Back to Journals » Infection and Drug Resistance » Volume 14
Carbonyl Cyanide 3-Chlorophenylhydrazone (CCCP) Exhibits Direct Antibacterial Activity Against Mycobacterium abscessus
Authors Chen S, Teng T, Zhang Z, Shang Y, Xiao H, Jiang G, Wang F, Jia J, Dong L, Zhao L, Chu N, Huang H
Received 27 January 2021
Accepted for publication 3 March 2021
Published 23 March 2021 Volume 2021:14 Pages 1199—1208
DOI https://doi.org/10.2147/IDR.S303113
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Suting Chen,1,* Tianlu Teng,1,2,* Zhuman Zhang,1 Yuanyuan Shang,1,2 Hua Xiao,1 Guanglu Jiang,1 Fen Wang,1 Junnan Jia,1 Lingling Dong,1 Liping Zhao,1 Naihui Chu,2 Hairong Huang1
1National Clinical Laboratory on Tuberculosis, Beijing Key Laboratory for Drug Resistant Tuberculosis Research, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Institute, Beijing, People’s Republic of China; 2Department of Tuberculosis; Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Institute, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hairong Huang; Naihui Chu Tel +86-10-89509159; +86-10-89509301
Email [email protected]; [email protected]
Objective: Treatment choices for Mycobacterium abscessus (M. abscessus) infections are very limited, and the prognosis is generally poor. Effective new antibiotics or repurposing existing antibiotics against M. abscessus infection are urgently needed. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP), a member of the lipophilic weak acid class, is known as an efflux pump inhibitor for Mycobacterium tuberculosis. The aim of this study was to determine the inhibitory activity of CCCP as a potential novel antibiotic against M. abscessus.
Methods: A total of 47 reference strains of different mycobacterial species and 60 clinical isolates of M. abscessus were enrolled. In vitro inhibitory activity of CCCP was accessed using microplates alamar blue method with the reference and clinical isolates. The activity of CCCP against intracellular M. abscessus residing within macrophage was also evaluated by intracellular colony numerating assay.
Results: CCCP exhibited good activity against M. abscessus clinical isolates in vitro, the minimum inhibitory concentration (MIC) ranged from 0.47 μg/mL to 3.75 μg/mL, with a MIC50 of 1.875 μg/mL and MIC90 of 3.75 μg/mL. At concentrations safe for the cells, CCCP exhibited highly intracellular bactericidal activities against M. abscessus and M. massiliense reference strains, with inhibitory rates of 84.8%± 8.8% and 72.5%± 13.7%, respectively. CCCP demonstrated bactericidal activity against intracellular M. abscessus that was comparable to clarithromycin, and concentration-dependent antimicrobial activity against M. abscessus in macrophages was observed. In addition, CCCP also exhibited good activities against most reference strains of rapidly growing mycobacterial species.
Conclusion: CCCP could be a potential candidate of novel antimicrobiological agent to treat M. abscessus infection.
Keywords: Mycobacterium abscessus, carbonyl cyanide 3-chlorophenylhydrazone, minimum inhibitory concentration, intracellular bactericidal activity
Introduction
Non-tuberculous mycobacteria (NTM) are opportunistic pathogens that exist ubiquitously in soil and water sources and usually cause serious lung infections in vulnerable population.1 Human lung infections caused by NTM have rapidly increased globally in the past decades.2,3 Among NTM species, Mycobacterium abscessus (M. abscessus) is one of the most frequently identified species with clinical significance.4 M. abscessus belongs to the fast-growing group of NTM that cause skin and soft tissue infections beside lung infection. It is estimated that this species caused 5–20% of all non-Mycobacterium tuberculosis (Mtb) infections globally. Furthermore, this rate is likely to be an underestimation since most of the countries do not routinely report these infectious diseases. The treatment of M. abscessus caused infections requires combinational usage of some of the following antibiotics: macrolide antibiotics, amikacin, cefoxitin and other drugs. However, poor prognosis of treatment is frequently confronted, whereas the cure rate only reaches 25–58%.5,6 As a consequence, M. abscessus infection is often considered as “antibiotic nightmare”.7 Therefore, new or repurposed drugs are urgently needed to change this clinical predicament.
Carbonyl cyanide 3-chlorophenylhydrazone (CCCP) functions as a proton carrier that reduces the transmembrane electrochemical gradient and causes an accumulation of antibiotics within bacterial cells. However, CCCP has generally been used only as an efflux pump inhibitors (EPIs).8–10 CCCP has been proven to reduce the minimum inhibitory concentration (MIC) of rifampicin, isoniazid for Mtb11 and improved the activity of clarithromycin (CLA) against CLA-resistant M. abscessus clinical strains.12 It is generally deem that CCCP, as an EPIs, is more effective when used in combination with antibiotics.13 We identified that CCCP alone had antibacterial activity against M. abscessus and possessed the potential to be considered as an independent antibiotic. In this study, we determined the susceptibility profile of M. abscessus clinical isolates to CCCP, investigated its bactericidal activity against M. abscessus in macrophages, and also analyzed its cytotoxicity. To our knowledge, this is the first report on the independent anti-M. abscessus activity of CCCP as a novel candidate antibiotic.
Materials and Methods
Ethical Approval
The study was approved by the Ethics Committee of Beijing Chest Hospital, Capital Medical University. All the reference strains and clinical strains were collected from the Beijing biobank of Clinical Resources on Tuberculosis located in Beijing Chest Hospital, Capital Medical University (Beijing, China); therefore the informed consents were waived.
Bacterial Strains and Culture
The M. abscessus clinical isolates were originally isolated from sputa of patients with lung infections. The strains were classified as NTM preliminarily with p-nitrobenzoic acid containing medium, and later identified into species by sequence alignments of 16S rRNA, hsp65, rpoB, 16–23S rRNA internal transcribed spacer in parallel. The reference strains including 29 rapidly growing mycobacteria (RGM) species, 18 slowly growing mycobacteria (SGM) species were obtained either from the American Type Culture Collection (ATCC) or from the German Collection of Microorganisms (DSM). All the bacterial strains studied were stored at −80°C in the Bio-bank in Beijing Chest Hospital, including reference strains and a total of 60 M. abscessus clinical isolates. M. abscessus clinical isolates consist of 40 M. abscessus subsp. abscessus and 20 M. abscessus subsp. massiliense. Bacterial suspensions were inoculated on the Middlebrook 7H10 (M7H10) agar plates or on the Lowenstein-Jensen (LJ) medium. The growth phenotypes of the clinical strains on the L-J medium were recorded.
Antimicrobial Susceptibility Testing
CCCP (CAS#555-60-2) and CLA (CAS#81103-11-9) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA) or Solarbio (Beijing, China), respectively. Both drugs were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 2400 μg/mL. MICs were determined according to the guidelines from the Clinical and Laboratory Standards Institute (CLSI).14 Cation-adjusted Mueller-Hinton broth (CAMHB) (BD, USA) enriched with 5% OADC was used for SGM, while CAMHB without OADC was used for RGM. CCCP was serially diluted (1:2) with final concentrations ranging from 0.115 to 60 μg/mL. NTM cultures were scraped from LJ media, homogenized and then adjusted to McFarland 0.5 with sterile saline, and then diluted 200-fold for inoculation. One hundred μL of diluted inoculum was added into each well. The plates were incubated at 37 °C for 3 to 4 days for RGM and 7 to 8 days for SGM until visible growth of mycobacteria in the control wells without CCCP. Twenty μL of alamarBlue (Invitrogen) and 50 μL of 5% Tween 80 were added to each well and the plates were re-incubated at 37°C for additional 24 h before assessing color change. Blue color indicated no mycobacterial growth and pink color indicated mycobacterial growth. The MIC was defined as the lowest drug concentration that inhibited the bacterial growth, observed by no color change from blue to pink. The MIC testing for each strain was performed in triplicate.
Minimum Bactericidal Concentration (MBC) Against M. abscessus
The reference strains and three clinical isolates for each of M. abscessus subsp. abscessus or subsp. massiliense were randomly selected for the assay. After 4 days’ incubation with CCCP at different concentrations, the wells in which the drug concentrations were higher than the MIC were resuspended and 100 μL medium of each well was inoculated onto 7H10-OADC plate. The plates were incubated at 37°C for 5 days, followed by counting the number of colonies. The MBC value was defined as the minimum drug concentration with no colony growth. An antibiotic was considered bactericidal when the MBC/MIC ratio was ≤4; otherwise, it was considered bacteriostatic.15
Cytotoxicity Assay
The toxicity of CCCP was assessed using THP-1 and U937 cells which were purchased from the Wuhan Procell Life Science&Technology Co., Ltd. Cells were seeded into 24-well plates and differentiated into macrophages with 100 nM PMA. After 24 h, cells were washed once and cultured in fresh RPMI medium (Gibco) with 10% fetal bovine serum (RPMI complete medium). CCCP solution was added into the wells with final concentrations ranging from 0.78 to 25 μg/mL, and incubated for additional 48 h. The same volume of DMSO was used to replace CCCP as control. The cytotoxicity of different concentrations of CCCP was monitored using a lactate dehydrogenase (LDH) cytotoxicity detection kit (Beyotime, Shanghai, China). The LDH activity in the cell culture supernatant were analysed and the absorbance value (Abs) under 490nm was recorded using Multiskan Go microplate reader (Thermo Fisher, USA). The cell survival rate (%), at each concentration was determined as follows: cell survival rate= (1-(Abs490 of treated cells-Abs490 of control cells)/(Abs490 of total lysis cells-Abs490 of control cells))×100%.
Intracellular Killing and Concentration-Kill Assay
U937 cells were seeded at 2×105 cells/well in a 24-well plate and differentiated with PMA. Cells were infected with M. abscessus reference strain (ATCC 19977) and M. massiliense reference strain (CCUG 48898) at a multiplicity of infection (MOI) of 1 or 10. At 2 h post-infection, the cells were gently washed three times with pre-warmed phosphate-buffered saline (PBS) to remove the extracellular bacteria. RPMI complete medium containing 250 μg/mL amikacin were added to kill the remaining extracellular bacteria for an additional 2 h.16 For intracellular killing assay, RPMI complete medium containing CCCP (6 μg/mL) or CLA (2 μg/mL) were added. Meanwhile, RPMI complete medium containing different concentrations of CCCP were supplied for the concentration killing assay. The culture medium with vehicle (DMSO) was included as growth controls. At 48 h post-infection, macrophages were extensively washed with PBS and lysed with 0.1% Tween 80, and serially diluted with 0.05% Tween 80. The number of CFUs was quantified by plating serial dilutions of lysates on M7H10 agar plates. The bacterial survival rate was calculated using the formula: viability=CFU of bacteria under the treatment of CCCP or CLA/CFU of bacteria treated with vehicle DMSO×100%.
Statistical Analysis
Data were analyzed using SPSS 24.0 software and GraphPad Prism 8.0 software. For antimicrobial susceptibility assay, non-parametric test was used to determine significant differences between groups. For cytotoxicity assay and intracellular bactericidal assay, one-way analysis of variance (ANOVA), followed by post hoc test, was used to determine significant differences between the groups. Differences were considered to be statistically significant for p value of <0.05.
Results
Activity Spectra of CCCP Against NTM Reference Strains
The MICs of CCCP against the 47 reference strains are shown in Table 1. CCCP showed excellent activities against most of the RGM reference strains. However, four RGM species had MICs of 3.75 μg/mL and 7.5 μg/mL, and 24 other RGM species had MICs of ≤ 1.875 μg/mL. Additionally, all of the 18 enrolled SGM species had MICs of ≥ 7.5 μg/mL.
 |
Table 1 MICs of CCCP for Reference Strains of 29 RGM Species and 18 SGM Species |
MICs and MBCs of CCCP Against M. abscessus Isolates
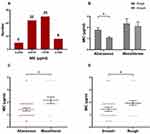
A total of 60 M. abscessus clinical isolates including 40 M. abscessus subsp. abscessus and 20 M. abscessus subsp. massiliense, were studied. The morphological observation indicated that 22 out of the 40 isolates of M. abscessus subsp. abscessus had smooth morphotypes, while 18 exhibited rough morphotypes. Similarly, 11 of the 20 M. abscessus subsp. massiliense isolates exhibited smooth colonies and 9 exhibited rough colonies. The MICs of CCCP against M. abscessus (ATCC19977) and M. massiliense (CCUG48898) were both 0.94 μg/mL. The MICs of CCCP against M. abscessus clinical isolates ranged from 0.47 to 3.75 μg/mL, the majority of these ranged from 0.9375 to 1.875 μg/mL (Figure 1A). The MIC50 and MIC90 of CCCP against M. abscessus subsp. abscessus were both 1.875 μg/mL. The MIC50 and MIC90 of CCCP against M. abscessus subsp. massiliense were 1.875 and 3.75 μg/mL, respectively. In contrast with M. abscessus subsp. abscessus, M. abscessus subsp. massiliense showed greater MIC to CCCP (P <0.05) (Figure 1C). Additionally, the MICs of CCCP against M. abscessus rough morphotype were greater than these of M. abscessus smooth morphotype (P <0.05) (Figure 1D). Specifically, the M. abscessus subsp. abscessus with smooth morphotype was the most sensitive group whereas the M. abscessus subsp. massiliense with rough morphotype was the most tolerant group to CCCP exposure (Figure 1B). The MBC/MIC ratio of CCCP against M. abscessus reference strains and 6 clinical isolates ranged from 4 to 8 (Table 2). Furthermore, 4 strains had MBC/MIC ratios > 4, one strain belonged to M. abscessus subsp. abscessus with smooth morphotype and another one belonged to M. abscessus subsp. massiliense with rough morphotype had MBC/MIC ratios = 4.
 |
Table 2 MIC, MBC Values and Antibacterial Activities of CCCP Against M. abscessus Complex |
Cytotoxicity Assay of CCCP in the THP-1 and U937 Cells
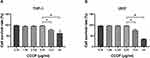
When the concentration of CCCP was lower than 6.25 μg/mL, the survival rates for both THP-1 and U937 cells were almost 97%. However, with the concentration of CCCP at 12.5 μg/mL or greater, the cells survival rates were less than 80% (Figure 2).
Intracellular Bactericidal Activity of CCCP Against M. abscessus in Macrophages
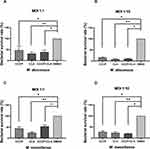
Bacterial survival rate was quantitated as shown in Figure 3. Both at MOI=1:1 and MOI=1:10, obvious reductions were observed in the colony number of M. abscessus (CFU/mL) treated with CCCP group (6 μg/mL) compared to the DMSO group. The intracellular growth inhibition rates of CCCP against both M. abscessus and M. massiliense reference strains at MOI of 10 were significantly higher than MOI of 1. Specifically, at MOI of 10, CCCP inhibited 84.8%±8.8% of intracellular bacterial growth against M. abscessus and 72.5%±13.7% of intracellular bacterial growth against M. massiliense. Similarly, compared with DMSO group, a pronounced decrease was observed in the colony number of M. abscessus treated with CLA group (2 μg/mL) (with inhibitory rates of 92.0%±4.0% in M. abscessus and 76.4%±11.7% in M. massiliense, respectively) and CCCP + CLA group (3 μg/mL CCCP and 1 μg/mL CLA) (with inhibitory rates of 90.4%±5.6% in M. abscessus and 79.4%±4.9% in M. massiliense, respectively). By gradually increasing the CCCP concentration, the intracellular bactericidal activity enhanced linearly, and uniform tendencies were observed at the MOI of 1 and 10 (Figure 4).
Discussion
Due to the intrinsic resistance of M. abscessus to most antibiotics, treatment of M. abscessus infections is highly challenging and often results in failure.17 Previous studies have shown that CCCP as an EPI can reduce the drug tolerance level of Mtb and NTM.11–13 However, antibacterial activity of CCCP as an independent antibiotic has never been reported. In this study, we evaluated the in vitro efficacy of CCCP against 46 NTM reference strains and observed that CCCP presented good activities against most of the RGM reference strains, including the notorious M. abscessus species. Then, we specifically tested more clinical M. abscessus isolates and surprisingly found that CCCP showed excellent intracellular bactericidal activity against M. abscessus. Moreover, variable inhibitory activities were presented against different subspecies or different morphotypes of M. abscessus. The M. abscessus subsp. abscessus with smooth morphotype was the most sensitive group to CCCP exposure while the M. abscessus subsp. massiliense was the vice versa. The MBC/MIC ratio of CCCP for M. abscessus is mainly indicative of bacteriostatic activity, although bactericidal activity might also be possible for the minority population strains of these species. The most important finding of our study is that CCCP has a strong intracellular bactericidal activity and this activity is concentration-dependent. The above results indicate that CCCP could be a promising drug candidate for the treatment of M. abscessus and worthy of further investigation.
In this study, RGM and SGM showed very different susceptibility profiles to CCCP treatment in vitro. CCCP presented good inhibitory activities against most of the enrolled RGM reference strains, but evidently weaker inhibitory activities against the enrolled SGM reference species. We speculate that the different growth and metabolism characteristics of RGM and SGM might cause this discrepancy. However, the mechanism underlying this phenomenon remains to be elucidated. Among the other enrolled RGM reference strains, M. chelonae had MIC to CCCP at 0.94 μg/mL, whereas M. fortuitum, another frequently isolated RGM species with clinical significance, had MIC at 3.75 μg/mL. This diversity within RGM is also worthy of further study.
Our study focused on evaluation of the antimicrobial activity against M. abscessus, which is one of the most frequently isolated NTM species with treatment difficulty. CCCP demonstrated good antimicrobial activities against both the reference strains and clinical strains of M. abscessus. A narrow MIC distribution range was obtained (0.47‒3.75 μg/mL) and only four 2-fold diluted concentrations were covered. 36.67% (22/60) and 41.67% (25/60) of the clinical isolates had MIC at 0.93 μg/mL or 1.83 μg/mL, respectively. A study using CCCP as an experimental control previously occasionally detected the MIC value of CCCP against M. abscessus ATCC19977 at 0.4 μg/mL,18 which was in accordance with our results. In this study, both the in vitro and intracellular testing demonstrated that CCCP has good and concentration-dependent antimicrobial activity against M. abscessus. Cytotoxicity assays indicated that THP-1 cells and U937 cells had high viability (>97%) when exposed to CCCP ≤6.25 µg/mL. The highest safe concentration was much greater than the greatest MIC of the enrolled M. abscessus clinical strains to CCCP, which favors the possibility of CCCP as a new antimicrobial agent. In the intracellular bactericidal experiments, CCCP plus CLA presented reasonable inhibitory activity in contrast to each of the drugs at doubled concentration. We presumed that the inhibition was mainly an outcome of the cumulative effect, while the possibility of the synergistic effect due to the canonical EPI function of CCCP was hard to be determined by our assay. We speculated that since the reference strains of M. abscessus were relatively susceptible to clarithromycin, which makes the efflux pump inhibition effect less obvious when it combined with CCCP.
M. abscessus, which consists of the three subspecies ie, M. abscessus subsp. abscessus, M. abscessus subsp. massiliense and M. abscessus subsp. bolletii, displays two colony morphology types: smooth and rough morphotypes. Previous studies reported that these three M. abscessus subspecies have different antibiotic resistance profiles.19 For example, M. abscessus subsp. massiliense was sensitive to doxycycline, but M. abscessus subsp. abscessus and M. abscessus subsp. bolletii were resistant to it. In addition, the sensitivities of the three subspecies to clarithromycin were not identical either. The major difference between M. abscessus subsp. abscessus and M. abscessus subsp. massiliense is that the latter does not have an intact erm(41) gene and thus does not have inducible macrolide resistance. Therefore, the treatment response may be better among patients infected with M. abscessus subsp. massiliense.20 Our study found that M. abscessus subspecies responded discrepantly when exposed to CCCP, the MIC for M. abscessus subsp. massiliense was generally one fold greater than this of M. abscessus subsp. abscessus. Since we do not have isolates of M. abscessus subsp. bolletii, we could not evaluate the activity of CCCP against this species.
Both smooth morphotype and rough morphotype can be found in subjects with M. abscessus infections.21 The smooth morphotype expresses glycopeptidolipid (GPL) on its cell surface while the rough morphotype expresses minimal amount of GPL on the cell wall.22–24 Biofilm formation and different colony morphotypes contribute to the persistence of M. abscessus in lung diseases. Correlations between morphotypes of M. abscessus and severity of disease were demonstrated in experimental and clinical studies, while rough morphotype has been considered more aggressive.25,26 However, the correlation between phenotype and drug susceptibility remains obscure. Gillian et al found that rough morphotype was more aggressive and formed biofilm-like aggregates, implying it was significantly more tolerant than smooth morphotype to acidic pHs and treatment with amikacin or azithromycin.27 Whereas several other studies did not find the colony morphology of M. abscessus having association with susceptibilities to clarithromycin, amikacin, or cefoxitin, and one study even found that the MIC of smooth morphotype was higher than rough morphotype to tigecycline.28 Our results showed that both morphotypes were susceptible to CCCP while smooth morphotype was more susceptible than rough morphotype. It would be an interesting study on whether CCCP has a stronger inhibitory activity against smooth morphotype resulting in a better therapeutic effect in vivo.
Our study has some limitations. Firstly, all the clinical isolates studied were obtained from a single institution. It may not represent all the characteristics of the M. abscessus isolates. Secondly, the bactericidal activity of CCCP against M. abscessus was only investigated in vitro and in macrophages, therefore the present result might not reflect the real reaction in vivo. Animal model study, pharmacokinetic/pharmacodynamic (PK/PD) studies and synergy of CCCP with other compounds should be further conducted to explore the antibacterial activity of CCCP against M. abscessus infection. Thirdly, although our result showed that the highest safe concentration was much greater than the greatest MIC value of the enrolled M. abscessus clinical strains, the toxicity of it is still a big concern.29–31 Due to its interfering with mitochondrial depolarization which would induce mitophagy process, applying CCCP in human should be extremely cautious.
In conclusion, CCCP has a strong antimicrobial activity against M. abscessus in vitro and in macrophages with less cytotoxicity. CCCP may be a promising drug candidate for M. abscessus treatment, for which very limited effective drug choice available nowadays.
Funding
The study was supported by National Major Science and Technology Projects of China (2018ZX10302-301-004), Beijing Natural Science Foundation (No.5192006), Tongzhou “Yun He” Talent Project (YHLD2018030), and Capital’s Funds for Health Improvement and Research (CFH2020-4-2163), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201824), Beijing Hospitals Authority Youth Programme (QML20201601), and Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20181602).
Disclosure
The authors declare no conflicts of interest.
References
1. Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of mycobacterium abscessus. Nat Rev Microbiol. 2020;18(7):392–407. doi:10.1038/s41579-020-0331-1
2. Cowman S, van Ingen J, Griffith DE, Loebinger MR. Non-tuberculous mycobacterial pulmonary disease. Eur Respir J. 2019;54(1):1900250. doi:10.1183/13993003.00250-2019
3. Koh WJ, Chang B, Jeong BH, et al. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in south korea. Tuberc Respir Dis (Seoul). 2013;75(5):199–204. doi:10.4046/trd.2013.75.5.199
4. Griffith DE. Mycobacterium abscessus and antibiotic resistance: same as it ever was. Clin Infect Dis. 2019;69(10):1687–1689. doi:10.1093/cid/ciz071
5. Chen J, Zhao L, Mao Y, et al. Clinical efficacy and adverse effects of antibiotics used to treat mycobacterium abscessus pulmonary disease. Front Microbiol. 2019;10:1977. doi:10.3389/fmicb.2019.01977
6. Kwak N, Dalcolmo MP, Daley CL, et al. Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur Respir J. 2019;54(1):1801991. doi:10.1183/13993003.01991-2018
7. Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother. 2012;67(4):810–818. doi:10.1093/jac/dkr578
8. Ardebili A, Talebi M, Azimi L, Rastegar Lari A. Effect of efflux pump inhibitor carbonyl cyanide 3-chlorophenylhydrazone on the minimum inhibitory concentration of ciprofloxacin in acinetobacter baumannii clinical isolates. Jundishapur J Microbiol. 2014;7(1):e8691. doi:10.5812/jjm.8691
9. Park YK, Ko KS. Effect of carbonyl cyanide 3-chlorophenylhydrazone (CCCP) on killing acinetobacter baumannii by colistin. J Microbiol. 2015;53(1):53–59. doi:10.1007/s12275-015-4498-5
10. Wang Y, Venter H, Ma S. Efflux pump inhibitors: a novel approach to combat efflux-mediated drug resistance in bacteria. Curr Drug Targets. 2016;17(6):702–719. doi:10.2174/1389450116666151001103948
11. Rodrigues L, Parish T, Balganesh M, Ainsa JA. Antituberculosis drugs: reducing efflux=increasing activity. Drug Discov Today. 2017;22(3):592–599. doi:10.1016/j.drudis.2017.01.002
12. Guo Q, Chen J, Zhang S, et al. Efflux pumps contribute to intrinsic clarithromycin resistance in clinical, mycobacterium abscessus isolates. Infect Drug Resist. 2020;13:447–454. doi:10.2147/IDR.S239850
13. Rindi L. Efflux pump inhibitors against nontuberculous mycobacteria. Int J Mol Sci. 2020;21(12):4191. doi:10.3390/ijms21124191
14. CLSI. Susceptibility Testing of Mycobacteria, Nocardia, and Other Aerobic Actinomycetes; Approved Standard, 2nd. Clsi Document M24-A2. CLSI; 2011.
15. Zhang S, Zou Y, Guo Q, et al. Ar-12 exhibits direct and host-targeted antibacterial activity toward mycobacterium abscessus. Antimicrob Agents Chemother. 2020;64(8). doi:10.1128/AAC.00236-20.
16. Viljoen A, Raynaud C, Johansen MD, et al. Verapamil improves the activity of bedaquiline against mycobacterium abscessus in vitro and in macrophages. Antimicrob Agents Chemother. 2019;63(9). doi:10.1128/AAC.00705-19.
17. Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36(1):67–78. doi:10.1016/j.ccm.2014.10.004
18. Ramis IB, Vianna JS, Silva Junior L, et al. In silico and in vitro evaluation of tetrahydropyridine compounds as efflux inhibitors in mycobacterium abscessus. Tuberculosis (Edinb). 2019;118:101853. doi:10.1016/j.tube.2019.07.004
19. Koh WJ, Jeon K, Lee NY, et al. Clinical significance of differentiation of mycobacterium massiliense from mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183(3):405–410. doi:10.1164/rccm.201003-0395OC
20. Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14(12):1139–1154. doi:10.1080/14787210.2016.1238304
21. Catherinot E, Roux AL, Macheras E, et al. Acute respiratory failure involving an r variant of mycobacterium abscessus. J Clin Microbiol. 2009;47(1):271–274. doi:10.1128/JCM.01478-08
22. Nessar R, Reyrat JM, Davidson LB, Byrd TF. Deletion of the mmpl4b gene in the mycobacterium abscessus glycopeptidolipid biosynthetic pathway results in loss of surface colonization capability, but enhanced ability to replicate in human macrophages and stimulate their innate immune response. Microbiology (Reading). 2011;157(4):1187–1195. doi:10.1099/mic.0.046557-0
23. Pawlik A, Garnier G, Orgeur M, et al. Identification and characterization of the genetic changes responsible for the characteristic smooth-to-rough morphotype alterations of clinically persistent mycobacterium abscessus. Mol Microbiol. 2013;90(3):612–629. doi:10.1111/mmi.12387
24. Rhoades ER, Archambault AS, Greendyke R, et al. Mycobacterium abscessus glycopeptidolipids mask underlying cell wall phosphatidyl-myo-inositol mannosides blocking induction of human macrophage TNF-α by preventing interaction with TLR2. J Immunol. 2009;183(3):1997–2007. doi:10.4049/jimmunol.0802181
25. Li B, Ye M, Zhao L, et al. Glycopeptidolipid genotype correlates with the severity of mycobacterium abscessus lung disease. J Infect Dis. 2020;221(Supplement_2):S257–S262. doi:10.1093/infdis/jiz475
26. Rottman M, Catherinot E, Hochedez P, et al. Importance of t cells, gamma interferon, and tumor necrosis factor in immune control of the rapid grower mycobacterium abscessus in c57bl/6 mice. Infect Immun. 2007;75(12):5898–5907. doi:10.1128/IAI.00014-07
27. Clary G, Sasindran SJ, Nesbitt N, et al. Mycobacterium abscessus smooth and rough morphotypes form antimicrobial-tolerant biofilm phenotypes but are killed by acetic acid. Antimicrob Agents Chemother. 2018;62(3). doi:10.1128/AAC.01782-17.
28. Ruger K, Hampel A, Billig S, et al. Characterization of rough and smooth morphotypes of mycobacterium abscessus isolates from clinical specimens. J Clin Microbiol. 2014;52(1):244–250. doi:10.1128/JCM.01249-13
29. Ni W, Li Y, Guan J, et al. Effects of efflux pump inhibitors on colistin resistance in multidrug-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2016;60(5):3215–3218. doi:10.1128/AAC.00248-16
30. Mlejnek P. Caspase-3 activity and carbonyl cyanide m-chlorophenylhydrazone-induced apoptosis in HL-60. Altern Lab Anim. 2001;29(3):243–249. doi:10.1177/026119290102900313
31. Soutar MPM, Kempthorne L, Annuario E, et al. FBS/BSA media concentration determines CCCP’s ability to depolarize mitochondria and activate PINK1-PRKN mitophagy. Autophagy. 2019;15(11):2002–2011. doi:10.1080/15548627.2019.1603549
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.