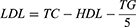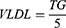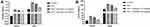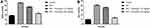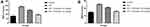Back to Journals » Journal of Inflammation Research » Volume 15
Cardioprotective Effect of Gossypin Against Myocardial Ischemic/Reperfusion in Rats via Alteration of Oxidative Stress, Inflammation and Gut Microbiota
Authors Cheng G , Zhang J, Jia S, Feng P, Chang F, Yan L, Gupta P, Wu H
Received 19 November 2021
Accepted for publication 11 January 2022
Published 5 March 2022 Volume 2022:15 Pages 1637—1651
DOI https://doi.org/10.2147/JIR.S348883
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Gong Cheng,1 Ji Zhang,2 Shuo Jia,2 Panpan Feng,3 Fengjun Chang,1 Li Yan,1 Pranay Gupta,4 Haoyu Wu1
1Department of Cardiology, Shaanxi Provincial People’s Hospital, Xian, 710068, People’s Republic of China; 2Department of Emergency, Shaanxi Provincial People’s Hospital, Xian, 710068, People’s Republic of China; 3Department of General Medicine, Shaanxi Provincial People’s Hospital, Xian, 710068, People’s Republic of China; 4Department of Pharmacology, Institut Teknologi Bandung, Bandung, Jawa Barat, Indonesia
Correspondence: Gong Cheng, Department of Cardiology, Shaanxi Provincial People’s Hospital, Xian, 710068, People’s Republic of China, Tel +8618729529996, Email [email protected]
Background: Myocardial ischemic/reperfusion (I/R) injury is a key prognostic factor after the myocardial infarction. However, at the time of reperfusion, the myocardial tissue has undergone for the necrosis and initiated the induction of oxidative stress and inflammation. The current study was to scrutinize the cardioprotective effect of gossypin against ISO-induced I/R injury in myocardial tissue and explore the possible underlying mechanism.
Methods: Sprague Dawley (SD) was used in the current protocol and ISO was used for induction the I/R in rat. The rats were divided into different groups and received the oral administration of gossypin treatment before the reperfusion. The body weight, heart weight and heart body weight ratio were estimated. The antioxidant, cardiac injury parameters, inflammatory cytokines, inflammatory mediators, gut microbiota and lipid parameters were estimated. At the end, heart tissue histopathological study was carried out.
Results: ISO-induced I/R rats received the gossypin treatment significantly (P < 0.001) enhanced the body weight and decreased the heart weight, along with suppressed the infarct size. Gossypin treatment significantly (P < 0.001) reduced the level of heart parameters, such as creatinine kinase-MB (CK-MB), lactate dehydrogenase (LDH), creatine kinase (CK), cardiac troponin I (CTn-I) and cardiac troponin T (CTn-T) in the serum. Gossypin treatment significantly (P < 0.001) altered the cardiac function, hepatic, antioxidant, inflammatory cytokines and inflammatory mediators. Gossypin significantly (P < 0.001) suppressed the MMP-2 and MMP-9 in ISO-induced I/R rats. Gossypin treatment considerably alleviated the gut dysbiosis through altered Firmicutes to Bacteroidetes (F/B) ratio and also maintained the relative abundance of Butyricicoccus, Clostridium IV, Akkermansia, Roseburia and Clostridium XIVs.
Conclusion: Based on result, we can conclude that gossypin is an alternative drug for the treatment of ISO-induced I/R in rats via alteration of oxidative stress, inflammatory reaction and gut microbiota.
Keywords: gossypin, ischemia-reperfusion, gut microbiota, inflammatory, antioxidant
Introduction
Coronary artery disease (CAD) is a global health problem that commonly induces the myocardial I/R.1 I/R is the leading cause of death among patients with coronary heart disease around the world. According to the World Health Organization (WHO) reports, I/R causes the mortality in 2020.2 As per the study of the Global Burden of Disease, 24% of men and 20% of women suffered from cardiovascular disease (CVD).3 Insufficient or irregular supply of blood and oxygen in the myocytes develops the I/R, which brings alterations in the biochemical, mechanical and basic properties of the heart tissue. Despite constant breakthroughs in the detection and treatment of coronary artery diseases, I/R continues to be leading cause of death, globally.2 I/R is a serious health issue and the leading cause of mortality and morbidity in the Western world, even in China.4 I/R-related morbidity and mortality have reached epidemic proportions, with 16.7 million deaths per year worldwide.5 During the I/R condition, increases the energy requirements and deficient oxygen supply to the heart myocytes. It involves various changes in the signaling and metabolic pathways that are involved in enhanced oxidative stress, excessive cytoplasmic/mitochondrial calcification, irreversible DNA injury, lipid peroxidation (LPO), dynamic cellular metabolism, altered antioxidant homeostasis and pathophysiology.6,7 Clinically, I/R diagnosis via observing the loss of ventricular functionality, electrocardiograph and blood pressure, coupled with boosted serum expression of cardiac-related proteins. I/R can induce other symptoms such as cardiac hypertrophy and myocardial fibrosis. The mechanism involved in the I/R associated with inflammation, apoptosis and oxidative stress.6,8,9
Isoprenaline (1-[3,4-dihydroxyphenyl]-2-isopropylamino ethanol hydrochloride) is a β-adrenoceptor agonist (catecholamine drug) widely used in the treatment of bronchial asthma, allergic emergencies, ventricular bradycardia, glaucoma and cardiac arrest.6,8,9 However, a large dose of ISO induces the stress in myocardium via reducing the energy of stored molecules in cardiomyocytes, which ultimately induces the infarcts such as irreversible cellular injury and necrosis.10–12 After autooxidation, ISO produces a huge quantity of free radicals, altering the tissue defense systems, such as antioxidant molecules or chemical scavengers and endogenous antioxidant enzymes, such as glutathione peroxidase (GPx), superoxide dismutase (SOD), glutathione-S-transferase (GST) and catalase (CAT).13,14 This is due to a reduction in the endogenous antioxidant system which increases the level of lipids in the heart tissue. It is well known that ISO-induced I/R model in the rodent similar to the human myocardial ischemia.15 Due to the similarity of this model, ISO model is widely used to evaluate the protective effect of various drugs against myocardial I/R.13,14
It is well known that the gut microbiota is made up of 5 phyla, such as Actinobacteria, Proteobacteria, Cerromicrobiota, Firmicutes and Bacteroidetes.16 All the 5 phyla of gut microbiota, Firmicutes (gram-positive bacteria) especially Lactobacillus and Clostridium and Bacteroidetes (gram-negative bacteria) especially Bacteroides and Prevotella, found more than 90% in the gut microbial community.17 During the cardiac disease, the level of gram positive and negative bacteria altered. The ratio of Firmicutes to Bacteroidetes varies person to person. The difference in the microbial diversity observed in the host genomes due to various factors such as lifestyle, food intake, regular intake antibiotics, cancer chemotherapy and the use of other drugs.18 The number of archaea and fungi found in the gut flora is less than 1%. The role of these microorganisms is to convert proteins and carbohydrates into short chain fatty acids (SCFAs) in the gut.19 In recent years, the alteration of gut microbiota and activation of inflammatory pathway have been concerned with the expansion of cardiac disease, with the concept of a gut-cardiac axis being proposed, although the connective relationship remains to be explored.16 Gut microbiota have been linked to systemic inflammation as a promoter or mediator.20 Bacterial translocation, dysbiosis and intestinal barrier dysfunction can induce inflammation in I/R.16 Therefore, the onset of I/R disease associated with cardiotoxicity may be prevented via alteration of gut microbiota.
The allopathic western drug mechanism is based on the lock-and-key model that targets a single metabolic/signaling pathway, and the resultant failure to treat the disease is governed by multiple molecular mechanisms.9,12 A lot of synthetic drugs were scrutinized against the I/R condition, but most of them exhibited the serious side/toxic effects and sometimes they cause the patient’s death.14,21 These synthetic drugs are associated with undesirable side effects that lead to serious health issues.6,22 Currently, researchers are focusing their research on the plant and its phytoconstituents to scrutinize the cardio-protection against I/R.13,14 Phytoconstituents with cardiac protective effects can be a safe and effective alternative medicine for the treatment of myocardial infarction.23 Flavonoids are specifically reported to possess anti-inflammatory and antioxidant effects.24 Gossypin (3,5,8,3,4-pentahydroxy-7-O-glucosyl flavone 8-glucoside) also known as gossypin-8-O glucoside (natural occurring bioflavonoid) isolated from the hibiscus vitifolius and belongs to the Malvaceae family.25,26 Gossypin exhibited the protective effect against various diseases via its antioxidant and anti-inflammatory effects.25,27 Gossypin showed a protective effect against the oxidative stress via improved the antioxidant defense system and activate the aminolevulinate dehydratase.28 Gossypin also protects the pancreatic β-cells from gluco-toxicity in diabetes.29 Additionally, gossypin exhibited a neuroprotective effect on the cerebral ischemia rat model.30 Nevertheless, until now, the cardioprotective effect of gossypin against ISO-induced I/R and the role of the gut microbiota have not been investigated. The aim of the current study was to scrutinize the cardioprotective effect of gossypin against ISO-induced I/R in rats and explore the underlying mechanism.
Materials and Methods
Chemical
ISO and gossypin were purchased from the Sigma Aldrich, USA. Total cholesterol (TC), high-density lipoprotein (HDL) and triglyceride (TG) kits were purchased from the Institute of Biological Engineering of Nanjing Jiancheng, Nanjing, China. LDH, CK-MB kits were procured from the Institute of Biological Engineering of Nanjing Jiancheng, Nanjing, China. Inflammatory cytokines and inflammatory mediators were purchased from the TaKaRa Corp, Japan.
Experimental Rodent
Sprague Dawley (SD) rats (200–225 g; sex – male; aged: 8–10 weeks) used in this protocol. The rats were kept in a well-ventilated animal house with maintained temperature (25°C), relative humidity (65%) and 12/12 h light and dark cycles. The rats were received the standard pellet diet and water ad libitum. All the experimental study was carried out accordance to the Institute guidelines and regulations. The animal protocol was approved from the Shaanxi Provincial People’s Hospital (SPPH200262-07).
Preparation of Toxicant and Tested Drug
ISO was used for induction the I/R. The toxicant was prepared using the saline. Tested drug (gossypin) suspension (1% DMSO) was used. All the toxicants and tested drugs are prepared freshly every time.
Induction of I/R
Subcutaneous injection of ISO (100 mg/kg) was used for induction the I/R.1 The rats were received the ISO treatment after every 24 h for 2 days. The tested drug dose was selected on the basis of preliminary dose dependent.
Experimental Protocol
Total 24 rats were divided into 4 groups, and each group contains the 6 rats. The groups are given below:
Group I: control.
Group II: ISO (100 mg/kg).
Group III: ISO (100 mg/kg) + gossypin (10 mg/kg) and
Group IV: ISO (100 mg/kg) + gossypin (20 mg/kg), respectively.
Group I and Group II received the oral administration of carboxyl methyl cellulose (CMC) (1%) using the oral gavage. All groups of rats received the oral administration of the above-mentioned treatment via intragastric tube for 30 days. Gossypin (tested drug) was prepared freshly via preparing the suspension of 1% CMC and tested group received the oral administration before 30 min, induction the myocardial ischemia injury. At the end of the experiment, hemodynamic parameters were recorded. Thereafter, the rats were killed under anesthetized conditions using urethane (1 g/kg, intra-peritoneally injected) to isolate blood. The heart tissues were rinsed in ice-cold 0.9% NaCl after harvesting.
Serum Preparation
At the end of the protocol, the blood samples from all groups of rats were collected into the dry test tube and left to coagulate at room temperature for 30 min. The blood sample centrifuged at 5000 rpm for 15 min.
For the preparation of plasma preparation, the collected blood samples centrifuged at 5000 rpm for 5 min and plasma was separated via aspiration.
Heart tissue was cut into small pieces and prepared the homogenate in appropriate buffer (pH = 7.0) to give homogenate (10%) (wt%/vol%) and homogenate centrifuged at 2000 rpm for 5 min at 0°C. After centrifuge, the supernatant was separated to estimate the biochemical parameters.
Cardiac Function
Previously reported method was used for the estimation of cardiac function using a pressure volume catheter.31 For the estimation of pressure and volume, the catheter was inserted into the carotid artery and progressed into the left ventricle. Different cardiac functions, viz., left ventricular end-systolic pressure (LVEDP), stroke work (SW) and left ventricular end-diastolic pressure (LVEDP), were estimated. PV loops were then changed under the conditions of preload adjustment, which was provoked by blockage of the inferior vena cava, to determine the region inside the PV loop and estimate ventricular function. Using the end-diastolic pressure volume relation (EDPVR) and end-systolic pressure volume relation (ESPVR), the slope of end-diastolic and end-systolic volume points, which are load-independent markers of myocardial ventricular and contractility compliance, was computed.
Cardiac and inflammatory cytokines Parameters
We measured the levels of CK, CK-MB, cTnT, LDH, cTnI and inflammatory cytokines in the rat serum using the standard kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Nitric Oxide (NO) and Inducible Nitric Oxide Synthase (iNOS)
iNOS and NO levels were determined via using the commercial kits following the manufacturer instruction (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Lipid Parameters
Lipid parameters, viz., TC, HDL and TG, were estimated using the commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s protocol. Low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) was estimated using the below formula
Antioxidant Parameters
Antioxidant parameters such as SOD, GPx, CAT and GSH were determined using the previously reported method with minor modification.21,32
Inflammatory Cytokines
Inflammatory cytokines, viz., INF-γ, TNF-α, TGF-β, IL-1β, IL-6 and IL-10, were estimated using the ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Matrix Metalloproteinases
Matrix metalloproteinases (MMP) such as MMP-2 and MMP-9 were estimated using the ELISA kits (Boster Biological Technology, Co, Ltd, California, USA) following the manufacturer instruction.
DNA Extraction and Estimation of 16 rRNA
QIA-amp FAST DNA Stool Mini Kit (Qiagen) was used for the isolation of DNA from the stool using the manufacturer’s protocol. 200 mg faecal samples were collected from the rats and homogenized in 1000 μL ASL lysis buffer and vortexing (2–3 min) in pre-sterilized Eppendorf tube and incubated in water bath for 15 min. After the incubation, the mixture was centrifuged at 15 g rpm for 5 min to separate the supernatant. The collected supernatant added in the 25 μL proteinase K, 200 μL AL solution and vortexed for 15 sec, incubated on water bath for 10 min. Finally, 1.5-mL sterile Eppendorf containers were used to combine the elution buffer (150 µL) with the columns, which were then incubated at room temperature for 10 minutes before being centrifuged for 1 minute at 14,000 rpm. The eluted DNA sample was analysed qualitatively and quantitatively using agarose gel electrophoresis and nanodrop techniques.
Histopathology
Heart tissue immediately removed after sacrifice the rats and heart tissue was soaked into the polyformaldehyde (4%) and fixed for 1 day. Afterthat, processed and embedded the heart tissue using the paraffin as per the standard protocol. Heart tissue (5 µm) cut, dehydrated, deparaffinized and stained with hematoxylin and eosin. A light microscope was used to observe the changes in the heart tissue using a digital camera.
Statistical Analysis
For statistical analysis, GraphPad Prism 8 software (Graph Pad Software) was utilised. One-way analysis of variance (ANOVA) was used to compare the differences in the statistical analysis. The different groups were compared using the Tukey multiple comparison test. If *<P 0.05, **<P 0.01 and ***<P 0.001 was considered significant.
Result
Effect on Infarct Size
No changes was observed in the heart tissue of normal rats. ISO-induced rats exhibited the increased infarct size and gossypin (10 and 20 mg/kg) treated rats exhibited the reduction of the infarct size (Figure 1).
Hemodynamic Parameters
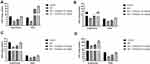
We estimated the hemodynamic parameters, such as LVESP, LVEDP, SW, DP, ESPVR and EDPVR in the different group of rats. ISO-induced I/R injury rats exhibited the reduced level of LVESP, SW, ESPVR, DP, EDPVR and enhanced level of LVEDP. Gossypin treated group rats showed the boosted level of LVESP, SW, ESPVR, DP, EDPVR and suppressed level of LVEDP (Figure 2).
Body Weight
Normal rats exhibited the boosted bodyweight and reduced heart tissue as compared to the ISO-induced I/R rats. ISO-induced rats showed the reduced bodyweight and enhanced heart weight. Due to increased heart weight, it also showed the increased heart/body weight ratio. Gossypin treated rats significantly (P < 0.001) increased the bodyweight and suppressed the heart weight and heart/body weight ratio (Figure 3).
Cardiac Parameters
During the cardiac disease, the cardiac parameters are suppressed due to the expansion of the disease. In this study, we estimated the cardiac parameters, such as CK-MB (Figure 4A), LDH (Figure 4B), CK (Figure 4C), CTn-l (Figure 4D) and CTn-T (Figure 4E) in the serum and the heart tissue. ISO group rats exhibited the increased level of CK-MB, LDH, CK, CTn-l and CTn-T in the serum and reduced level of CK-MB, LDH, CK, CTn-l and CTn-T in the heart tissue. Gossypin treated rats significantly (P < 0.001) suppressed the level of CK-MB, LDH, CK, CTn-l and CTn-T in the serum and boosted the level of CK-MB, LDH, CK, CTn-l and CTn-T in the heart tissue (Figure 4).
Lipid Parameters
ISO induced rats exhibited the increased levels of TG, TC, LDL, VLDL and reduced level of HDL. Gossypin significantly (P < 0.001) suppressed the level of TG, TC, LDL, VLDL and increased the level of HDL (Figure 5).
Antioxidant Parameters
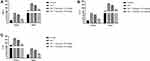
Oxidative stress plays an important role in the expansion of cardiac disease. During the cardiac disease, start the production of free radcials, which further induces the oxidative stress and altered the antioxidant enzymes. The similar result was observed in this experimental study. Where, ISO-induced group rats exhibited the reduced level of SOD (Figure 6A), GPx (Figure 6B), GSH (Figure 6C), CAT (Figure 6D) and gossypin treatment significantly (P<0.001) increased the level of SOD, GPx, GSH, CAT in the erythrocytes and cardiac tissue (Figure 6).
Figure 7 ISO-induced rats exhibited the enhanced level of TBARS and LOOH in the serum and heart tissue. Gossypin treatment significantly (P < 0.001) suppressed the level of TBARS and LOOH in the serum and heart tissue (figure 7).
iNOS and NO
ISO-induced I/R rats exhibited the increased level of iNOS and NO as compared to normal and tested group rats. Gossypin treatment significantly (P < 0.001) suppressed the level of iNOS and NO (Figure 8).
MMP Level
ISO-induced I/R group rats showed the increased level of MMP-2 and MMP-9 and gossypin treatment significantly (P < 0.001) decreased the level of MMP-2 and MMP-9 (Figure 9).
Inflammatory Cytokines and Inflammatory Parameter
ISO-induced I/R group rats exhibited the increased level of TNF-α, IL-1β, IL-6 in the serum and heart tissue. Gossypin significantly (P < 0.001) suppressed the level of inflammatory cytokines in the serum and heart tissue (Figure 10).
The level of inflammatory parameter such as NF-κB play significant role in cardiac disease. ISO-induced I/R group rats exhibited an increased level of NF-κB and gossypin treatment significantly (P < 0.001) decreased the level of NF-κB in the serum and heart tissue (Figure 11). The suppression of inflammatory reaction via gossypin suggesting the antiinflammatory effect.
Gut Microbiota
To estimate the cardiac protective effect of gossypin, we estimated the gut microbiota of all groups of rats. The normal group of rats exhibited the normal abundance of different bacteria. ISO group of rats exhibited an increased abundance of Bifidobacterium, Unidentified-Desulfovibrionaceae and reduced level of Unidentified-Prevoteliaccce, Parabacteroides; gossypin treated rats suppressed the abundance of Bifidobacterium, Unidentified-Desulfovibrionaceae and boosted the level of Unidentified-Prevoteliaccce, Parabacteroides (Figure 12A). F/B level was boosted in the ISO induced I/R group rats and gossypin treated rats significantly (P<0.001) suppressed the F/B ratio (Figure 12B). ISO group rats exhibited the increased adundance of Firmicutes, Candidatus_Saccharibacteria, Unclassified Bacteria and suppressed the level of Bacteroidetes, Actinobacteria, Tenericutes, Verrucomicrobia. Gossypin considerably suppressed the abundance of Firmicutes, Candidatus_Saccharibacteria, Unclassified Bacteria and increased the abundance of Bacteroidetes, Actinobacteria, Tenericutes, Verrucomicrobia (Figure 12C). ISO group rats exhibited the increased abundance of Firmicutes and reduced abundance of Bacteroidetes, Proteobacteria and gossypin significantly (P<0.001) altered abundance of Firmicutes and Bacteroidetes, Proteobacteria (Figure 12D). Gossypin treatment significantly (P<0.001) suppressed the abundance of Unclassified Lachnospiraceae, Clostridium IXVa, Akkermansia (Figure 12E).
Histopathology
The effect of gossypin on the heart histopathology was also investigated to estimate its effect on the micro-structural level. Normal group rats did not exhibit any cell death, histo-architecture and inflammation (Figure 13A). ISO-induced group rats exhibited the apoptosis of muscle fibres with edema, necrosis (Figure 13B) and gossypin (10 and 20 mg/kg) remarkably suppressed the apoptosis of muscle fibres with edema and necrosis (Figure 13C and D).
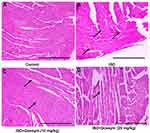 |
Figure 13 Effect of Gossypin on the histopathology of ISO induced MI rats. (A) Normal control, (B) ISO control, (C) ISO+Gossypin (10 mg/kg) and (D) ISO+Gossypin (20 mg/kg). All group contains 6 rats. |
Discussion
To eliminate the global burden of CVD, particularly I/R, it is necessary to find non-toxic and safe cardio-protective drugs. ISO-induced I/R animal model exhibits similar cytological and metabolic alterations to those observed in human I/R. ISO is a synthetic catecholamine (β-adrenergic agonist) has been observed to induce like myocardial necrosis, increase LPO and suppress endogenous antioxidants, enhance cardiac malfunctioning and histological alteration of myocardium that have been defined as comparable to human I/R.4 Various reports suggest that ISO-induced I/R occurs due to alteration in normal hemostasis redox state, which leads to promoting the free radicals and pre-oxide that cause the cardiac cell injury.33,34 Cardiac tissue is highly susceptible to oxidative stress owing to low antioxidant enzyme activity. Antioxidant enzymes and free radical scavengers reduce cardiac dysfunction, constrain infarction size and limit MI progression.33 In this study, we scrutinized the cardioprotective effect of gossypin against the ISO-induced I/R and explored the underlying mechanism.
ISO-induced I/R rats exhibited an increased heart weight to body weight ratio, which may be attributed to an increase in the water level in the intramuscular space, i.e. edema. The water content increase of 1% may be resultant suppress the cardiac function (10%). Previous report suggests that ISO-induced I/R rats demonstrated an increased heart weight and heart weight/body weight ratio.35 Furthermore, gossypin treatment considerably reduced the heart weight and heart weight to body weight ratio. These findings are also corroborated by prior reports.35
CK-MB is a gold indicator of cardiac tissue and is used as an indicator for estimation of myocardial injury.20 The increase level of CK-MB and other diagnostic parameters boosted in the serum showed cardiomyocyte injury, which is an indicator of I/R. Cardiac specific markers such as troponins I and troponin T are highly sensitive and specific markers for cardiac disease and are also used as diagnostic markers for I/R disease.36 CTn-T is the specific marker of I/R and CTn-I is a constituent of myofibrillar cardiac tissue.37 Previous research suggests that the stabilization of cardiac muscle membrane injury is considered as a novel approach to treat ISO-induced I/R.38 The administration of ISO enhances the level of CK, CK-MB, LDH, CTn-1 and CTn-T in the serum and reduces the level in the heart tissue. This alteration might be due to ISO-induced alteration in myocardial film permeability as well as integrity and cardiac infarction. These changes occur in the membrane due to increased free radical production and damage the myocardial membrane.39,40 The Gossypin treatment considerably suppressed the levels of CK, CK-MB, LDH, CTn-I and CTn-T in the serum and improved the heart tissue due to its antioxidant potential and stabilizing the membrane injury.
It is well known that during coronary heart disease, increases the lipid level. A similar result was found in the ISO group of rats. They showed an enhanced level of lipid parameters, suggesting the induction of coronary heart disease.38,39 A dramatically increased level of TG, VLDL, LDL, TC and suppressed level of HDL was observed after the ISO treatment. These changes in lipid profile are due to enhancement of lipid biosynthesis through increased cyclic adenosine monophosphate (cAMP) levels.39
Oxidative damage and mitochondrial dysfunction are associated with increased LPO and reduced endogenous antioxidant enzymes in the myocardial tissue.41,42 The enzymatic antioxidant myocardial system works in a coordination manner to maintain the cellular redox balances. During the ISO treatment, a disturbance occurred in the redox balance in the myocardial. LPO is a crucial pathogenic process in the condition of cardiac injury and the effect of ISO on LPO in the myocardial tissue of rodents is well established.41,42 The peroxidative injury is exerted through the quinone formation (intermediates of ISO), which reacts with the molecular oxygen to generate the ROS and induces the membrane LPO.41 In this experimental protocol, gossypin considerably suppressed the ISO mediated TBARS (LPO marker) in myocardial tissue, suggesting a potent antioxidant effect to protect the myocardium. Gossypin may donate hydrogen atoms to the free radicals during the ISO-induced I/R injury and terminate the free radical-chain reactions. The changes in the redox balance during the ISO in the myocardial tissue are well documented.41,43 In the current study, the reduction of GSH, SOD, GPx and SOD were observed in the heart tissue. This reduction occurred due to the utilization of more cellular enzymatic and non-enzymatic antioxidants to scavenge the free radicals generated during ISO treatment.41 However, gossypin treatment significantly boosted the level of endogenous antioxidant enzymes in the cardiac tissue. ISO group rats received gossypin considerably altered the level of antioxidant enzymes, suggesting an antioxidant effect.
Inflammatory signaling pathways play an important role in the development and progression of CVD and a lot of synthetic drugs, especially anti-inflammatory agents, are used to reduce myocardial injury.38,44 A recent report suggests that during myocardial injury, excessive generation of inflammatory cytokines is observed. IL-1β can increase the infiltration of neutrophils and inflammatory mediators, further inducing the inflammatory reaction and ultimately causing tissue injury.38,45 IL-1β is related to the intensity of inflammation and is also used as an indicator of disease severity.46 ISO-induced I/R rats showed the boosted level of IL-1β and gossypin significantly suppressed the level of IL-1β in the serum and cardiac tissue. The result suggests the anti-inflammatory effect of gossypin leads to the reduction of activation and cascade of inflammatory cytokines, NF-κB and iNOS. Inflammatory cytokines such as IL-6 and TNF-α mainly responsible for the myocardial injury, which depends on the activation of NF-κB. Normally, NF-κB is prevented from inhibitory kappa B (IκB) family activation.38,46 Consequently, IκB fails to maintain the level of NF-κB and activates the NF-κB and its translocation from the cytosol to the nucleus and binds to the promoter sequence of target genes and induces the transcription of inflammatory cytokines.38 IL-6 involved in numerous inflammatory reactions such as neutrophil recruitment and maturation.42 During the cardiac disease, the level of IL-6 increased and it suppressed the catalase activity and enhanced the inflammatory and toxic effects.38 The results showed that gossypin treatment considerably reduced the levels of IL-6, IL-1β, TNF-α and NF-κB in the heart tissue as well as serum as compared to I/R control group rats, suggesting an anti-inflammatory effect, which protects the heart from I/R injury. Flavonoids are reported to have promising preventive effects against various inflammatory reactions and have the capability to alter the key signaling pathways involved in inflammation (pro and anti-inflammatory cytokines).47 Gossypin (flavonoids) suppressed the inflammatory cytokines and inflammatory mediators, suggesting an anti-inflammatory effect against ISO-induced I/R in rats.
MMPs are the proteolytic enzymes involved in the degradation of the extracellular matrix and alteration of cardiomyocytes in both non-infarcted and infarcted myocardium (process of cardiac remodelling), which constitutes the anatomic substrate for the development of congestive heart failure and suggests cardiac death.48 MMP-2 and 9 play an important role in postmyocardial infarction and left ventricular remodeling since they are activated in the myocardial tissue after I/R. MMP-2 (gelatinase A) is commonly observed in all types of cells and degrades into collagen type IV (significant component of the basement membrane) and extracellular matrix protein and denatured collagen.49 Previous research suggests that MMP-2 dysfunction has a cardioprotective effect on oxidative stress via alteration of mitochondrial respiration and increased lipid peroxidation the I/R in rodents.50 During acute I/R, MMP-9 in the infarcted tissue is derived from the neutrophils and may act directly or indirectly on the ventricular tissue (as protease), but it also enhances neutrophil infiltration and exacerbates and degranulates the ischemic insult.49 The suppression of MMP-2 is related to left ventricular toxic remodeling with higher survival. The inhibition of MMP-9 leads to suppress the myocardial rupturing after acute I/R and lower left ventricular dilation due to less reorganization of collagen in the infarct area in rodents.50 Gossypin treatment significantly suppressed MMP-2 and MMP-9 via its potent antioxidant potential, ameliorating cardiac dysfunction and enhancing cardiac repair following I/R.
Literature suggests that the gut microbiota plays an important role in the progression and expansion of I/R disease.51 During the cardiotoxicity, the suppression of gut microbiota was observed in the diversity and richness of different phylum of bacteria and dietary supplements altered the gut microbiota and helped to reduce the cardiotoxicity. Targeting the gut microbiota is a potential approach to treat ISO induced I/R.16 Gossypin treatment considerably removed the deleterious effects of gut dysbiosis such as changes in gut microbiota, reducing inflammation and gut leakiness. ISO induced I/R rat, faecal microbiota transplantation involved in the gut microbiome enhanced the cardiomyopathy and dysregulation of the gut. Targeting gut microbiota may be considered as a novel approach to treating I/R.16 In this study, gossypin treatment considerably avoided dysbiosis of gut microbiota and removed the intestinal barrier integrity. In this study, ISO induced I/R group rats showed the changes in the richness and diversity of gut microbiota and gossypin treatment altered the gut microbiota diversity and richness.
Gossypin treatment considerably protected the gut microbiota composition and relative abundance of different bacteria phyla and genera. The results showed that gossypin treatment considerably enhanced the Bacteroidetes and Firmicutes number and ratio of F/B. The ratio of Firmicutes to Bacteroidetes is used as an indicator of dysbiosis gut microbiota. ISO-induced I/R rats showed a reduction in the F/B ratio and gossypin considerably improved the F/B ratio, suggesting the balancing of gut microbiota dysbiosis is a safe state. Firmicutes are made up 84 species and belong to the Clostridium genera. Among all the species of Firmicutes, Tyzzerella, Coprococcus, Ruminococcus and Lachnospiraceae are linked with the various complications of CVD.16 It is well proven that alteration in the level of Firmicutes induces the CVD lifetime. Bacteroidetes play a crucial role in heart functioning. In this study, ISO group rats increased the Firmicutes level, suggesting that gut microbiota relate to the various signaling pathways, including the trimethylamine (TMA)/trimethylamine N-oxide (TMAO) and SCFAs pathways.52 Gossypin significantly enhanced the levels of Clostridium, Roseburia, Lachnospiraceae and Roseburia; all the bacteria generate anti-inflammatory metabolites which help to change and decrease the pH level in the gut and also suppress excessive bacterial growth and suggesting an anti-inflammatory effect.
Both bacteria Bacteroides and Firmicutes demonstrated an anti-inflammatory effect and played a significant role in the intestinal defence barrier via boosting the TJ protein and mucin productions, leading to higher villi and having longer nutrient absorption capacity.53 Gossypin considerably enhanced the relative abundance of unclassified Clostridiales, which generate butyrate and suppress the activation of NLR Family Pyrin Domain Containing 1 (NLRP1) inflammasome and prevent the cardiac disease. Akkermansia is widely used to host secreted the mucus O-glycoprotein (MOGs) and secreted the huge amount from the goblet cells.16 Gossypin treatment considerably maintained the number of goblet cell and relative abundance of Akkermansia, which further enhanced the protection and repairment of mucus barrier.
Conclusion
Collectively, we can say that gossypin considerably suppressed the hemodynamic parameters, infarct size, heart weight, body weight/heart ratio and increased the body weight. Gossypin treatment considerably suppressed the cardiac parameters, oxidative stress and inflammatory reaction. Gossypin treatment considerably suppressed the MMP-2 and MMP-9 level. The alteration of gut microbiota by gossypin prevents ISO induced myocardial reperfusion in rats via modulation of gut microbiota.
Abbreviation
I/R, Ischemic/reperfusion; SD, Sprague Dawley; LAD, Left anterior descending coronary artery; LVESP, Left ventricular end-systolic pressure; SW, Stroke work; DP, Diastolic pressure; ESPVR, End-systolic pressure volume relation; EDPVR, End-diastolic pressure volume relation; LVEDP, Left ventricular end-diastolic pressure; LDH, Lactate dehydrogenase; CKMB, Creatine Kinase MB; CK, Creatine kinase; CTn-I, Cardiac troponin I; CTn-T, Cardiac troponin T; SOD, Superoxide dismutase; GPx, Glutathione peroxidase; GSH, Glutathione; CAT, Catalase; TBARS, Thiobarbituric acid reactive substances; LOOH, Lipid Hydroperoxide; MMP-2, Matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; CAD, Coronary artery disease; WHO, World Health Organization; LPO, Lipid peroxidation; GST, Glutathione-S-transferase; TC, Total cholesterol; HDL, High-density lipoprotein; TG, Triglyceride; CMC, Carboxyl methyl cellulose; NO, Nitric oxide; iNOS, Inducible nitric oxide synthase; LDL, Low-density lipoprotein; VLDL, Very low density lipoprotein; MMP, Matrix metalloproteinases; TMA, Trimethylamine; TMAO, Trimethylamine N-oxide; NLRP1, NLR Family Pyrin Domain Containing; MOGs, Mucus O-glycoprotein.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Panda S, Kar A, Biswas S. Preventive effect of Agnucastoside C against isoproterenol-induced myocardial injury. Sci Rep. 2017;7(1). doi:10.1038/s41598-017-16075-0
2. Fan Y. Cardioprotective effect of rhapontigenin in isoproterenol-induced myocardial infarction in a rat model. Pharmacology. 2019;103(5–6):291–302. doi:10.1159/000496800
3. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010
4. Adedapo AA, Adeoye BO, Oyagbemi AA, Omobowale TO, Yakubu MA. Cardioprotective effects of the ethanol leaf extract of andrographis paniculata in isoprotereno l-induced myocardial infarction in rats. FASEB J. 2017;31(1Supplement 1). Available from: http://www.fasebj.org/content/31/1_Supplement/1070.12.abstract?sid=63efa7fd-3bba-4fc9-9138-51c75457d9b9%0Ahttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed18&NEWS=N&AN=616904111. Accessed January 12, 2022.
5. Sreeniwas Kumar A, Sinha N. Cardiovascular disease in India: a 360 degree overview. Med J Armed Forces India. 2020;76(1):1–3. doi:10.1016/j.mjafi.2019.12.005
6. Ganapathy R, Ramachandran A, Shivalingaiah SB, et al. Cardioprotective potential of polyphenols rich Thraatchathi Chooranam against isoproterenol induced myocardial necrosis in experimental rats. BMC Complement Med Ther. 2020;20(1). doi:10.1186/s12906-020-03124-x
7. Zhu L, Wei T, Chang X, et al. Effects of salidroside on myocardial injury in vivo in vitro via regulation of Nox/NF-κB/AP1 pathway. Inflammation. 2015;38(4):1589–1598. doi:10.1007/s10753-015-0134-0
8. Raja S, Ramya I, Ravindranadh K. A review on protective role of phytoconstituents against isoproterenol induced myocardial necrosis. Int J Pharmacogn Phytochem Res. 2016;8(5):848–864.
9. Murugesan S, Pandiyan A, Saravanakumar L, Moodley K, Mackraj I. Protective role of wild garlic on isoproterenol-induced myocardial necrosis in Wistar rats. J Ethnopharmacol. 2019;237:108–115. doi:10.1016/j.jep.2019.03.049
10. Krishna RG, Sundara rajan R. Cardioprotective and antioxidant effects of Bougainvillea glabra against isoproterenol induced myocardial necrosis in albino rats. Int J Phytomedicine. 2018;10(1):45. doi:10.5138/09750185.2200
11. Singh N, Kumar R. Effect of nicorandil and amlodipine on bio-chemical parameters during isoproterenol induced myocardial necrosis in rats. Indian J Clin Biochem. 2003;18(1):99–102. doi:10.1007/BF02867674
12. Raja S, Ramya I. Cardioprotective potential of methanol extract of Polygonum glabrum on isoproterenol induced myocardial necrosis in rats. Int J Phytomedicine. 2017;9(3):518. doi:10.5138/09750185.2125
13. Wang Z, Zhang F, Liu W, Sheng N, Sun H, Zhang J. Impaired tricarboxylic acid cycle flux and mitochondrial aerobic respiration during isoproterenol induced myocardial ischemia is rescued by bilobalide. J Pharm Anal. 2021. doi:10.1016/j.jpha.2020.08.008
14. Wang C, Peng D, Liu Y, Yu Z, Guo P, Wei J. Agarwood alcohol extract ameliorates isoproterenol-induced myocardial ischemia by inhibiting oxidation and apoptosis. Cardiol Res Pract. 2020;2020:1–10. doi:10.1155/2020/3640815
15. Fan S, Zhang J, Xiao Q, et al. Cardioprotective effect of the polysaccharide from Ophiopogon japonicus on isoproterenol-induced myocardial ischemia in rats. Int J Biol Macromol. 2020;147:233–240. doi:10.1016/j.ijbiomac.2020.01.068
16. Huang K, Liu Y, Tang H, et al. Glabridin prevents doxorubicin-induced cardiotoxicity through gut microbiota modulation and colonic macrophage polarization in mice. Front Pharmacol. 2019;10(February). doi:10.3389/fphar.2019.00107
17. Huang Z, Gan J, Jia L, et al. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials. 2015;48:26–36. doi:10.1016/j.biomaterials.2015.01.013
18. Magne F, Gotteland M, Gauthier L, et al. The Firmicutes/Bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12(5):1474. doi:10.3390/nu12051474
19. Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8836–8847. doi:10.3748/wjg.v21.i29.8787
20. Naqvi S, Asar TO, Kumar V, et al. A cross-talk between gut microbiome, salt and hypertension. Biomed Pharmacother. 2021;134:111156. doi:10.1016/j.biopha.2020.111156
21. Viswanadha VP, Dhivya V, Somasundaram B, et al. The role of mitochondria in piperine mediated cardioprotection in isoproterenol induced myocardial ischemia. Curr Pharm Des. 2020;27(26):2975–2989. doi:10.2174/1381612826666200909125750
22. Verma VK, Arya D, Bhatia J. Morin ameliorates isoproterenol induced myocardial necrosis in rats. Proc Annu Meet Jpn Pharmacol Soc. 2018;WCP2018:
23. Fathima SN, Vasudeva Murthy S. Current pharmacological status of cardioprotective plants against isoproterenol induced myocardial infarction. Asian J Pharm Clin Res. 2018;11(4):17–27. doi:10.22159/ajpcr.2018.v11i4.24158
24. Ciumărnean L, Milaciu MV, Runcan O, et al. The effects of flavonoids in cardiovascular diseases. Molecules. 2020;25(18):18. doi:10.3390/molecules25184320
25. Bhardwaj JS, Manandhar S, Chatterjee S, et al. Protective effects of gossypin in colchicine-induced cognitive dysfunction and oxidative damage in rats. Res J Pharm Technol. 2020;13(11):5189–5196. doi:10.5958/0974-360X.2020.00907.5
26. Tanyeli A, Eraslan E, Güler MC, Kurt N, Akaras N. Gossypin protects against renal ischemia-reperfusion injury in rats. Kafkas Univ Vet Fak Derg. 2020;26(1):89–96. doi:10.9775/kvfd.2019.22396
27. Cinar I, Yayla M, Tavaci T, et al. In vivo and in vitro cardioprotective effect of gossypin against isoproterenol-induced myocardial infarction injury. Cardiovasc Toxicol. 2021;22(1):52–62. doi:10.1007/s12012-021-09698-3
28. Gautam P, Flora SJS. Oral supplementation of gossypin during lead exposure protects alteration in heme synthesis pathway and brain oxidative stress in rats. Nutrition. 2010;26(5):563–570. doi:10.1016/j.nut.2009.06.008
29. Venkatesan T, Sorimuthu Pillai S. Antidiabetic activity of gossypin, a pentahydroxyflavone glucoside, in streptozotocin-induced experimental diabetes in rats. J Diabetes. 2012;4(1):41–46. doi:10.1111/j.1753-0407.2011.00145.x
30. Kardeş S, Karakuş E, Gelen V, et al. Neuroprotective effect of gossypin on glutamate-induced excitotoxic neuronal death in SH-SY5Y cell line. Toxicol Lett. 2016;258:S286. doi:10.1016/j.toxlet.2016.06.1997
31. Ji L, Tian Z, Liu Y, Liu M. Protective effect of casticin in myocardial ischemia/reperfusion injury in rats via attenuation of oxidative stress and inflammation. Arch Med Sci. 2021. doi:10.5114/aoms/127588
32. Raish M. Momordica charantia polysaccharides ameliorate oxidative stress, hyperlipidemia, inflammation, and apoptosis during myocardial infarction by inhibiting the NF-κB signaling pathway. Int J Biol Macromol. 2017;97:544–551. doi:10.1016/j.ijbiomac.2017.01.074
33. Fathiazad F, Tamarzadeh N, Alsos D, Garjani A, Vaez H. The effect of astragaloside IV on isoproterenol-induced myocardial infarction in rats. Pharm Sci. 2019;25(2):100–110. doi:10.15171/PS.2019.16
34. Goyal SN, Sharma C, Mahajan UB, et al. Protective effects of cardamom in isoproterenol-induced myocardial infarction in rats. Int J Mol Sci. 2015;16(11):27457–27469. doi:10.3390/ijms161126040
35. Govindasami S, Uddandrao VVS, Raveendran N, Sasikumar V. Therapeutic potential of biochanin-a against isoproterenol-induced myocardial infarction in rats. Cardiovasc Hematol Agents Med Chem. 2020;18(1):31–36. doi:10.2174/1871525718666200206114304
36. Ouyang B, Li Z, Ji X, Huang J, Zhang H, Jiang C. The protective role of lutein on isoproterenol-induced cardiac failure rat model through improving cardiac morphology, antioxidant status via positively regulating Nrf2/HO-1 signalling pathway. Pharm Biol. 2019;57(1):529–535. doi:10.1080/13880209.2019.1649436
37. Li H, Song F, Duan LR, et al. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci Rep. 2016;6. doi:10.1038/srep23693
38. Huang H, Geng Q, Yao H, et al. Protective effect of scutellarin on myocardial infarction induced by isoprenaline in rats. Iran J Basic Med Sci. 2018;21(3):267–276. doi:10.22038/ijbms.2018.26110.6415
39. Mundugaru R, Udaykumar P, Senthilkumar S, Bhat S. Cardioprotective activity of fruit of Garcinia pedunculata on isoprenaline-induced myocardial infarction in rat. Bangladesh J Pharmacol. 2016;11(1):231–235. doi:10.3329/bjp.v11i1.24941
40. Abdelhalim AT, Nur NM, Mansour S, Ibrahim A. Cardioprotective effect of vitamin e against myocardial infarction induced by isoprenaline in albino rats. Asian J Pharm Clin Res. 2018;11(6):273–276. doi:10.22159/ajpcr.2018.v11i6.24999
41. Shen Z, Geng Q, Huang H, et al. Antioxidative and cardioprotective effects of schisandra chinensis bee pollen extract on isoprenaline-induced myocardial infarction in rats. Molecules. 2019;24(6):1090. doi:10.3390/molecules24061090
42. Zhang X, Li X, Wang C, et al. Ameliorative effect of ferruginol on isoprenaline hydrochloride-induced myocardial infarction in rats. Environ Toxicol. 2021;36(2):249–256. doi:10.1002/tox.23030
43. Zarkasi KA, Zainalabidin S, Jen-Kit T, Hakimi NH, Ramli NZ, Jubri Z. Tocotrienol-rich fraction modulates cardiac metabolic profile changes in isoprenaline-induced myocardial infarction rats. Sains Malays. 2020;49(2):357–373. doi:10.17576/jsm-2020-4902-14
44. Zaafan MA, Abdelhamid AM. The cardioprotective effect of astaxanthin against isoprenaline-induced myocardial injury in rats: involvement of TLR4/NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2021;25(11):4099–4105. doi:10.26355/eurrev_202106_26052
45. Tawfik MK, Ghattas MH, Abo-Elmatty DM, Abdel-Aziz NA. Atorvastatin restores the balance between pro-inflammatory and anti-inflammatory mediators in rats with acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2010;14(6):499–506.
46. Badavi M, Mard SA, Dianat M, Dashtbozorgi N. Crocin attenuates oxidative stress and inflammation in myocardial infarction induced by isoprenaline via PPARγ activation in diabetic rats. J Diabetes Metab Disord. 2020;19(2):1517–1525. doi:10.1007/s40200-020-00686-y
47. Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12(2):457. doi:10.3390/nu12020457
48. Reinhardt D, Sigusch HH, Henße J, Tyagi SC, Körfer R, Figulla HR. Cardiac remodelling in end stage heart failure: upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart. 2002;88(5):525–530. doi:10.1136/heart.88.5.525
49. Periasamy S, Mo FE, Chen SY, Chang CC, Liu MY. Sesamol attenuates isoproterenol-induced acute myocardial infarction via inhibition of matrix metalloproteinase-2 and −9 expression in rats. Cell Physiol Biochem. 2011;27(3–4):273–280. doi:10.1159/000327953
50. Boarescu P-M, Chirilǎ I, Bulboacǎ AE, et al. Effects of curcumin nanoparticles in isoproterenol-induced myocardial infarction. Oxid Med Cell Longev. 2019;2019:1–13. doi:10.1016/j.mjafi.2019.12.005
51. Kho ZY, Lal SK. The human gut microbiome - a potential controller of wellness and disease. Front Microbiol. 2018;9(AUG). doi:10.3389/fmicb.2018.01835
52. Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes Rev. 2013;14(12):950–959. doi:10.1111/obr.12068
53. Luissint AC, Parkos CA, Nusrat A. Inflammation and the intestinal barrier: leukocyte–epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology. 2016;151(4):616–632. doi:10.1053/j.gastro.2016.07.008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.