Back to Journals » Medical Devices: Evidence and Research » Volume 13
Cardiac Electronic Devices: Future Directions and Challenges
Authors Kotalczyk A , Kalarus Z , Wright DJ, Boriani G , Lip GYH
Received 16 July 2020
Accepted for publication 2 September 2020
Published 25 September 2020 Volume 2020:13 Pages 325—338
DOI https://doi.org/10.2147/MDER.S245625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Agnieszka Kotalczyk,1,2 Zbigniew Kalarus,2 David Justin Wright,1 Giuseppe Boriani,3 Gregory Y H Lip1,2
1Liverpool Centre for Cardiovascular Science, University of Liverpool and Liverpool Heart and Chest Hospital, Liverpool, UK; 2Department of Cardiology, Congenital Heart Diseases and Electrotherapy, Medical University of Silesia, Silesian Centre for Heart Diseases, Zabrze, Poland; 3Cardiology Division, Department of Biomedical, Metabolic, and Neural Sciences, University of Modena and Reggio Emilia, Policlinico di Modena, Modena, Italy
Correspondence: Gregory Y H Lip Email [email protected]
Abstract: Cardiovascular implantable electronic devices (CIEDs) are essential management options for patients with brady- and tachyarrhythmias or heart failure with concomitant optimal pharmacotherapy. Despite increasing technological advances, there are still gaps in the management of CIED patients, eg, the growing number of lead- and pocket-related long-term complications, including cardiac device–related infective endocarditis, requires the greatest care. Likewise, patients with CIEDs should be monitored remotely as a part of a comprehensive, holistic management approach. In addition, novel technologies used in smartwatches may be a convenient tool for long-term atrial fibrillation (AF) screening, especially in high-risk populations. Early detection of AF may reduce the risk of stroke and other AF-related complications. The objective of this review article was to provide an overview of novel technologies in cardiac rhythm–management devices and future challenges related to CIEDs.
Keywords: cardiovascular implantable electronic devices, CIEDs, pacemaker, implantable cardioverter–defibrillator, cardiac resynchronization therapy, remote monitoring, wearable technology
Introduction
Modern cardiology develops and progresses through innovations in technology and a deeper understanding of the pathophysiology of heart diseases. Indeed, marked advances have been made since 1958, when the first pacemaker was implanted.1 Over the decades, cardiovascular implantable electronic devices (CIEDs) have become the cornerstone of management for patients with brady- or tachyarrhythmias and heart failure (HF) with reduced ejection fraction (EF).2–5 This is associated with the emergence of complex stimulation systems — pacemakers (PMs), implantable cardioverter–defibrillators (ICDs), cardiac resynchronization therapy (CRT) — and the growing number of patients with different indications treated with CIEDs. Indeed, rhythm-management devices may improve the life expectancy and quality of life of these patients.1
Impressive progress in the field of cardiac pacing has led to technical improvements in existing devices and leads, and new ones are constantly emerging. Despite this development, the systems may have a downside associated with early and late (>3 months after implantation) complications of using such CIEDs. Many are related to the weakest links, ie, the transvenous lead and subcutaneous pocket.6,7 Wireless technology and optimization of pacing systems have emerged to minimize potential CIED side effects. The effective interrogation and monitoring of patients with CIEDs to detect arrhythmias or system malfunction assumes even great importance.8 The objective of this review article was to provide an overview of novel technologies in cardiac rhythm–management devices and future directions and challenges related to CIEDs.
Search Strategy
We performed a comprehensive literature search using electronic databases (PubMed, ClinicalTrials.gov) to identify relevant studies and systematic reviews reporting on cardiac rhythm–management devices. The following search terms were included (individually and in combination): cardiac implantable electronic devices, pacemaker, implantable cardioverter–defibrillator, cardiac resynchronization therapy, leadless cardiac pacemaker, wearable cardioverter–defibrillator, subcutaneous cardioverter–defibrillator, substernal lead, remote monitoring, atrial high-rate episodes, smartwatch, and wearable technology. Selected articles, clinical trials, and guideline documents were reviewed for inclusion.
Who is Appropriate for CIEDs?
A challenge is whether a patient appropriately qualifies for CIED implantation, despite the current guidelines.2–5 SCD-HeFT9 was a randomized controlled trial (RCT) conducted among 2,521 patients with HF, left ventricular EF (LVEF) ≤35%, and New York Heart Association class II or III. It was found that ICD therapy was related to a 23% reduction of mortality compared with patients treated with amiodarone or placebo.9 However, the recent results from the DANISH trial10 did not show a survival benefits among patients with nonischemic HF with ICD implanted as primary prevention of sudden cardiac death (SCD). The exception was the subgroup of patients aged <68 years, in which the risk of all-cause death was significantly lower in the ICD group than the control group (HR 0.64, 95% CI 0.45–0.90). Importantly, 31% of deaths were not related to cardiovascular events, which may also indicate less benefit from ICDs in older and frail patients.10 Lee et al11 reported that the presence of ICDs significantly reduced the rate of all-cause death (HR 0.640, 95% CI 0.448–0.915) in patients with ischemic cardiomyopathy, but not among individuals with nonischemic cardiomyopathy (HR 0.984, 95% CI 0.641–1.509) during follow-up of 3.5±1.8 years.11
Beyond recommendations about ICD therapy in patients with ischemic cardiomyopathy and poor LVEF, diagnostic algorithms for the identification of patients with relatively preserved LV contractility at increased risk of major arrhythmic events have been proposed. The PRESERVE EF study12 was performed among 575 patients of mean age 57 years and LVEF 50.8%. Participants were assessed in two steps: if there were abnormalities on ECG (eg, premature ventricular complexes, unsustained ventricular tachycardia, late potentials, prolonged QTc), patients were referred to programmed ventricular stimulation (PVS). For those with induced ventricular tachyarrhythmia (VT), ICDs were implanted. The primary end point was the occurrence of a major arrhythmic event: sustained ventricular tachycardia/fibrillation, appropriate ICD therapy, or SCD. The study found that 35.5% had abnormal ECG findings, and 27% of those were inducible with PVS. ICDs were implanted in 37 patients (90.2% of inducible subgroup). During the 32-month follow-up, there were no SCDs among ICD patients, whereas nine appropriate ICD shocks were observed. A previous study13 showed that patients with hypertrophic cardiomyopathy and noninducible arrhythmia with PVS had longer event-free survival. Inducibility with PVS was an independent predictor of SCD or appropriate ICD therapy among patients with hypertrophic cardiomyopathy.13
While the effectiveness of ICD therapy in patients with nonischemic dilated cardiomyopathy and reduced LVEF (≤35%) is debated, the selection of patients with dilated cardiomyopathy and well-maintained LV contractility (LVEF >35%) at risk of malignant cardiac arrhythmic events who may gain a survival benefit from ICD therapy represents another challenging area. Gatzoulis et al14 used a two-step algorithm in another study — ReCONSIDER; (NCT04246450) — which is an ongoing prospective observational trial among patients with nonischemic cardiomyopathy aiming to recognize those with a truly high risk of SCD.14 CMR GUIDE (NCT01918215)15 is an ongoing RCT to assess myocardial fibrosis and related risk of SCD among patients with LVEF 36%–50% and evidence of fibrosis on optimal HF therapy. Patients are randomized to receive ICD (as primary SCD prevention) or an implantable loop recorder (ILR). The composite primary end point is time to SCD or hemodynamically significant VT.15
Another debated issue is the optimal selection criteria for CRT responders, especially among patients with HF, without typical left bundle–branch block.16 New strategies, such as leadless pacing, optimization of LV-lead position, multipolar LV pacing, alternative right ventricular (RV) pacing, eg, His-bundle pacing or cardiac contractility modulation, may positively impact on further CIED therapy.17 MORE-CRT MPP-PHASE II (NCT02006069)18 is an RCT to assess the impact of multipoint pacing in nonresponders to 6 months of standard biventricular pacing. Preferential LV-only pacing is also considered an alternative to standard biventricular pacing.19 A prospective randomized study of CRT with preferential adaptive LV-only pacing (AdaptResponse, NCT02205359)20 is assessing if the new pacing algorithm reduces the incidence of the combined end point of all-cause mortality and HF decompensation compared with conventional CRT among patients eligible for CRT. The AdaptivCRT algorithm optimizes the pacing method and atrioventricular/interventricular delays, based on the current patient’s activity and intrinsic conduction.20
His-bundle pacing is the most physiological form of ventricular pacing, and appears to be a safe and effective method during long-term follow-up.21 This approach is considered superior to standard RV pacing and may also improve clinical outcomes in patients with CRT indications.22 The His-SYNC (NCT02700425)23 pilot trial was the first RCT comparing His-bundle pacing for CRT (His-CRT) vs biventricular pacing (BiV-CRT) among 41 patients with standard indications for CRT. At 6-month follow-up, His-CRT resulted in QRS narrowing with a nonsignificant trend toward a higher rate of echocardiographic response (91% vs 54%, p=0.078) compared with BiV-CRT; however, there were no significant differences in mortality or cardiovascular hospitalization between the groups.23 As such, large multicenter RCTs are necessary to evaluate the clinical efficacy of His-bundle pacing and also comparing His-CRT and BiV-CRT. It is also open whether patients who qualify for CRT have a survival benefit from ICD. RESET-CRT (NCT03494933) is an ongoing RCT to compare clinical outcomes among patients with a CRT PM vs CRT defibrillator.
A recent European Heart Rhythm Association (EHRA) consensus document on management of arrhythmias and cardiac electronic devices in critically ill and postsurgery patients highlighted the risks and challenges among CIED patients with a terminal illness.24 In an EHRA survey,25 73% of patients declared that CIED implantation improved their quality of life, whereas 36% had concerns about the device, mostly related to ICD shocks, daily activities, or impairment of the device. Indeed, the final decision about CIED implantation should take into account the patient’s age, frailty, cardiac condition, and other comorbidities, concomitant with personal values and preferences.
Therefore, it is often necessary to create novel algorithms for the selection of CRT responders, individualized risk scores for SCD, or procedure-related complications, which may result in a highly individualized approach and targeted CIED implantation. The future may bring patient-specific digital models to calculate the risk–benefit profile and create a simulation — virtual implantation. This might check whether the procedure is feasible and which device is favorable for each patient, but also guide a lead during the real procedure. In addition, CIEDs may be considered a cotreatment of other morbidities, such as hypertension.NCT03757377 is an ongoing RCT evaluating a new PM algorithm that may be useful for patients with indications for antibradycardia pacing and persistent hypertension despite pharmacotherapy.
The Weakest Links
Transvenous leads represent a major source of CIED complications — not only dislocation or mechanical damage but also tricuspid regurgitation, venous occlusion, superior vena cava syndrome, cardiac perforation, cardiac device–related infective endocarditis (CDRIE) — and subcutaneous pockets: hematoma, decubitus, inflammation.6,7,26 Palmisano et al27 reported a higher risk of all-cause death among patients with CIEDs and early complications — pneumothorax (HR 8.731, 95% CI 1.42–53.63) and pocket hematoma (HR 2.515, 95% CI 1.07–5.94) — whereas CDRIE was most markedly related to increased risk of cardiovascular death (HR 4.025, 95% CI 1.5–10.78) during median follow-up of 56.9 months. An EHRA international consensus on how to prevent, diagnose, and treat CIED-infections28 states that prevention and careful consideration before implantation are the best treatment for CDRIE. Indeed, leadless cardiac PMs (LCPs) and extravascular cardioverter-defibrillators have been designed to minimize complications. Also, an absorbable, antibiotic-eluting envelope has been created to use with CIEDs as a prophylactic strategy to prevent CDRIE.29 These new technologies have already found a place in everyday clinical practice.
Leadless Cardiac Pacemakers
An LCP is a small (volume 0.8 cm3) single-chamber PM that is implanted directly into the RV by a special catheter and introducer sheath via transfemoral access.30 Therefore, it does not require the subcutaneous pocket or transvenous lead.31 The first LCP was Nanostim (St Jude Medical), implanted worldwide between 2013 and 2016. The device was recalled in 2016, due to battery failures, but the concept of LCPs has been widely accepted.32
At present, the only type of LCP available on the market is the Micra transcatheter pacing system. The pacing mode is similar to transvenous PMs, so an LCP may be used as an alternative device.30,33 However, the system is limited to the RV component, meaning that an LCP may be indicated only for patients requiring single-chamber pacing, eg, permanent atrial fibrillation (AF) with bradycardia or for those with low expected stimulation percentage.30 As such, patients with missing or difficult venous access, with a history of CDRIE, and indications for ventricular single-chamber pacing are considered good LCP candidates. Importantly, the potential benefits of LCPs must be confronted with the limited data on the long-term follow-up, and also the procedure of device replacement or retrieval is still debated.34 According to a national expert consensus document of the Austrian Society of Cardiology, LCP retrieval should not be recommended as a routine procedure and should be limited only to specific issues, ie, endocarditis or system upgrades.34 One worldwide experience of 40 successful device retrievals revealed that it may be feasible and safe if performed with a special sheath and a snare catheter and introduced via femoral access. The most common reasons for extraction included elevated pacing threshold, endovascular infection, and indications for a system upgrade to a transvenous device.35 If it is necessary to replace the battery, a new LCP may be implanted next to old devices without extraction of previous ones; however, current clinical experience is very limited.34
The results of the Micra Transcatheter Pacing Study36 showed the safety and efficacy(primary safety and efficacy end points were reached in 96% and 98.3%, respectively) of LCPs among 725 patients who had undergone device implantation. Likewise, the Micra Post-Approval Registry37 reported a high rate of successful LCP implantations (99.1%) with a low risk of major complications (2.7%) among 1,817 patients. During follow-up of 12-months postprocedure, complication rates in LCP patients were significantly lower (HR 0.37, 95% CI 0.27–0.52) than a historical transvenous PM group. The most common complications in the LCP group were pacing issues (0.72%), groin injury (0.61%), cardiac effusion/perfusion (0.44%), and infection (0.17%).37 In another study, El-Chami et al38 reported on the safety and feasibility of LCPs, also in patients after PM extraction and a recent CDRIE.
Piccini et al39 compared clinical outcomes among 720 patients successfully implanted with LCPs, based on ventricular pacing indications: individuals with AF (68.3%) and those without AF (31.7%). Reasons for selecting LCPs in the non-AF group included an expectation of infrequent pacing (66.2%) and advanced age (27.2%). During 24 months of follow-up, there were no significant differences between the groups in occurrence of the composite primary outcome (cardiac failure, PM syndrome, or LCP-related syncope).39 In another study, the safety and mortality of LCP implantation was assessed and stratified by whether patients were precluded from transvenous PMs.40 It was found that 19.4% of patients were ineligible for traditional PMs because of venous access issues or prior CDRIE. Both acute and total mortality at 36 months (2.75% vs 1.32% [p=0.022] and 38.1% vs 20.6% [p<0.001], respectively) were significantly higher in the precluded patients than the non-precluded; however, the mortality rate among precluded patients was similar to historical transvenous PM group.40
Despite concerns regarding LCPs in frail elderly patients, because of implant-sheath size and risk of perforation, Micra implantation also appears to be safe and feasible among those individuals.41,42 However, RCTs directly comparing the efficacy and safety of LCPs vs transvenous PMs are needed. In the EHRA prospective survey,43 the overall use of LCPs in daily clinical practice remains low, constituting only 9% of all procedures and 36% of single-chamber PM implants. LCPrecipients were more often male (74% vs 54%) and had a history of valvular heart disease (45% vs 35%), AF (65% vs 23%), and other comorbidities (66% vs 52%) than those with transvenous single-chamber PMs, but no significant association was observed with patients’ age.43 LCP implantation was successful in 98% of recipients, and the only procedure-related complication was groin hematoma.44 Indeed, leadless devices are still in development, and there are also the prototypes of dual-chamber systems,45 which may be used in a wider group of patients. As such, LCPs are a potential game changer for modern CIEDs.
MARVEL (NCT03157297)46 was a recent study of a new LCP algorithm to synchronize ventricle pacing with atrial sensing (synchronous atrioventricular [AV] pacing). Consequently, MARVEL 2 (NCT03752151)47 revealed that the new algorithm provided successful AV-synchrony pacing (mean 89.2%) among 75 LCP recipients with sinus rhythm and AV block. Notably, the atrial sensing algorithms were safe, and there were neither pauses nor episodes of PM-mediated tachycardia.47 The technology is currently used in a new Micra AV device (approved by the US Food and Drug Administration in January 2020), broadening potential indications to LCP implantation.48 Further innovations, such as compatibility with extravascular ICDs, leadless CRT, renewable batteries, or less invasive implantation procedures, are highly anticipated.
Wireless Cardiac Resynchronization Therapy
The SELECT-LV study49 investigated the clinical efficacy and safety of wireless stimulation endocardially for CRT (WiSE-CRT) pacing via an LV endocardial electrode and a pulse generator (implanted subcutaneously). The trial was conducted among 35 patients with HF and indications for biventricular pacing who were nonresponders to traditional CRT or implantation of a coronary sinus lead was not possible. The feasibility and efficacy of WiSE-CRT were reported. Rates of successful implantation and effective CRT pacing were high (97.1%), with a substantial improvement (84.8%) in the clinical composite score at 6 months; however, the rate of early serious complications was 31.5% at 1-month postprocedure follow-up (8.6% within 24 hours and 22.9% between 24 hours and 1 month).49 According to recent data from a Multicenter International Registry of the WiSE-CRT pacing system,50 implantation of the device was feasible in 94.4% of patients and 70% of those reported improvement in of HF symptoms. Complication rates differed among the centers: 4.4%, 18.8%, and 6.7% within <24 hours, 1–30 days, and 1–6 months, respectively.50 Likewise, the SOLVE-CRT trial51 is an ongoing RCT assessing the safety and effectiveness of the WiSE-CRT in nonresponders to traditional CRT.
Extravascular Cardioverter–Defibrillators
Wearable Cardioverter–Defibrillators
European Society of Cardiology (ESC) guidelines for the management of patients with ventricular arrhythmias and prevention of SCD5 state that a wearable cardioverter-defibrillator (WCD) attached to a vest may be considered a bridge in patients with transient impaired LVEF, post–myocardial infarction (<40 days), postpartum cardiomyopathy, myocarditis (until recovery), or for those awaiting heart transplantation. VEST52 was an RCT of 2,302 subjects with acute myocardial infarction and LVEF ≤35% designed to compare a WCD group vs a no-device group, with both groups receiving optimal pharmacotherapy. The study found that the WCD did not have a significant impact on the composite primary end point (SCD and death from sustained VT) during 90 days postinfarct.52 Adherence to wearing the defibrillator was an important issue that conditioned the results of the VEST study, as highlighted by the on-treatment and per-protocol analyses that found a benefit of the wearable defibrillator in the group of patients selected for high compliance to apply the device.53 Data from the WEARIT-II Registry54 showed a high rate of VT — 3% among patients with ischemic and congenital heart disease and 1% among nonischemic patients — during 3 months of WCD use. In total, there were 120 VT episodes recorded in 41 patients, and 54% of those received appropriate WCD therapy. In sum, 840 patients (42%) were implanted with an ICD, and LVEF improvement was the most common reason for ICD removal.54 The EHRA survey55 reported that WCDs were used as a temporary solution, mostly for patients after CDRIE and awaiting ICD reimplantation or with transient LVEF impairment, after recent myocarditis/myocardial infarction, or before heart transplantation. Notably, the most common contraindications for WCD use were life expectancy <12 months and noncompliance.55
Subcutaneous Cardioverter–Defibrillator
Subcutaneous cardioverter-defibrillators (sICDs) are implantable devices comprising a subcutaneous pulse generator and subcutaneous lead to deliver a shock as VT therapy. According to the current ESC guidelines, sICDs may be an alternative for patients who require an ICD but do not have an indication for ventricular pacing, CRT, or antitachycardia pacing.5 The American Heart Association guidelines4 recommend sICDs for patients without proper venous access or at high risk of CDRIE. Boersma et al56 reported low periprocedural complication rates, with 99.6% successfully implanted devices and high defibrillation efficacy (99.2% during defibrillation testing) among 1,116 patients who had undergone sICD implantation as primary prevention of SCD. In another study, Boersma et al57 indicated a low risk of infection in sICD recipients, even in individuals with a history of CDRIE and explanted transvenous ICDs.
Results from an EHRA prospective survey on sICD use showed that sICDs were favorable among younger patients and those with lead-related complications or elevated risk/history of CDRIE, taking into consideration patient preferences and active lifestyle. Of note, need for ventricular antitachycardia pacing, CRT, or permanent pacing were benefiting, the transvenous ICD.58 Implantation time and periprocedural complication rates were similar in both subgroups.59 Since 2010, when sICD became available for patients, this technology has been evolving.60 Various ongoing clinical trials have been designed to assess new extravascular systems, eg, EV ICD (NCT04060680) and ASE (NCT03802110), for testing new shock configurations.
Substernal Leads
ASD261 was the first human study to evaluate a novel approach to ICD therapy — substernal leads. Pacing threshold, sensing, and defibrillation efficacy were assessed among 79 patients who had undergone lead implantation. The lead was placed into the substernal space via subxiphoid access, and a defibrillation-patch electrode or active can emulator (subcutaneous) was set in the left mid-axillary line. It was found that R-wave amplitudes were compliant with ICD sensing (median R-wave 2.4 mV), successful ventricular pacing rates were 97.4%, and defibrillation efficacy was >80% with a single shock of 30 J. The lower shock energy (compared with the sICD’s 80 J) may be beneficial for battery longevity.61 Indeed, substernal lead therapy may be feasible, and results are promising. Nevertheless, neither long-term follow-up data nor risk of infection and lead extraction are available. Indeed, substernal lead therapy may be feasible, and results are promising. Nevertheless, neither long-term follow-up data nor risk of infection and lead extraction are available (Table 1).
 |
Table 1 Comparison of Implantable Cardioverter Defibrillators |
Remote Monitoring of Patients with Cardiac Electronic Implantable Devices
Patients with CIEDs usually have routine in-person appointments to verify whether the device is functioning adequately and for assessment of clinical findings every 6–12 months.62 In addition, unscheduled clinic visits may be necessary in cases of system malfunction or worsening of health status.62 However, the conventional monitoring of CIEDs, resulting in limited contact with patients, is insufficient and outdated. Digital health-care models and remote control of devices are the future of modern medicine and cardiology. They involve patients taking an active role in their own clinical care, and such a personalized approach, including shared decision-making, is crucial (Table 2).63
 |
Table 2 Expected Advantages of Remote Monitoring of Cardiac Electronic Implantable Devices |
Teletransmission systems transfer data recorded from the patient’s device to a database, where the data are available to the health-care team. New systems transmit the data via the patient’s smartphone or tablet. As such, telemonitoring allows assessment of the relevant technical parameters of the device, ie, battery status, electrode function, and system compatibility, on an ongoing basis. It also provides key clinical information, such as stimulation percentage, stored arrhythmic episodes, or current intracardiac electrograms.8,62 The daily telemetric care of a large population of such CIED patients may be considered triage of high-risk patients, with ongoing selection of individuals who require urgent medical intervention.64
According to expert consensus, remote monitoring should be available for all patients with CIEDs as part of the standard follow-up strategy, but in particular for patients with HF and cardiac arrhythmias.8,62 There is urgent need for integrated medical care for patients with HF. Telemetric data may help to recognize current clinical status and device alarm. Of note, telemetric care is crucial to diagnose and monitor episodes of arrhythmia, especially life-threatening episodes of VT and ICD-delivered shocks (appropriate or inappropriate). As a result, the health-care team may efficiently modify pharmacotherapy or plan any further treatment strategy (Table 3). Importantly, the effectiveness of the therapy may be assessed on an ongoing basis.64 A survey from the Health Economics Committee of the EHRA25 showed that early recognition of AF among PM patients, lead failure in ICD patients, and HF worsening in CRT patients were considered essential advantages of remote monitoring.
 |
Table 3 Remote Monitoring of Patients with Cardiac Resynchronization Therapy |
The opportunity comes from dual-chamber devices that can also monitor atrial rhythm, allowing the possibility of recognizing arrhythmias and AF. These recorded events are called atrial high-rate episodes (AHREs), more often of short duration, lasting a few seconds to minutes.65,66 The key issue is to diagnose real episodes of AF or atrial flutter, and thus it is necessary to evaluate stored current intracardiac electrograms and exclude false episodes (Table 4).65–68 Among 2,718 individuals with HF and CIEDs, AHREs were found in 34.8%, whereas AF was confirmed in 91% by atrial electrograms.69 In an observational study of 304 patients with HF and CRT with a defibrillator, AHREs were found in 57.9% of patients within 2.5 years postimplantation.67 After inspection, 89.2% of AHREs were truly AF and 62% of these were AF de novo.67
 |
Table 4 Atrial High–rhythm Episodes: Common Causes of Atrial Fibrillation Misdetections, |
A significant benefit of telemonitoring may be obtained by patients with previously undetected “silent” AF. An active search for real arrhythmia and related risk factors is a significant part of individualized approaches among patients with CIEDs. Miyazawa et al70 revealed that age ≥65 years, diabetes mellitus, congestive HF, and left atrial volume index >34 mL/m2 were independent factors of AF de novo in patients with CIEDs. Indeed, detecting asymptomatic AF with an adequate therapeutic response may prevent potential complications, eg, stroke/thromboembolic events and AF-related morbidity. However, there remains a gap in the data regarding the management of patients with AHREs without a proper AF diagnosis.
How much AHRE is too much? This is the big question that derives from lack of knowledge on what the number of AHREs is and their minimum duration, which actually increases significantly the thromboembolism risk.68,71–73 One study among patients with dual-chamber CIEDs showed an increased risk of thromboembolism (HR 3.40, 95% CI 1.38–8.37) and all-cause death (HR 3.47, 95% CI 1.51–7.95) among patients with AHRE.74 Of note, the West Birmingham Atrial Fibrillation Project reported that among CIED patients, a higher risk of thromboembolic events was related to comorbidities (ie, CHA2DS2VASc score), but not AHRE per se.75 Indeed, Pastori et al76 reported that AHREs ≥5 minutes (HR 1.788, 95% CI 1.247–2.562), diabetes mellitus (HR 1.909, 95% CI 1.358–2.683), HF (HR 2.203, 95% CI 1.527–3.178), and coronary artery disease (HR 1.862, 95% CI 1.293–2.681) were significantly related to the risk of major adverse cardiovascular events in patients with CIEDs. Furthermore, Boriani et al77 found that among patients with AF duration ≥5 minutes and CIEDs, female sex (HR 3.43, 95% CI 1.05–11.18) and history of coronary artery bypass surgery(HR 4.34, 95% CI 1.44–13.13), but not higher burden of AF, were independent predictors of stroke.77
New-onset AF is detected earlier in patients with remote monitoring than standard clinical care.78–80 Also, treatment of arrhythmia is initiated significantly earlier (3 vs 54 days), but does not improve clinical outcomes of HF patients in term of stroke and bleeding prevention, based on introduction or termination of oral anticoagulation (OAC).69 Perino et al81 conducted a study of 2,101 patients with CIEDs on remote monitoring and AF duration >6 minutes (detected by device). Among these patients, OAC users had a significantly lower risk of stroke than a no-anticoagulation group (HR 0.68, 95% CI 0.47–0.97). Of note, a reduction in strokes with anticoagulation was reported in patients with AF duration >24 hours (HR 0.27, 95% CI 0.14–0.51).81
There is a beneficial effect of OAC in stroke prevention in CIED patients with documented AF, while evidence of benefit is missing for AHRE (without formal AF diagnosis).65,68,71,72,82,83 An ongoing RCT, NOAH-AFNET 6 (NCT02618577),84 is comparing edoxaban vs aspirin or no treatment among patients with AHRE (without AF) and two or more stroke risk factors. The primary outcome is time to first ischemic event or cardiovascular-related death.84 The ARTESiA (NCT01938248)85 trial is comparing apixaban vs aspirin in terms of stroke and systemic embolism in this group of patients.
The CASTLE-AF86 trial was designed to compare the clinical outcomes of AF patients with HF and implanted ICDs. Subjects were randomized to catheter ablation or guideline-adherent medical therapy. The major exclusion criteria were heart-transplant candidacy or planned cardiovascular intervention. Patients who underwent catheter ablation had significantly lower risk of any-cause death (13.4% vs 25.0%, HR 0.53, 95% CI 0.32–0.86), cardiovascular-related death (11.2% vs 22.3%, HR 0.49, 95% CI 0.29–0.84), and hospitalization due to worsening of HF (20.7% vs 35.9%; HR 0.56, 95% CI 0.37–0.83), than the medical therapy group.86
Noseworthy et al87 assessed the generalizability of the CASTLE-AF trial among patients with AF and HF treated with ablation (n=7,465) or standard medical therapy (n=282,366). They found that only 7.8% of patients would have been eligible for CASTLE-AF. Catheter ablation was related to a lower risk of the primary outcome among all patients (HR 0.81, 95% CI 0.76–0.87) vs standard medical therapy, specifically in the CASTLE-AF–eligible subgroup (HR 0.82, 95% CI 0.70–0.96), but not in patients who met the exclusion criteria.87 Likewise, the CABANA88 trial revealed that among AF patients, catheter ablation did not significantly lower the primary composite end point of death, disabling stroke, major bleeding, or cardiac arrest compared with a medical therapy group. In an observational real-world patient study,89 assessing catheter ablation was related to a reduction in the composite end point vs medical therapy (HR 0.75, 95% CI 0.70–0.81). The risk reduction associated with ablation was higher among CABANA-eligible patients (HR 0.70, 95% CI 0.63–0.77).89
The possibility of remote monitoring may facilitate comprehensive care management for patients with CIEDs.90 However, RCT-based data are heterogeneous in terms of telemonitoring effectiveness, ie, improvement of outcomes for HF patients with CIEDs (Table 5).91–95 The benefits of monitoring may be dependent on the health-care team’s reaction to the transmitted data. Therefore, remote monitoring should not be considered a treatment per se, but may allow for a more appropriate medical response to device alerts.95,96 Consequently, further developments should be focused on improving the technical issues and efficiency of telemonitoring, eg, artificial intelligence to for triage of high-risk patients or integration of the data with electronic medical records or extra features to monitor potential comorbidities. Another challenge is the accessibility, feasibility, and adherence to therapeutic protocols by both groups — physicians and patients. Further studies are needed to identify novel functions with a positive impact on clinical outcomes.97
 |
Table 5 Studies of Remote Monitoring of Cardiac Electronic Implantable Devices |
Novel Approaches Incorporating Cardiac Rhythm–Monitoring Technology
Implantable Loop Recorder
The ILR is a type of long-term cardiac rhythm–monitoring device that is inserted underneath the chest skin. It is a safe and effective tool in the diagnosis of unexplained syncope, arrhythmias, or cryptogenic stroke, and is indicated for patients with recurring symptoms but too infrequent to be diagnosed with conventional ECG monitoring techniques.98,99 In patients with recurrent symptomatic palpitations, the ILR may be a useful diagnostic approach for evaluation.100 Of note, recent studies of ILRs have shown their feasibility to detect and record asymptomatic AF. One RCT (CRYSTAL-AF)101 was conducted to compare ECG monitoring with an ILR vs conventional follow-up among 441 patients after cryptogenic stroke. The primary end point was time to first AF detection within 6 months. ILRs were superior to standard monitoring in detecting AF: AF was detected in 8.9% of patients in the ILR group vs 1.4% of patients in the control group (HR 6.4, 95% CI 1.9–21.7) during 6 months of follow-up.101 The LOOP (NCT02036450) study was designed to determine whether initiation of OAC if AF were detected with ILRs would reduce the risk of stroke among 597 patients aged ≥70 years with stroke risk factors. First insights from the LOOP study revealed that during 40 months of follow-up, AF was detected in 209 (35%) patients.102 In addition, AF was silent in 90% of patients at debut, and 87% never noticed AF-related symptoms. The average heart rate during AF was only moderately elevated to 96 beats/minute, whereas average daytime sinus rate was 72 beats/minute.103
Wearable Technology
Wearable technologies provide the possibility of continuous rhythm monitoring and display a real-time heart rate, as well as a review of heart-rate trends.104 For example, Dörr et al105 conducted a study using smartwatches among 508 patients of mean age 76.4 years, 46.6% of whom had previously diagnosed AF. High sensitivity, specificity, and accuracy (93.7%, 98.2%, and 96.1%, respectively) in detecting AF was observed. The authors speculated that AF detection may be feasible with high diagnostic precision using commercial smartwatches. Likewise, attaining proper signal quality during algorithm use was highlighted as the main limitation.105
The possibility of cardiac rhythm monitoring via smartwatches was assessed in two large population-based cohort studies in the US and China. The Apple Heart Study106,107 involved 419,297 participants aged ≥22 years without a history of AF. The idea of the study was to find out if a mobile application could recognize irregular heart rate and AF from data collected on the Apple Watch. During follow-up, an irregular pulse was detected in 0.52% of participants. Consequently, they were monitored by ECG patch for 7 days for arrhythmia, and AF was diagnosed in 34%. Notably, AF was observed more often among participants aged ≥65 years (3.2% vs 0.16% in participants aged <40 years) and men (0.7% vs 0.26% in women). Likewise, 76% of notified participants contacted their health-care provider, 33% were referred to a specialist, and 36% required further investigations.
The Huawei Heart Study108 conducted in China on 187,912 participants of mean age 35 years found that 424 (0.23%) individuals were notified of suspected AF, and AF was finally confirmed by clinical evaluation in 87% of those. Researchers highlighted that AF suspicion and identification significantly increased with age. Of note, the majority of patients entered a program of integrated AF management, and approximately 80% of high-risk patients were successfully anticoagulated.108 Subsequent to the Huawei Heart Study, mAFA II109 was a cluster RCT to assess the impact of integrated care using a mobile AF application (mAFA) compared with usual care on clinical outcomes among 3,324 AFpatients with two or more stroke risk factors.109 The trial demonstrated that rates of composite outcome (ischemic stroke/systemic thromboembolism, death, and rehospitalization) and rehospitalization were lower among mAFA patients vs the usual-care control group (1.9% vs 6.0%, HR 0.39, 95% CI 0.22–0.67 and 1.2% vs 4.5%, HR 0.32, 95% CI 0.17–0.60, respectively).109 In an ancillary analysis of mAFAII, proactive assessment of bleeding risks using the HAS-BLED score resulted in mitigation of modifiable bleeding risk factors, fewer bleeding events and an increase in OAC uptake compared to usual care.110 Another approach was illustrated with the NOMED-AF (NCT03243474) study,111 which assessed the prevalence of AF and concomitant comorbidities in a population aged ≥65 years. Long-term ECG monitoring was enabled by special vests equipped with electrodes and recorder. Data were transmitted to the monitoring platform every 24 hours. The study identified high-risk populations, requiring more intense AF screening.111
The data available show that wearable technology using photoplethysmographic sensors, which detect blood volume changes, may recognize irregular heart rhythm, including previously unknown AF (Table 6).106–108 As such, smartwatches may be convenient tools for long-term AF screening in large populations, especially in high-risk patients. Early AF detection may reduce the burden of stroke and other AF-related complications. Future studies may demonstrate the utility of wearable technology in AF integrated care and optimize a holistic approach within individuals with AF. Likewise, the advance of artificial intelligence may support treatment and assist in diagnosis, management, and prediction of occurrence of arrhythmia or other heart diseases.112
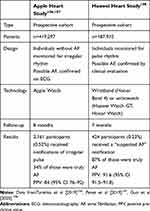 |
Table 6 Wearable Technologies – an Insight from Observational Studies |
Conclusion
The future of cardiac rhythm–management devices looks bright, but incorporation and adoption of new technologies remains a challenge. There is an immense need for an individualized approach with targeted CIED implantation and personalized risk stratifications. Further studies are needed to identify novel strategies and technologies with a positive impact on patient diagnosis and treatment. Novel solutions may see a move away from transvenous leads to leadless systems, with combinations of different individualized pacing and monitoring functions. Relevant developments and innovations of devices, implantation processes, and postprocedure follow-up may improve clinical outcomes among patients.
Disclosure
ZK reports personal fees from Boehringer Ingelheim, Bayer, and Pfizer outside the submitted work, DJW consultancy and speaker fees for Medtronic, Boston Scientific, and St Jude and research grants from Boston Scientific, GYHL consultancy for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi Sankyo and speaking for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi Sankyo (no fees directly received personally), and GB small speaker’s fees from Medtronic, Boston, Biotronik, Boehringer, and Bayer outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Wilkoff BL, Auricchio A, Brugada J, et al. HRS/EHRA Expert Consensus on the Monitoring of Cardiovascular Implantable Electronic Devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations: developed in partnership with the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA); and in collaboration with the American College of Cardiology (ACC), the American Heart Association (AHA), the European Society of Cardiology (ESC), the Heart Failure Association of ESC (HFA), and the Heart Failure Society of America (HFSA). Endorsed by the Heart Rhythm Society, the European Heart Rhythm Association (a registered branch of the ESC), the American College of Cardiology, the American Heart Association . Europace. 2008;10(6):707–725.
2. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37(27):2129–2200m. doi:10.1093/eurheartj/ehw128
3. Brignole M, Auricchio A, Baron-Esquivias G, et al. 213 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2013;34(29):2281–2329.
4. Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138(13):e272–e391.
5. Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the Europe. Eur Heart J. 2015;36(41):2793–2867.
6. Kirkfeldt RE, Johansen JB, Nohr EA, Dan Jørgensen O, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35(18):1186–1194. doi:10.1093/eurheartj/eht511
7. Bongiorni MG, Burri H, Deharo JC, et al. 2018 EHRA expert consensus statement on lead extraction: recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: endorsed by APHRS/HRS/LAHRS. Europace. 2018;20:1217. doi:10.1093/europace/euy050
8. Slotwiner D, Varma N, Akar JG, et al. HRS expert consensus statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Hear Rhythm. 2015;12(7):e69–e100.
9. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. doi:10.1056/NEJMoa043399
10. Køber L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375(13):1221–1230. doi:10.1056/NEJMoa1608029
11. Lee TC, Qian M, Mu L, et al. Association between mortality and implantable cardioverter‐defibrillators by aetiology of heart failure: a propensity‐matched analysis of the WARCEF trial. ESC Hear Fail. 2019;6(2):297–307. doi:10.1002/ehf2.12407
12. Gatzoulis KA, Tsiachris D, Arsenos P, et al. Arrhythmic risk stratification in post-myocardial infarction patients with preserved ejection fraction: the PRESERVE EF study. Eur Heart J. 2019;40(35):2940–2949. doi:10.1093/eurheartj/ehz260
13. Gatzoulis KA, Georgopoulos S, Antoniou CK, et al. Programmed ventricular stimulation predicts arrhythmic events and survival in hypertrophic cardiomyopathy. Int J Cardiol. 2018;254:175–181. doi:10.1016/j.ijcard.2017.10.033
14. Gatzoulis KA, Dilaveris P, Arsenos P, et al. Arrhythmic risk stratification in nonischemic dilated cardiomyopathy: the ReCONSIDER study design – A two-step, multifactorial, electrophysiology-inclusive approach. Hell J Cardiol. 2020. doi:10.1016/j.hjc.2020.03.008
15. Selvanayagam JB, Hartshorne T, Billot L, et al. Cardiovascular magnetic resonance-guided management of mild to moderate left ventricular systolic dysfunction (CMR GUIDE): study protocol for a randomized controlled trial. Ann Noninvasive Electrocardiol. 2017;22(4):12420. doi:10.1111/anec.12420
16. Salden OAE, Vernooy K, van Stipdonk AMW, Cramer MJ, Prinzen FW, Meine M. Strategies to improve selection of patients without typical left bundle branch block for cardiac resynchronization therapy. JACC Clin Electrophysiol. 2020;6(2):129–142. doi:10.1016/j.jacep.2019.11.018
17. Halbfass P, Sonne K, Nentwich K, Ene E, Deneke T. Current developments in cardiac rhythm management devices. Clin Res Cardiol. 2018;107(S2):100–104. doi:10.1007/s00392-018-1313-4
18. Leclercq C, Burri H, Curnis A, et al. Rationale and design of a randomized clinical trial to assess the safety and efficacy of multipoint pacing therapy: more response on cardiac resynchronization therapy with multipoint pacing (MORE-CRT MPP-PHASE II). Am Heart J. 2019;209:1–8. doi:10.1016/j.ahj.2018.12.004
19. Dilaveris P, Antoniou CK, Manolakou P, et al. Comparison of left ventricular and biventricular pacing: rationale and clinical implications. Anatol J Cardiol. 2019;22(3):132–139.
20. Filippatos G, Birnie D, Gold MR, et al. Rationale and design of the adapt response trial: a prospective randomized study of cardiac resynchronization therapy with preferential adaptive left ventricular-only pacing. Eur J Heart Fail. 2017;19(7):950–957. doi:10.1002/ejhf.895
21. Zanon F, Abdelrahman M, Marcantoni L, et al. Long term performance and safety of His bundle pacing: a multicenter experience. J Cardiovasc Electrophysiol. 2019;30(9):1594–1601. doi:10.1111/jce.14063
22. Sharma PS, Vijayaraman P, Ellenbogen KA. Permanent His bundle pacing: shaping the future of physiological ventricular pacing. Nat Rev Cardiol. 2020;17(1):22–36. doi:10.1038/s41569-019-0224-z
23. Upadhyay GA, Vijayaraman P, Nayak HM, et al. On-treatment comparison between corrective His bundle pacing and biventricular pacing for cardiac resynchronization: a secondary analysis of the His-SYNC Pilot Trial. Hear Rhythm. 2019;16(12):1797–1807. doi:10.1016/j.hrthm.2019.05.009
24. Boriani G, Fauchier L, Aguinaga L, et al. European Heart Rhythm Association (EHRA) consensus document on management of arrhythmias and cardiac electronic devices in the critically ill and post-surgery patient, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Card. EP Eur. 2018;21(1):7–8.
25. Mairesse GH, Braunschweig F, Klersy K, Cowie MR, Leyva F. Implementation and reimbursement of remote monitoring for cardiac implantable electronic devices in Europe: a survey from the health economics committee of the European Heart Rhythm Association. EP Eur. 2015;17(5):814–818.
26. Poole JE, Gleva MJ, Mela T, et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122(16):1553–1561. doi:10.1161/CIRCULATIONAHA.110.976076
27. Palmisano P, Guerra F, Dell’Era G, et al. Impact on all-cause and cardiovascular mortality of cardiac implantable electronic device complications: results from the POINTED registry. JACC Clin Electrophysiol. 2020;6(4):382–392. doi:10.1016/j.jacep.2019.11.005
28. Blomström-Lundqvist C, Traykov V, Erba PA, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections—endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), th. EP Eur. 2019;22(4):515–549.
29. Tarakji KG, Mittal S, Kennergren C, et al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med. 2019;380(20):1895–1905. doi:10.1056/NEJMoa1901111
30. Sideris S, Archontakis S, Dilaveris P, et al. Leadless cardiac pacemakers: current status of a modern approach in pacing. Hell J Cardiol. 2017;58(6):403–410. doi:10.1016/j.hjc.2017.05.004
31. Wiles BM, Roberts PR. Design and evaluation of the micra transcatheter pacing system for bradyarrhythmia management. Future Cardiol. 2019;15(1):9–15. doi:10.2217/fca-2018-0077
32. Sperzel J, Hamm C, Hain A. Nanostim—leadless pacemaker. Herzschrittmachertherapie und Elektrophysiologie. 2018;29(4):327–333. doi:10.1007/s00399-018-0598-3
33. Groner A, Grippe K. The leadless pacemaker. J Am Acad Physician Assist. 2019;32(6):48–50. doi:10.1097/01.JAA.0000554750.85170.d4
34. Steinwender C, Lercher P, Schukro C, et al. State of the art: leadless ventricular pacing: a national expert consensus of the Austrian Society of Cardiology. J Interv Card Electrophysiol. 2020;57(1):27–37. doi:10.1007/s10840-019-00680-2
35. Afzal MR, Daoud EG, Cunnane R, et al. Techniques for successful early retrieval of the Micra transcatheter pacing system: a worldwide experience. Hear Rhythm. 2018;15(6):841–846. doi:10.1016/j.hrthm.2018.02.008
36. Reynolds D, Duray GZ, Omar R, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374(6):533–541. doi:10.1056/NEJMoa1511643
37. El-Chami MF, Al-Samadi F, Clementy N, et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Hear Rhythm. 2018;15(12):1800–1807. doi:10.1016/j.hrthm.2018.08.005
38. El‐Chami MF, Johansen JB, Zaidi A, et al. Leadless pacemaker implant in patients with pre‐existing infections: results from the Micra postapproval registry. J Cardiovasc Electrophysiol. 2019;30(4):569–574. doi:10.1111/jce.13851
39. Piccini JP, Stromberg K, Jackson KP, et al. Patient selection, pacing indications, and subsequent outcomes with de novo leadless single-chamber VVI pacing. Europace. 2019;21(11):1686–1693. doi:10.1093/europace/euz230
40. Garg A, Koneru JN, Fagan DH, et al. Morbidity and mortality in patients precluded for transvenous pacemaker implantation: experience with a leadless pacemaker. Hear Rhythm. 2020. doi:10.1016/j.hrthm.2020.07.035
41. Tachibana M, Banba K, Matsumoto K, Ohara M. The feasibility of leadless pacemaker implantation for superelderly patients. Pacing Clin Electrophysiol. 2020;43(4):374–381. doi:10.1111/pace.13894
42. Pagan E, Gabriels J, Khodak A, et al. Safety of leadless pacemaker implantation in the very elderly. Hear Rhythm. 2020. doi:10.1016/j.hrthm.2020.05.022
43. Boveda S, Marijon E, Lenarczyk R, et al. Factors influencing the use of leadless or transvenous pacemakers: results of the European Heart Rhythm Association Prospective Survey. EP Eur. 2020;22(4):667–673.
44. Lenarczyk R, Boveda S, Mansourati J, et al. Peri-procedural management, implantation feasibility, and short-term outcomes in patients undergoing implantation of leadless pacemakers: European snapshot survey. EP Eur. 2020;22(5):833–838.
45. Bereuter L, Gysin M, Kueffer T, et al. Leadless dual-chamber pacing: a novel communication method for wireless pacemaker synchronization. JACC Basic Transl Sci. 2018;3(6):813–823. doi:10.1016/j.jacbts.2018.07.009
46. Chinitz L, Ritter P, Khelae SK, et al. Accelerometer-based atrioventricular synchronous pacing with a ventricular leadless pacemaker: results from the Micra atrioventricular feasibility studies. Hear Rhythm. 2018;15(9):1363–1371. doi:10.1016/j.hrthm.2018.05.004
47. Steinwender C, Khelae SK, Garweg C, et al. Atrioventricular synchronous pacing using a leadless ventricular pacemaker: results from the MARVEL 2 study. JACC Clin Electrophysiol. 2020;6(1):94–106.
48. Medtronic MicraTM AV MC1AVR1 device manual. Available from: https://www.medtronic.com/us-en/healthcare-professionals/products/cardiac-rhythm/pacemakers/micra-pacing-system.html.
49. Reddy VY, Miller MA, Neuzil P, et al. Cardiac resynchronization therapy with wireless left ventricular endocardial pacing: the SELECT-LV study. J Am Coll Cardiol. 2017;69(17):2119–2129. doi:10.1016/j.jacc.2017.02.059
50. Sieniewicz BJ, Betts TR, James S, et al. Real-world experience of leadless left ventricular endocardial cardiac resynchronization therapy: a multicenter international registry of the WiSE-CRT pacing system. Hear Rhythm. 2020;17(8):1291–1297. doi:10.1016/j.hrthm.2020.03.002
51. Singh JP, Abraham WT, Auricchio A, et al. Design and rationale for the stimulation of the left ventricular endocardium for cardiac resynchronization therapy in non-responders and previously untreatable patients (SOLVE-CRT) trial. Am Heart J. 2019;217:13–22. doi:10.1016/j.ahj.2019.04.002
52. Olgin JE, Pletcher MJ, Vittinghoff E, et al. Wearable cardioverter–defibrillator after myocardial infarction. N Engl J Med. 2018;379(13):1205–1215. doi:10.1056/NEJMoa1800781
53. Olgin JE, Lee BK, Vittinghoff E, et al. Impact of wearable cardioverter‐defibrillator compliance on outcomes in the VEST trial: as‐treated and per‐protocol analyses. J Cardiovasc Electrophysiol. 2020;31(5):1009–1018. doi:10.1111/jce.14404
54. Kutyifa V, Moss AJ, Klein H, et al. Use of the wearable cardioverter defibrillator in high-risk cardiac patients data from the prospective registry of patients using the wearable cardioverter defibrillator (WEARIT-II registry). Circulation. 2015;132(17):1613–1619. doi:10.1161/CIRCULATIONAHA.115.015677
55. Lenarczyk R, Potpara TS, Haugaa KH, et al. The use of wearable cardioverter-defibrillators in Europe: results of the European Heart Rhythm Association survey. EP Eur. 2016;18(1):146–150.
56. Boersma LV, El-Chami MF, Bongiorni MG, et al. Understanding outcomes with the EMBLEM S-ICD in primary prevention patients with low EF study (UNTOUCHED): clinical characteristics and perioperative results. Hear Rhythm. 2019;16(11):1636–1644. doi:10.1016/j.hrthm.2019.04.048
57. Boersma L, Burke MC, Neuzil P, et al. Infection and mortality after implantation of a subcutaneous ICD after transvenous ICD extraction. Hear Rhythm. 2016;13(1):157–164. doi:10.1016/j.hrthm.2015.08.039
58. Boveda S, Lenarczyk R, Fumagalli S, et al. Factors influencing the use of subcutaneous or transvenous implantable cardioverter-defibrillators: results of the European Heart Rhythm Association prospective survey. EP Eur. 2018;20(5):887–892.
59. Lenarczyk R, Boveda S, Haugaa KH, et al. Peri-procedural routines, implantation techniques, and procedure-related complications in patients undergoing implantation of subcutaneous or transvenous automatic cardioverter-defibrillators: results of the European Snapshot Survey on S-ICD Implantation. EP Eur. 2018;20(7):1218–1224.
60. Kaya E, Rassaf T, Wakili R. Subcutaneous ICD: current standards and future perspective. IJC Hear Vasc. 2019;24.
61. Boersma LVA, Merkely B, Neuzil P, et al. Therapy from a novel substernal lead: the ASD2 study. JACC Clin Electrophysiol. 2019;5(2):186–196. doi:10.1016/j.jacep.2018.11.003
62. Yee R, Verma A, Beardsall M, Fraser J, Philippon F, Exner DV. Canadian Cardiovascular Society/Canadian Heart Rhythm Society Joint Position Statement on the use of remote monitoring for cardiovascular implantable electronic device follow-up. Can J Cardiol. 2013;29(6):644–651. doi:10.1016/j.cjca.2012.11.036
63. Frederix I, Caiani EG, Dendale P, et al. ESC e-Cardiology Working Group Position Paper: overcoming challenges in digital health implementation in cardiovascular medicine. Eur J Prev Cardiol. 2019;26(11):1166–1177. doi:10.1177/2047487319832394
64. Liberska A, Kowalski O, Mazurek M, et al. Day by day telemetric care of patients treated with cardiac resynchronisation therapy: first Polish experience. Kardiol Pol. 2016;74:741–748. doi:10.5603/KP.a2016.0019
65. Hohnloser HS, Capucci A, Fain E, et al. ASymptomatic atrial fibrillation and Stroke Evaluation in pacemaker patients and the atrial fibrillation Reduction atrial pacing Trial (ASSERT). Am Heart J. 2006;152(3):442–447. doi:10.1016/j.ahj.2006.02.016
66. Ziegler PD, Glotzer TV, Daoud EG, et al. Detection of previously undiagnosed atrial fibrillation in patients with stroke risk factors and usefulness of continuous monitoring in primary stroke prevention. Am J Cardiol. 2012;110(9):1309–1314. doi:10.1016/j.amjcard.2012.06.034
67. Jȩdrzejczyk-Patej E, Lenarczyk R, Mazurek M, et al. Can we rely on machines? Device-detected atrial high rates correspond well with atrial arrhythmias in cardiac resynchronization recipients. Europace. 2016;18(3):436–444. doi:10.1093/europace/euv095
68. Gorenek B, Bax J, Boriani G, et al. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management. EP Eur. 2017;19(9):1556–1578.
69. Martin DT, Bersohn MM, Waldo AL, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36:1660–1668. doi:10.1093/eurheartj/ehv115
70. Miyazawa K, Kondo Y, Nakano M, et al. Risk factors for the development of incident atrial fibrillation in patients with cardiac implantable electronic devices. Eur J Intern Med. 2018;52:54–59. doi:10.1016/j.ejim.2018.02.019
71. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962.
72. Mairesse GH, Moran P, Van Gelder IC, et al. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiolog. Europace. 2017;19(10):1589–1623. doi:10.1093/europace/eux177
73. Khan AA, Boriani G, Lip GYH. Are atrial high rate episodes (AHREs) a precursor to atrial fibrillation? Clin Res Cardiol. 2020;109(4):409–416. doi:10.1007/s00392-019-01545-4
74. Miyazawa K, Pastori D, Li YG, et al. Atrial high rate episodes in patients with cardiac implantable electronic devices: implications for clinical outcomes. Clin Res Cardiol. 2019;108(9):1034–1041. doi:10.1007/s00392-019-01432-y
75. Li YG, Miyazawa K, Pastori D, Szekely O, Shahid F, Lip GYH. Atrial high-rate episodes and thromboembolism in patients without atrial fibrillation: the West Birmingham atrial fibrillation project. Int J Cardiol. 2019;292:126–130. doi:10.1016/j.ijcard.2019.04.055
76. Pastori D, Miyazawa K, Li Y, et al. Atrial high-rate episodes and risk of major adverse cardiovascular events in patients with cardiac implantable electronic devices. Clin Res Cardiol. 2020;109(1):96–102. doi:10.1007/s00392-019-01493-z
77. Boriani G, Lip GYH, Pietro R, et al. The increased risk of stroke/transient ischemic attack in women with a cardiac implantable electronic device is not associated with a higher atrial fibrillation burden. Europace. 2017;19(11):1767–1775. doi:10.1093/europace/euw333
78. Lorenzoni G, Folino F, Soriani N, Iliceto S, Gregori D. Cost-effectiveness of early detection of atrial fibrillation via remote control of implanted devices. J Eval Clin Pract. 2014;20(5):570–577.
79. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up. Circulation. 2010;122(4):325–332. doi:10.1161/CIRCULATIONAHA.110.937409
80. Amara W, Montagnier C, Cheggour S, et al. Early detection and treatment of atrial arrhythmias alleviates the arrhythmic burden in paced patients: the SETAM study. Pacing Clin Electrophysiol. 2017;40(5):527–536. doi:10.1111/pace.13062
81. Perino A, Fan J, Askari M, et al. How much atrial fibrillation is too much? Treatment benefit of anticoagulation based on threshold of device-detected Af. J Am Coll Cardiol. 2019;73(9):290. doi:10.1016/S0735-1097(19)30898-8
82. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30(10):1114–1130.
83. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154(5):1121–1201. doi:10.1016/j.chest.2018.07.040
84. Kirchhof P, Blank BF, Calvert M, et al. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the Non–vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH–AFNET 6) trial. Am Heart J. 2017;190:12–18. doi:10.1016/j.ahj.2017.04.015
85. Lopes RD, Alings M, Connolly SJ, et al. Rationale and design of the Apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137–145. doi:10.1016/j.ahj.2017.04.008
86. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417–427. doi:10.1056/NEJMoa1707855
87. Noseworthy PA, Van Houten HK, Gersh BJ, et al. Generalizability of the CASTLE-AF trial: catheter ablation for patients with atrial fibrillation and heart failure in routine practice. Hear Rhythm. 2020;17(7):1057–1065. doi:10.1016/j.hrthm.2020.02.030
88. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. doi:10.1001/jama.2019.0693
89. Noseworthy PA, Gersh BJ, Kent DM, et al. Atrial fibrillation ablation in practice: assessing CABANA generalizability. Eur Heart J. 2019;40(16):1257–1264. doi:10.1093/eurheartj/ehz085
90. Ontario HQ. Remote monitoring of implantable cardioverter-defibrillators, cardiac resynchronization therapy and permanent pacemakers: a health technology assessment. Ont Health Technol Assess Ser. 2018;18(7):1–199.
91. Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2020;384(9943):583–590. doi:10.1016/S0140-6736(14)61176-4
92. Sardu C, Santamaria M, Rizzo MR, et al. Telemonitoring in heart failure patients treated by cardiac resynchronisation therapy with defibrillator (CRT-D): the TELECART study. Int J Clin Pract. 2016;70(7):569–576. doi:10.1111/ijcp.12823
93. Boriani G, Da Costa A, Quesada A, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail. 2017;19(3):416–425. doi:10.1002/ejhf.626
94. Morgan JM, Kitt S, Gill J, et al. Remote management of heart failure using implantable electronic devices. Eur Heart J. 2017;38(30):2352–2360. doi:10.1093/eurheartj/ehx227
95. Tajstra M, Sokal A, Gadula-Gacek E, et al. Remote supervision to decrease hospitalization rate (RESULT) study in patients with implanted cardioverter-defibrillator. EP Eur. 2020;22(5):769–776.
96. Braunschweig F, Anker SD, Proff J, Varma N. Remote monitoring of implantable cardioverter-defibrillators and resynchronization devices to improve patient outcomes: dead end or way ahead? EP Eur. 2019;21(6):846–855.
97. Goette A, Auricchio A, Boriani G, et al. EHRA White Paper: knowledge gaps in arrhythmia management - Status 2019. Europace. 2019;21(7):993–994.
98. Brignole M, Moya A, de Lange FJ, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39(21):1883–1948.
99. Seidl K, Rameken M, Breunung S, et al. Diagnostic assessment of recurrent unexplained syncope with a new subcutaneously implantable loop recorder. Europace. 2000;2(3):256–262. doi:10.1053/eupc.2000.0108
100. Giada F, Gulizia M, Francese M, et al. Recurrent Unexplained Palpitations (RUP) study. Comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
101. Sanna T, Diener H-C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486. doi:10.1056/NEJMoa1313600
102. Diederichsen SZ, Haugan KJ, Brandes A, et al. Incidence and predictors of atrial fibrillation episodes as detected by implantable loop recorder in patients at risk: from the LOOP study. Am Heart J. 2020;219:117–127. doi:10.1016/j.ahj.2019.09.009
103. Diederichsen SZ, Haugan KJ, Brandes A, et al. Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J Am Coll Cardiol. 2019;74(22):2771–2781. doi:10.1016/j.jacc.2019.09.050
104. Ip JE. Evaluation of cardiac rhythm abnormalities from wearable devices. JAMA. 2019;321(11):1098–1099. doi:10.1001/jama.2019.1681
105. Dörr M, Nohturfft V, Brasier N, et al. The WATCH AF trial: smartWATCHes for detection of atrial fibrillation. JACC Clin Electrophysiol. 2019;5(2):199–208. doi:10.1016/j.jacep.2018.10.006
106. Turakhia MP, Desai M, Hedlin H, et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: the Apple Heart Study. Am Heart J. 2019;207:66–75. doi:10.1016/j.ahj.2018.09.002
107. Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–1917. doi:10.1056/NEJMoa1901183
108. Guo Y, Wang H, Zhang H, et al. Mobile Photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74(19):2365–2375. doi:10.1016/j.jacc.2019.08.019
109. Guo Y, Lane DA, Wang L, et al. Mobile Health (mHealth) technology for improved screening, patient involvement and optimising integrated care in atrial fibrillation: the mAFA (mAF‐App) II randomised trial. Int J Clin Pract. 2019;73(7). doi:10.1111/ijcp.13352.
110. Guo Y, Lane DA, Chen Y, Lip GYH. Regular bleeding risk assessment associated with reduction in bleeding outcomes: the mAFA-II randomized trial. Am J Med. 2020. doi:10.1016/j.amjmed.2020.03.019
111. Kalarus Z, Balsam P, Bandosz P, et al. NOninvasive monitoring for early detection of Atrial fibrillation: rationale and design of the NOMED-AF study. Kardiol Pol. 2018;76(10):1482–1485. doi:10.5603/KP.a2018.0193
112. Pevnick JM, Birkeland K, Zimmer R, Elad Y, Kedan I. Wearable technology for cardiology: an update and framework for the future. Trends Cardiovasc Med. 2018;28(2):144–150. doi:10.1016/j.tcm.2017.08.003
113. Uslan DZ, Sohail MR, St Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med. 2007;167(7):669–675. doi:10.1001/archinte.167.7.669
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
