For the detection of signal intensity of reactive bands, a phosphorimager (45-710; Bio-Rad, Hercules, CA, USA) was used.
For ELISA, the iPSC-derived cardiomyocytes (Cor.4U) were harvested in nonenzymatic dissociation solution (Sigma), then washed and transferred to U-bottom microtiter plates (1×105 cells per well), and treated as previously described.29 Briefly, after blocking with PBS containing 6% bovine serum albumin (Sigma-Aldrich), cells were treated with the anti-ErbB2 antibodies diluted in PBS 1x containing bovine serum albumin 3% for 90′ at 25°C. Then the cells were washed twice in PBS 1x and incubated with horseradish peroxidase-conjugated anti-human IgG (Fc-specific). Binding values were determined from the absorbance at 450 nm and reported as the mean of at least three determinations.
In vitro cardiotoxicity assays
To evaluate the in vitro cardiotoxic effects of trastuzumab, pertuzumab, TDM1 or doxorubicin (EBEWE Italia srl, Rome, Italy), H9C2 and HFC cell lines were seeded in 96-well microtiter plates at a density of 1×104 cells/well. Cor.4U cells were seeded in Human Fibronectin Cellware 96-well plates (Corning, Bedford, MA, USA) at a density of 2.5×104 cells/well and incubated for 16 hours at 37°C to allow for cell adhesion in the plates.
The medium was then replaced with fresh medium containing increasing concentrations of trastuzumab, pertuzumab, TDM1 or doxorubicin (25, 50, 100 and 200 nM) for 72 hours.
For the determination of cardiotoxicity, cells were either tested by MTT, as previously described,29 or counted by the trypan blue exclusion test.
In order to evaluate the cardioprotective effects of ranolazine (Ranexa; A. Menarini Industrie Farmaceutiche Riunite S.r.l. Florence, Italy), doses comparable with those used clinically in humans were used.30 Cells were seeded in an MW96 plate as described above and then treated with 10 μM of ranolazine for 72 hours. Cells were then treated for further 72 hours in the absence or the presence of 200 nM of trastuzumab, pertuzumab, TDM1 or doxorubicin. Cell viability was evaluated by both trypan blue exclusion test and MTT assay as described above; the values were obtained from three independent experiments and expressed as the percentage of viable cells treated with the drugs with respect to untreated control cells. SDs were calculated by using the results obtained for each experiment, and the p-values were calculated by using a two-tailed Student’s t-test.
Cell morphology analysis
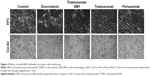
HFC cells were seeded in six-well microtiter plates at a density of 6×105 cells/well. Cor.4U cells were seeded in Human Fibronectin Cellware 96-well plates at a density of 2.5×104 cells/well and incubated for 16 hours at 37°C. Cells were then treated for 72 hours in the absence or the presence of 200 nM of trastuzumab, pertuzumab, TDM1 or doxorubicin and were observed by light microscopy (DM IL; Leica) and photographed (DFC320; Leica) to analyze cell morphology (magnification 1:20).
Evaluation of in vivo cardiotoxic effects of TDM1
C57/BL6 mice, 6–8 weeks old, were purchased from Harlan (San Pietro al Natisone, Italy). Mice were housed (six per cage) and maintained in conditions with 12 h of light and were provided food and water ad libitum at all times. The experimental protocols were in accordance with the EU Directive 2010/63/EU for animal experiments and the institutional guidelines of the Italian Ministry of Health Animal Care and Use Committee. All animal experiments were approved by the local ethic committee “Organismo preposto al benessere degli animali”.
After 1 week, the mice were randomized for weight and enrolled in treatment groups. To evaluate the cardiac function in vivo, mice were treated with 100 μL of saline solution or with TDM1 (44.4 mg/kg). Mice were anesthetized with tiletamine (0.09 mg/g), zolazepam (0.09 mg/g) and 0.01% atropine (0.04 mL/g). At day 0 and after 7 days, fractional shortening (FS) and ejection fraction (EF) were measured by M/B mode echocardiography. These measurements were repeated after 5 days of ranolazine treatment (305 mg/kg/day), starting at the end of TDM1 treatment.
Results
ErbB2 expression in HFC and differentiated adult cardiomyocytes
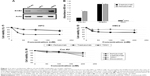
To evaluate the levels of expression of ErbB2 receptor in adult-like cardiomyocytes and compare them to those of HFC, a Western blotting analysis of cell extracts from human differentiated iPSC-derived cardiomyocytes (Cor.4U), HFC and H9C2 cardiomyoblasts was carried out by using a commercial anti-ErbB2 antibody. In Cor.4U cell line, endowed with a phenotype that resembles the adult cardiac beating phenotype, the levels of ErbB2 were comparable to those of HFC (Figure 1A), even though they were slightly lower than those observed in H9C2 cardiomyoblasts.
To test whether the receptor was expressed on the cell surface of iPSC-derived cardiomyocytes in a native conformation, such as that recognized by the anti-ErbB2 antibodies trastuzumab and pertuzumab, an ELISA was performed by incubating the Cor.4U cells in the absence or the presence of 200 nM of each antibody. In parallel assays, ErbB2-positive SKBR3 and ErbB2-negative A431 tumor cells were used as positive and negative controls, respectively. As shown in Figure 1B, both the antibodies tested were able to bind to ErbB2 receptor expressed on the surface of Cor.4U cells, thus confirming that the receptor is expressed in a correct conformation and that this cell line can be useful for studying the effects of ErbB2-targeted therapeutics.
In vitro cardiotoxic effects of anti-ErbB2 antibodies
Since TDM1 and pertuzumab have been recently approved by the FDA for clinical use, we decided to verify whether they are less toxic than trastuzumab on cardiac cells in vitro.
In a first attempt, we tested the cardiotoxic effects of pertuzumab and TDM1 on H9C2 and HFC cells in vitro by using trastuzumab and doxorubicin as controls. Briefly, the cells were treated for 72 hours in the absence or the presence of increasing concentrations of trastuzumab, pertuzumab or TDM1 (25, 50, 100 and 200 nM) and then were tested by MTT. As shown in Figure 1C and D, pertuzumab and trastuzumab reduced HFC viability by about 20%. Surprisingly, a more drastic reduction (reaching about 60%) of viability was observed in TDM1-treated cells. Indeed, TDM1 showed a stronger effect also on H9C2 cardiomyoblasts (40% vs 20% of trastuzumab) in a similar fashion to pertuzumab.
To test whether these effects were only due to an inhibition of cell growth of proliferating cardiomyoblasts and fetal cardiomyocytes, we decided to test the effects of these novel drugs on human iPSC-derived cardiomyocytes that resemble human adult differentiated cardiomyocytes with beating phenotype.
Thus, the cardiotoxic effects of anti-ErbB2 antibodies were tested on Cor.4U cells in the same conditions described above for H9C2 and HFC cells by using doxorubicin as a control.
As shown in Figure 1E, Cor.4U cells showed a decrease in viability of about 10% when treated with trastuzumab or pertuzumab at a concentration of 200 nM. A more significant decrease in cell viability was observed when these cells were treated with TDM1 or doxorubicin (15% and 25%, respectively).
The difference in the effects of the antibodies on cell viability values between HFC and adult-like cardiomyocytes can be explained by considering that the former cells are still proliferating and their growth can also be inhibited by the anti-ErbB2 antibodies, whereas the Cor.4U cells are terminally differentiated and, thus, their proliferation cannot be affected.
More interestingly, TDM1 and doxorubicin strongly affected the morphologic features also of both HFC and Cor.4U cells (Figure 2), thus confirming that they are toxic for these cells.
This hypothesis is also supported by their ability to alter the beating phenotype of differentiated cardiomyocytes (Videos S1–3) differently from trastuzumab and pertuzumab (data not shown).
In vitro cardioprotective effects of ranolazine
To evaluate whether ranolazine is able to protect cardiomyocytes from the cardiotoxic effects of trastuzumab, pertuzumab and TDM1, all the three HFC, H9C2 and Cor.4U cell lines were pretreated in the absence or the presence of 10 μM of ranolazine for 72 hours and then further treated for an additional 72 hours in the absence or the presence of 200 nM of trastuzumab, pertuzumab, TDM1 or doxorubicin. Cells were either counted by the trypan blue exclusion test or tested by MTT assay to determinate the cell viability, and the results reported in Figure 3 are those obtained by trypan blue exclusion test as this method appeared to be more sensitive to the toxicity of the monoclonal antibodies with respect to MTT, used in Figure 1.
As shown in Figure 3, ranolazine had a significant cardioprotective effect on all the cells treated either with trastuzumab, pertuzumab or TDM1. Indeed, ranolazine significantly reduced trastuzumab toxicity by increasing the cell viability from 60% to 80% in H9C2 cells and from 80% to 100% in HFC cells.
Moreover, ranolazine blunted pertuzumab and TDM1 cardiotoxicity by increasing the cell viability of about 40% in H9C2 and 20% in HFC and about 15% in H9C2 and 25% in HFC, respectively. Ranolazine also significantly protected the differentiated cardiomyocytes from the cardiotoxic effects of doxorubicin, trastuzumab, TDM1 and pertuzumab (Figure 4).
TDM1 induces cardiac dysfunction in mice attenuated by ranolazine
We tested here for the first time the effects of TDM1 on the heart function in mice. To achieve this aim, echocardiography was performed in groups of six mice before and after 7 days of treatment with TDM1. As shown in Figure 5, TDM1 significantly reduced (about 10%) both FS and EF after 7 days of treatment.
The protective role of ranolazine on TDM1 cardiotoxicity was then tested in parallel in two groups of six mice pretreated with TDM1 for 7 days. At the end of TDM1 treatment, the mice were further treated with ranolazine (305 mg/kg/day) or with 100 μL of saline solution (control) for 5 days, as described in the “Materials and methods” section. As shown in Figure 5, FS and EF were only partially reduced (about 5%) in mice treated with ranolazine compared to the mice treated with TDM1 as a single agent, thus confirming that ranolazine can attenuate the cardiotoxic effects of TDM1 also in vivo in a mouse model.
Discussion
Trastuzumab, the most widely used drug in targeted breast cancer therapy, clearly improves the survival of treated patients, but on the other hand, it has shown cardiotoxic effects both in clinical trials and in retrospective studies on the real population.5–7 Thus, new drugs specific for ErbB2 receptor and approved by the FDA, such as pertuzumab and trastuzumab-DM1,20,21 have been considered as novel therapeutic drugs, for increasing the efficiency and safety of breast cancer immunotherapy.
Indeed, the addition of pertuzumab, a humanized mAb currently used for metastatic breast cancer therapy, to trastuzumab and docetaxel in patients with ErbB2-positive metastatic breast cancer significantly improved the median overall survival, showing the efficacy of this drug combination.31,32 However, pertuzumab-based therapy is not free of some toxic side effects, occurring in almost 70% of treated patients,33 and unfortunately, it also increases trastuzumab-associated cardiotoxicity.23
Trastuzumab-DM1, a new immunoagent developed for the treatment of ErbB2-positive breast cancer, has been designed to become a more potent cytotoxic drug than trastuzumab as it is made up of trastuzumab linked to DM1, which inhibits cell division and induces cell death.25–27 TDM1 has been approved by the FDA for the treatment of ErbB2-positive breast cancer patients already treated with trastuzumab and taxane-based therapy.34
Since the clinical trials with TDM1 have been performed on selected patients who did not show cardiotoxic effects during the previous treatment with trastuzumab, it could have been hard to highlight the potential toxic effects of its treatment on cardiac cells.
For this reason, the aim of our study was to test, for the first time, the cardiotoxic effects of TDM1 and pertuzumab in vitro on different cultures of cardiomyocytes and in vivo on a mouse model.
In particular, here, we used for the first time adult-like iPSC-derived cardiomyocytes (Cor.4U) that perfectly resemble human adult differentiated cardiomyocytes with beating phenotype, in addition to cardiomyoblasts and HFC, already used for our previous studies.29,35
All the treated cardiac cell lines were significantly affected by treatment with all the tested drugs. Surprisingly, the novel immunoconjugate TDM1 showed stronger inhibitory effects on cardiac cells with respect to trastuzumab and pertuzumab, not only by reducing more significantly the cell viability of both HFC and adult-like cardiomyocytes (Cor.4U) but also by changing the morphology of these cells, differently from trastuzumab and pertuzumab. Moreover, TDM1 strongly altered the functionality of cardiac cells as it was able to affect the beating phenotype of adult-like cardiomyocytes in vitro and reduce the FS and EF in vivo in a mouse model.
The unpredicted cardiotoxicity of TDM1 can be easily explained by considering that it could be likely due to the biologic ability of trastuzumab to block ErbB2, combined with the additional toxic effects of a toxin, such as DM1.
We also further investigated the protective effects, both in vitro and in vivo, of ranolazine, a drug in clinical use to treat chronic angina and ischemia, acting as an inhibitor of the late Na+ ion current in cardiac cells,14 which has already shown cardioprotective effects for doxorubicin and trastuzumab treatment by reducing the effects of oxidative stress.18,19 Here, we tested whether it could also be used to revert the cardiotoxic effects of the novel approved drugs. We found that ranolazine attenuated not only the cardiotoxic side effects of trastuzumab but also those of pertuzumab and TDM1, when used in combinatorial treatments on all the cardiac cell lines used in our study. In particular, ranolazine blunted pertuzumab and TDM1 cardiotoxicity by increasing the cell viability of both fetal and differentiated cardiomyocytes.
Similar results were also obtained in vivo in a mouse model, as ranolazine was able to reduce the alteration of FS and EF induced by TDM1, thus confirming its potential cardioprotective role, already evidenced for trastuzumab in previously published reports19 and for TDM1 as well.
Conclusion
In conclusion, we demonstrated that it is possible to predict eventual cardiotoxic effects of approved anticancer drugs early by using simple models that can be applied to a larger panel of therapeutics, in order to anticipate the appearance of future adverse side effects. Furthermore, these in vitro and in vivo approaches can also be useful to screen cardioprotective agents in advance, so that they could be appropriately used in combination treatments to avoid the onset of unwanted cardiotoxic side effects.
Acknowledgments
The authors wish to thank Dr Giovanna Piscopo and Dr Claudio Arra for their kind support. This work was funded by the “Ricerca Corrente” grant from the Italian Ministry of Health.
Author contributions
CDL and NM conceived the project. CDL and RP wrote the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Grüllich C. Immunotherapy as modern tumor treatment. Radiologe. 2017;57(10):822–825. | ||
Stewart B, Wild CP. World Cancer Report 2014, International Agency for Research on Cancer. Lyon Cedex: International Agency for Research on Cancer; 2014. | ||
Slamon DJ, Clark GM. Human breats cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. | ||
Stebbing J, Copson E, O’Reilly. Herceptin (Trastuzumab) in advanced breast cancer. Cancer Treat Rev. 2000;26(4):287–290. | ||
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al; ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–2801. | ||
Bowles EJ, Wellman R, Feigelson HS, et al; Pharmacovigilance Study Team. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104(17):1293–1305. | ||
Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60(24):2504–2512. | ||
Lim SL, Lam CS, Segers VF, Brutsaert DL, De Keulenaer GW. Cardiac endothelium-myocyte interaction: clinical opportunities for new heart failure therapies regardless of ejection fraction. Eur Heart J. 2015;36(31):2050–2060. | ||
De Keulenaer GW, Doggen K, Lemmens K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res. 2010;106(1):35–46. | ||
D’Uva G, Aharonov A, Lauriola M, et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17(5):627–638. | ||
Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–270. | ||
Timolati F, Ott D, Pentassuglia L, et al. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol. 2006;41(5):845–854. | ||
Luo M, Anderson ME. Mechanisms of altered Ca2+ handling in heart failure. Circ Res. 2013;113(6):690–708. | ||
Boden WE, O’Rourke RA, Teo KK, et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–1516. | ||
Margos P, Margos N, Mokadem N, Patsiotis I, Kranidis A. Ranolazine: safe and effective in a patient with hypertensive cardiomyopathy and multiple episodes of electrical storm. Clin Case Rep. 2017;5(7):1170–1175. | ||
Sendón JL, Lee S, Cheng ML, Ben-Yehuda O; CARISA Study Investigators. Effects of ranolazine on exercise tolerance and angina frequency in patients with severe chronic angina receiving maximally-tolerated background therapy: analysis from the Combination Assessment of Ranolazine in Stable Angina (CARISA) randomized trial. Eur J Prev Cardiol. 2012;19:952–959. | ||
Ahmad S, Ahmad A, Hendry-Hofer TB, et al. Sarcoendoplasmic reticulum Ca(2+) ATPase. A critical target in chlorine inhalation-induced cardiotoxicity. Am J Respir Cell Mol Biol. 2015;52(4):492–502. | ||
Tocchetti CG, Carpi A, Coppola C, et al. Ranolazine protects from doxorubicin-induced oxidative stress and cardiac dysfunction. Eur J Heart Fail. 2014;16(4):358–366. | ||
Riccio G, Antonucci S, Coppola C, et al. Ranolazine attenuates trastuzumab induced heart dysfunction by modulating ROS production. Front Physiol. 2018;9:38. | ||
Baron JM, Boster BL, Barnett CM. Ado-trastuzumab emtansine (TDM1): a novel antibody-drug conjugate for the treatment of HER2-positive metastatic breast cancer. J Oncol Pharm Pract. 2015;21(2):132–142. | ||
Gao J, Swain SM. Pertuzumab for the treatment of breast cancer: a safety review. Expert Opin Drug Saf. 2016;15(6):853–863. | ||
Fleeman N, Bagust A, Beale S, et al. Pertuzumab in combination with trastuzumab and docetaxel for the treatment of HER2-positive metastatic or locally recurrent unresectable breast cancer. PharmacoEconomics. 2015;33(1):13–23. | ||
Van Ramshorst MS, van Werkhoven E, Honkoop AH, et al; Dutch Breast Cancer Research Group (BOOG). Toxicity of dual HER2-blockade with pertuzumab added to anthracycline versus non-anthracycline containing chemotherapy as neoadjuvant treatment in HER2-positive breast cancer: the TRAIN-2 study. Breast. 2016;29:153–159. | ||
Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:2698–2704. | ||
Remillard S, Rebhun LI, Howie GA, et al. Antimitotic activity of the potent tumor inhibitor maytansine. Science. 1975;189:1002–1005. | ||
Cassady JM, Chan KK, Floss HG, et al. Recent developments in the maytansinoid antitumor agents. Chem Pharm Bull. 2004;52:1–26. | ||
Widdison WC, Wilhelm SD, Cavanagh EE, et al. Semisynthetic maytansine analogues for the targeted treatment of cancer. J Med Chem. 2006;49:4392–4408. | ||
Fedele C, Carvalho S, Riccio G, et al. Effects of a human compact anti-ErbB2 antibody on gastric cancer. Gastric Cancer. 2014;17(1):107–115. | ||
Riccio G, Esposito G, Leoncini E, et al. Cardiotoxic effects, or lack thereof, of anti-ErbB2 immunoagents. FASEB J. 2009;23(9):3171–3178. | ||
Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. | ||
Swain SM, Baselga J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Eng J Med. 2015;372(8):724–734. | ||
Amiri-Kordestani L, Wedam S, Zhang L, et al. First FDA approval of neoadjuvant therapy for breast cancer: pertuzumab for the treatment of patients with HER2-positive breast cancer. Clin Cancer Res. 2014;20(21):5359–5364. | ||
Soyano AE, Reynolds G, Moreno-Aspitia A, et al. Rifaximin for pertuzumab-related GI toxicities. Front Oncol. 2017;7:168. | ||
Martínez MT, Pérez-Fidalgo JA, Martín-Martorell P, et al. Treatment of HER2 positive advanced breast cancer with TDM1: a review of the literature. Crit Rev Oncol Hematol. 2016;97:96–106. | ||
Fedele C, Riccio G, Coppola C, et al. Comparison of preclinical cardiotoxic effects of different ErbB2 inhibitors. Breast Cancer Res Treat. 2012;133(2):511–521. |
Supplementary materials
Video S1 Cor.4U-not-treated: beating phenotype of untreated Cor.4U cells.
Video S2 Cor.4U-doxorubicin: effects of doxorubicin on cardiac cell beating phenotype of Cor.4U cells treated for 72 hours.
Video S3 Cor.4U-TDM1: effects of TDM1 on cardiac cell beating phenotype of Cor.4U cells treated for 72 hours.
Abbreviation: TDM1, trastuzumab-emtansine.
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.