Back to Journals » Journal of Hepatocellular Carcinoma » Volume 8
Carbon Ion Radiotherapy in the Management of Hepatocellular Carcinoma
Authors Abousaida B, Seneviratne D, Hoppe BS, Ko SJ, Asaithamby A, Cucinotta FA, Kirwan JM, Mody K, Toskich B, Ashman JB, Hallemeier CL, Krishnan S
Received 20 May 2021
Accepted for publication 8 September 2021
Published 24 September 2021 Volume 2021:8 Pages 1169—1179
DOI https://doi.org/10.2147/JHC.S292516
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Laura A. Dawson
Belal Abousaida,1,* Danushka Seneviratne,1,* Bradford S Hoppe,1 Stephen J Ko,1 Aroumougame Asaithamby,2 Francis A Cucinotta,3 Jessica M Kirwan,4 Kabir Mody,5 Beau Toskich,6 Jonathan B Ashman,7 Christopher L Hallemeier,8 Sunil Krishnan1
1Department of Radiation Oncology, Mayo Clinic Florida, Jacksonville, FL, USA; 2Department of Radiation Oncology, University of Texas Southwestern Medical Center, Dallas, TX, USA; 3School of Integrated Health Sciences, University of Las Vegas, Las Vegas, NV, USA; 4Department of Radiation Oncology, University of Florida, Gainesville, FL, USA; 5Division of Hematology and Medical Oncology, Department of Internal Medicine, Mayo Clinic Florida, Jacksonville, FL, USA; 6Division of Interventional Radiology, Department of Radiology, Mayo Clinic Florida, Jacksonville, FL, USA; 7Department of Radiation Oncology, Mayo Clinic Arizona, Phoenix, AZ, USA; 8Department of Radiation Oncology, Mayo Clinic Rochester, Rochester, MN, USA
*These authors contributed equally to this work
Correspondence: Sunil Krishnan
Department of Radiation Oncology, Mayo Clinic Florida, Jacksonville, FL, 32224, USA
Tel +1 713-202-8063
Email [email protected]
Abstract: Localized hepatocellular carcinoma (HCC) that is unresectable and non-transplantable can be treated by several liver-directed therapies. External beam radiation therapy (EBRT) is an increasingly accepted and widely utilized treatment modality in this setting. Accelerated charged particles such as proton beam therapy (PBT) and carbon ion radiation therapy (CIRT) offer technological advancements over conventional photon radiotherapy. In this review, we summarize the distinct advantages of CIRT use for HCC treatment, focusing on physical and biological attributes, and outline dosimetric and treatment planning caveats. Based on these considerations, we posit that HCC may be among the best indications for use of CIRT, as it allows for maximizing tumoricidal doses to the target volume while minimizing the dose to the organs at risk.
Keywords: carbon ion therapy, hepatocellular carcinoma, hypofractionation
Introduction
Hepatocellular carcinoma (HCC) ranks seventh worldwide among the highest frequently diagnosed cancers and ranks second in cancer-related deaths. It is predominantly prevalent in Asia and Africa, accounting for more than 70% of the disease burden worldwide.1 There were nearly 900,000 people diagnosed with HCC and over 800,000 deaths from HCC globally in 2020. Despite novel therapeutic advances, 10–15% of HCC patients could achieve 5-year overall survival (OS).2 Likely reflecting the widespread use of preventative hepatitis B virus (HBV) vaccinations, the prevalence of hepatitis B and C induced HCC is decreasing in high socio-economic index nations. However, HCC associated with long standing alcoholic cirrhosis and non-alcoholic fatty liver disease has been rising steadily in Western nations over the last decade.3
Surgical resection, thermal ablation, and liver transplant remain the gold standard treatments for primary HCC and may yield favorable clinical outcomes. Unfortunately, no more than 30% of localized HCC patients could be eligible for surgical treatment due to underlying liver dysfunction, or deteriorating performance status or medical comorbidities.4 Local tumor control while preserving liver function can potentially decrease HCC-related mortality and improve OS.5 Minimally invasive techniques can be applied as an alternative treatment option for those who are not surgically eligible. These include transcatheter arterial chemoembolization (TACE), during which chemotherapeutics are infused selectively to the tumor through the feeding blood vessel, yttrium-90 transarterial radioembolization (TARE), wherein the feeding vessel is embolized with radiolabeled beads, and radiofrequency ablation.6
Radiotherapy was evaluated as a potential non-invasive treatment for HCC in different stages. HCC is a radiosensitive tumor occurring in a radiosensitive organ that is often diseased and chronically inflamed. Radiotherapy has historically been underutilized for HCC due to concerns regarding the risk of normal liver toxicity. Nevertheless, pioneering research in the arena of partial liver radiation in the 1980s and 1990s in addition to TARE in the past two decades set the stage for delivering ablative doses to the area of interest while at the same time reducing the dose to the adjacent areas. By using highly conformal techniques including stereotactic ablative body radiotherapy and intensity modulated radiation therapy (IMRT), tumor-directed but uninvolved liver sparing therapy has become easier to administer, resulting in a resurgence of interest in the use of this non-invasive modality to treat HCC. Several trials have proven the efficacy and tolerability of different photon-based techniques of EBRT, particularly in complex cases involving large lesions, vascular invasion, and recurrent disease. In early stage inoperable HCC, radiation therapy has also been used to bridge patients to liver transplant.7 Photon-based radiotherapy for HCC is constrained by the possibility of triggering radiation-induced liver disease (RILD) and bowel toxicity, uncertainties posed by organ motion, and the difficulty of achieving an effective dose within the tumor while maintaining the mean dose to uninvolved liver as low as possible.8 Achieving a low mean liver dose is particularly important in X-ray based therapies given the extensive low dose bath associated with photon therapy. However, this may be less of a concern when utilizing high linear energy transfer (LET) radiation therapy which has no low dose bath and deposits a high dose within the target.9
Although not widespread, accelerated particle therapies such as PBTand CIRT have been investigated in selected centers for the management of HCC since the 1980s, and early reports regarding the efficiency of proton therapy in HCC were published in the 1990s.10 Since then, charged particle therapies have become more widely adopted in the treatment of HCC, as these modalities allow for the delivery of highly localized tumor-targeted radiation to enhance tumor control, while maximally sparing the normal liver via increased dose conformality to the tumor and the absence of an exit dose beyond the tumor along the beam path.11 This review seeks to provide a general overview of CIRT, as well as explore the advantages and pitfalls associated with its use in the management of HCC.
The Relevance of Physical Properties of CIRT in the Treatment of HCC
The elastic and inelastic collisions of charged particles with the atomic nuclei and electrons of tissues allow for the release of its energy in the tissues while heavy ions transverse through tissues and eventually grind to a halt. At the very end of its range, they deposit most of their energy over a short distance, creating the characteristic Bragg peak. In addition to the interaction with electrons, the Coulomb field of charged particles is also repulsed by the electric field of the nucleus of atoms within tissues, causing deflections in its path. Multiple Coulomb scattering events result in lateral beam scattering, explaining the penumbra of a charged particle beam. This penumbra is smaller for heavier particles. Another interaction is the inelastic collision with atomic nuclei wherein the heavy ion is fragmented into multiple lighter ions that travel in the same general direction as the incident ion to create the characteristic fragmentation tail that persists beyond the Bragg peak12 (Figure 1).
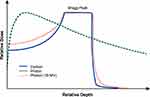 |
Figure 1 Percentage depth dose curves for carbon, proton, and photon beams. |
It is important to note that although CIRT and PBT are often thought to be similar, their physical and biological properties differ significantly. Compared to PBT, CIRT has a narrower lateral penumbra, as noted above.11 CIRT is also different from PBT in the creation of the characteristic fragmentation tail. Although the tail constitutes a small fraction of overall dose, the high LET of these light ions can contribute considerably to their biological dose. And compared to PBT where there is no measurable distal dose beyond the Bragg peak, the physical dose profile of CIRT is distinctly altered by the presence of this fragmentation tail. Similar to passively scattered PBT, ridge filters are used to widen the narrow Bragg peak and encompass the tumor volume within the spread out Bragg peak (SOBP). Raster-scanning technology facilitates modulating the intensity of CIRT beam energy to achieve higher conformality of dose to the tumor.12–15 Most published data for CIRT of HCCs utilized passive scattering methods. Yet, the promising results reported suggest that in settings of large tumor size and considerable organ motion, a passively scattered beam may be sufficient. Nonetheless, the optimal field formation technique (passive scattering versus spot scanning) for the treatment of HCC warrants further investigation.16
The treatment of HCC requires the delivery of highly targeted radiation therapy to a large mass while limiting radiation dose to the surrounding normal liver and nearby GI tract. The unique physical features of carbon ions including their Bragg peak characteristics, narrow lateral penumbra, and steep dose gradients may serve as ideal attributes for HCC treatment wherein sparing even a low dose bath to the uninvolved liver could be advantageous when there is underlying liver dysfunction.15 Similar conformality achieved with ablative TARE or “radiation segmentectomy” has demonstrated increased pathologic necrosis rates,17–19 providing further justification for employing CIRT as a means to achieve conformality, high radiation doses to the tumor, and efficient sparing of the uninvolved liver. Hypothesizing that an increased tumor dose will translate into improved LC and, in turn, improved OS, one could conjecture that CIRT tumor control is superior to that of the photon and PBT. However, there is no conclusive evidence that this is indeed true. Clearly, randomized data is not available currently and non-randomized comparisons are fraught with biases. The closest data that we are aware of is a report from the Massachusetts General Hospital where retrospective analysis of HCC treatment concluded that proton therapy resulted in better OS compared to photon therapy (median OS was 31 months versus 14 months, p=0.008). This was not attributed to a decrease in locoregional recurrence but reduced the risk of non-classic RILD (odds ratio=0.2; 95% CI=0.08–0.8; p=0.03). The ongoing prospective randomized NRG study comparing protons to photons may answer this question and by extension shed light on the potential benefit of CIRT.20
The Relevance of Biological Properties of CIRT in the Treatment of HCC
The high ionization density along the carbon ion beam track result in greater LET, and, in turn, a higher likelihood of complex and clustered DNA damage. These DNA lesions arise along a single track of ionizing radiation and are comprised of two or more distinct lesions within 1–2 helical turns (~10–20 base pairs) of DNA. Individual lesions may be abasic sites, base damage, or singleor double strand DNA breaks. The complexity of such multiply damaged DNA sites contributes to the difficulty with repairing them and therefore the persistence of unrepaired DNA damage, readily visualized as widespread γH2AX foci on 3D structured illumination microscopy following CIRT.21 Often the damage is so catastrophic that it results in chromothripsis or chromosome shattering where genome rearrangements and chromosome translocations can contribute to immense genome instability. Both homologous and non-homologous end joining DNA repair pathways are significantly impaired by high LET irradiation, rendering typically radioresistant cells more sensitive to radiotherapy. CIRT has been shown to cause a higher proportion of damage left unrepaired by homologous recombination mechanisms, because it creates short DNA fragments that remain unbound to the Ku70/Ku80 heterodimer.22 Consequent to this increased DNA damage, CIRT has a higher relative biological effectiveness (RBE) compared to PBT (2–4 vs 1.1).23 RBE correlates with the dose per fraction with a higher dose per fraction resulting in a lower RBE. Another factor affecting the value of RBE is the repair capacity of tissues after radiation; tissues with a higher repair capacity show lower RBE and tissues with a higher α/β ratio show lower RBE. One of the most important factors determining the radiosensitivity of tissues to low LET radiation is the oxygenation status where hypoxic tissue are known to be more radioresistant, but in CIRT hypoxic tissues are not significantly more resistant to treatment than normoxic tissue. Lastly, CIRT results in greater cell cycle delays in the G2 phase and its efficacy is less cell cycle dependent.24
The intent of treatment is to achieve maximum RBE within the tumor while limiting impact within the normal liver. CIRT can be delivered in ultra-hypofractionated regimens which allow for decreasing RBE, particularly in normal liver tissues, which may ultimately limit the risk of RILD. These distinctive biological properties of carbon ions, particularly their ability to achieve a high RBE in tumor while allowing for a favorable dosimetric profile within normal tissues, present significant advantages over alternative forms of radiation therapy in the treatment of HCC. LET and RBE of various radiation modalities is shown in Table 1.
 |
Table 1 LET and RBE of Various Radiation Modalities |
Dose Prescription for CIRT
Scaling of physical CIRT dose along a beam to biological dose requires careful benchmarking and indexing since the RBE along this path can vary as much as 2–4-fold. This is more complex than proton RBE calculations because an arbitrary value of 1.1 works fairly well for most portions of the Bragg curve besides the very distal portion. However, with CIRT the RBE varies considerably across a long segment of the beam path and cannot be arbitrarily assigned a uniform value. To account for this, Japanese investigators performed CIRT irradiation experiments with human salivary gland cells, as representative of early responding tissues, placed along different points along an SOBP and scaled each voxel of an SOBP using relative cellular cytotoxicity of treatment at that depth. This allowed a downward slanting physical dose profile of CIRT SOBP to result in a flat RBE profile across a tumor. To then generate absolute RBE values, the classical mixed-effects (or Kanai) model used a scaling factor that assigned a value of 3 for a LET of 80 keV/µm. This assumption was predicted on an appreciation of biological equivalence of this LET to that of 16 fast neutron treatments of 0.9 Gy. This served the clinical practice well for many years until the advent of intensity modulated CIRT both in Japan and Europe. Here, the models diverged and were refined using slightly different assumptions and mathematical formalisms, although both recognize that ionization density at a microscopic scale defines spatial dose deposition. The Japanese developed the microdosimetric kinetic model (MKM) where RBE was defined within each microscopic spatial “domain” as a function of α and β parameters of the linear quadratic model, with α being proportional to LET and β being independent of LET.15 The α and β parameters were derived from human salivary gland cell line irradiation experiments to generate relative RBE values. Absolute RBE values were generated by cross-referencing prior RBEs within the middle of a 6 cm SOBP from a 350 MeV/nucleon carbon ion beam. The German local effects model (LEM) indexed RBE to in vitro survival data from neuronal cells (late responding normal tissue with an α/β ratio of 2) in an effort to account for normal tissue complications more so than tumor control. The LEM model has been refined and modified (LEM I–IV) over the years and are increasingly complex, often resulting in simple versus complex breaks in both DNA strands with euchromatin versus heterochromatin, etc. Naturally, the biological or RBE-weighted dose (denoted as Gy(RBE)), used for prescribing CIRT treatment in Japan and Europe, are slightly different; yet, the mathematical formulas have been relatively robust at modeling a complex problem of variable LET along a carbon beam path.26,27
Advantages of CIRT in the Management of HCC
Hypofractionation and Dose Escalation in HCC
There have been limited studies regarding the use of CIRT in HCC. Below, a comprehensive review of available studies reporting the use of CIRT in this disease cohort is provided.
The National Institute of Radiological Sciences (NIRS) has carried out several clinical studies since the 1990s using five different fractionation schedules in order to establish the optimal dose of CIRT in a series of dose escalation studies. The first study, Phase I/II trial, used a 15-fraction regimen with doses ranging from 49.5 to 79.5 Gy (RBE), delivered over 5 weeks in 24 patients (10 stage II, 6 stage III, and 8 stage IVA). After a median follow-up of 71 months, the study showed nearly no adverse effects and demonstrated a complete response in 41.7% of patients, a partial response in 29.2%, stable disease in 12.5%, and progression in 16.7% of patients.28 The subsequent Phase I/II study used a 2-fraction regimen with doses ranging from 32–37 Gy(RBE). The preliminary findings of this study indicated an overall tumor response rate of 96% at 6 months after CIRT with no adverse effects observed at 1 year.29
These preliminary NIRS studies established that hypofractionated CIRT was safe and effective in the treatment of HCC. Nonetheless, many patients experienced both in-field and out-of-field disease recurrence on long-term follow-up, though treatment was well tolerated. Further dose escalation was evaluated by Yasuda et al26 in 57 patients with localized HCC (72% newly diagnosed, 28% persistent after prior TACE or RFA treatment or recurrent) receiving 45 Gy(RBE) delivered in 2 days. One-, 3-, and 5-year local control (LC) rates were 98%, 91%, and 91%, respectively, even after failure of prior TACE or RFA treatment. Consistent with these results but with a slightly more fractionated course of radiation, Shibuya et al30 analyzed outcomes of 21 patients (11 with tumors measuring >5 cm and 10 measuring <5 cm, Child-Pugh A or B, without major vascular invasion or extrahepatic metastases) treated between 2012 and 2016 to a dose of 60 Gy(RBE) in four fractions. Progression-free survival rates were 100% and 92.3% at 1- and 2-years, respectively. No patient experienced acute grade 3/4 toxicities, but three experienced late grade 3 toxicities; Child–Pugh score worsened in two patients at 3months. Similarly, Kasuya et al31 performed two trials, a phase I/II trial (protocol 9603) and a Phase II trial (protocol 0004). The initial trial used doses of 69.6 Gy(RBE), 58.0 Gy(RBE), and 52.8 Gy(RBE) delivered in progressive hypofractionation schedules from 12 to four fractions. The recommended phase II dose was four fractions of 13.2 Gy(RBE) each and was utilized for an expansion cohort of 124 patients. In-field and out-of-field tumor recurrence rates were 2.2%, 4.5%, and 8.4% at 1, 3, and 5 years, respectively.
In a multicentric retrospective study of 174 previously treated patients who received <5 fractions of CIRT between 2005 and 2014, LC rates were 94.6%, 87.7%, and 81.0% at 1, 2, and 3 years, respectively. Only 10 patients experienced grade 3/4 toxicities and a mere 1.7% of patients developed RILD.32 The results of the PROMETHEUS clinical trial are awaited as they investigated the feasibility of escalating the dose of CIRT from 40 to 56 Gy(RBE) in four fractions using active raster scanning.33
In a retrospective comparison of PBT and CIRT done by Komatsu et al,34 343 patients who had 386 tumors were studied and they concluded that the rate of LC was 90.8% and the average OS rate was 38.2% at 5 years for both groups, with those numbers being 90% and 38%, respectively, for those receiving PBT and 93% and 36.3% for those undergoing CIRT.
Selected clinical studies regarding the use of CIRT in HCC are documented in Table 2.
 |
Table 2 Literature Review of Studies Reporting Outcomes After CIRT for Hepatocellular Carcinoma |
Treatment Planning Considerations for CIRT in HCC
CIRT affords excellent dose conformality, sharp penumbra, and steep dose gradients around the target volume to allow (i) the delivery of ablative doses to the tumor, ie, a higher tumor control probability and (ii) lower dose to surrounding liver tissue (mean liver dose and V5–V20) and organs at risk, ie, lower probability of RILD. Another advantage of CIRT that adds to the better conformality of CIRT is the usage of the least number of fields and low exit dosage in comparison to the multiple fixed beams or arcs used in IMRT or SBRT. CIRT and SBRT plans were compared in 10 respiratory gated HCC patients, the ability to cover the PTV with 90% of the prescribed dose was found to be higher in CIRT [59.6±0.2 Gy(RBE)] versus SBRT groups [56.6 Gy] (p<0.05).35 The CIRT group also showed a better homogeneity index and a better conformality index, and the maximal dose to the gastrointestinal tract was lower for CIRT than SBRT (8.4±4.3 Gy(RBE) versus 17.4±7.1 Gy).35 A multitude of photon and radioembolization trials demonstrated that LC was enhanced with the escalation of dose delivered to tumor and a similar correlation between RILD and mean radiation dose to “normal” liver.17–19,26,36–38
Design and delivery of treatment fields for HCC CIRT require careful consideration of some practical issues. The exquisite sensitivity of the range of carbon beams to changes in Hounsfield units along their path mandates that planning be performed on non-contrast scans that mimic the tissue configurations at the time of treatment. Similarly, it is customary to minimize beams traversing through the lungs or bowel and terminating in critical normal organs like the bowel, spinal cord, and central biliary structures, since slight changes in air content of these structures may significantly change penetrance of the beam. Likewise, metal fiducials have the potential to shield dose downstream of them and create unwanted cold spots in the dose distributions; carbon and polymer fiducials are preferable and create fewer artifacts. The sharp penumbra of carbon ion beams may under-treat tumors with ill-defined infiltrative margins. Lastly, respiratory motion management is critical since the range of uncertainty of carbon ions is exacerbated when tissue planes and densities vary over the time frame that the beam is on.39
HCC CIRT in Special Scenarios
As a consequence of better dose conformity and maximal sparing of normal liver tissue, CIRT may be especially well suited for salvaging patients who recur locally following prior high-dose radiotherapy; the higher RBE within the tumor and the high dose-conformality sparing “normal” liver may both be advantageous. Reirradiation using CIRT has been reported in recurrent head and neck cancers, base of skull chordomas, pancreatic cancers, and rectal cancers.40–43 Although no such HCC reirradiation series has been reported, in a report of 57 patients who received CIRT, four developed local failure and one was salvaged with another four fractions of 13.2 Gy(RBE) of CIRT and no treatment-related adverse events reported at 5 years.26
Tumor size is an independent predictor of objective response rates, LC rates, in-field recurrence-free rates, and OS rates in photon radiotherapy of HCC.44–46 In a representative retrospective study, 141 patients underwent SBRT and they were grouped in accordance with the longest diameter of the lesion into small (<4 cm), intermediate (4–10 cm), and large (>10 cm) and had objective response rates of 96.15%, 90.90%, and 76.47%, respectively (p ≤0.0001). The 3 year LC rates of 97%, 72%, and 82%, respectively, were significantly different (p =0.0035), but the 3 year OS difference was not significant.44 In dosimetric comparisons of protons versus IMRT, proton treatments achieve better sparing of the liver and gastrointestinal tract than photons. This benefit was more evident for larger tumors, with tumors larger than 6.3 cm having an estimated risk of RILD of 94.5% with photons compared to 6.2% with PBT.47 PBT was also effective in controlling larger (>5 cm) HCCs with PVTT, achieving 66% OS at 2 years.48 By extrapolation, it seems reasonable to assume that CIRT would be beneficial for patients with larger tumors since the volume of “normal” liver to spare is smaller and the lack of a low dose radiation bath could minimize the risk of RILD and permit greater dose escalation to the tumor itself. There are, however, no published reports with objective data confirming this assumption.
Even among elderly patients with limited capacity of liver function recovery, the use of short course CIRT has been shown to be safe. For instance, in an analysis of 31 patients over the age of 80 treated with CIRT, only one patient progressed from Child-Pugh A to Child-Pugh B within the first 3 months of CIRT.49
Radiation Induced Liver Disease
As a major adverse effect of liver irradiation is the development of RILD, most CIRT studies have quantified and reported results that include this information. Yet, the majority of these studies were retrospective, and they reported hepatotoxicity in different ways with no unified criteria for the severity of the hepatotoxicity. This makes the comparison between studies highly challenging. Nonetheless, there are some unmistakable trends that are seen. For instance, despite 45 Gy(RBE) being delivered in 2 consecutive days, one CIRT study noted no acute or late RILD (considered when the Child-Pugh score increases by ≥2 after a median follow-up of 51 months.26 In a combined analysis of two prospective trials involving hypofractionated CIRT, 29% of patients experienced an increase in Child-Pugh score ≥1 at 3 months, but this declined to 22% at 6 months, indicating that acute liver injury from CIRT may be transient and can recover over time. An increase by ≥2 in Child-Pugh was only reported in 3% at 3 months and 5% at 6 months.31
Limitations of CIRT in HCC
The greatest impediment to widespread adoption of CIRT for HCC is the high cost of construction and operation of treatment facilities; this is compounded by the lack of FDA approval, approved billing codes, and insurance coverage in the US. Another limitation is the lack of consensus in RBE modeling or objective liver or gastrointestinal tract specific preclinical data to build these models on. As noted previously, the lack of a low dose bath is especially useful for tumors where the remaining liver tissue represents the dose limiting structure. However, when the dose-limiting normal structure is the gastrointestinal tract, the benefit of CIRT may be less evident since point dose or focal high dose (especially distal to the Bragg peak if the beam exits into this mucosa) dictates toxicity.50
Conclusions
The incidence of HCC is growing globally, with the majority of patients in populations without active screening protocols presenting with advanced tumors, oftentimes with associated portal vein thrombosis within a background of considerable liver dysfunction. Given the complexity of HCC treatments and the need to achieve a delicate balance between disease control and limiting toxicity, there is a critical need for therapies which achieve adequate LC with minimal impact on the surrounding liver. Due to the associated low dose radiation bath, traditional photon radiotherapy may not adequately spare non-tumor-bearing liver; CIRT offers an alternative form of radiotherapy that delivers highly conformal high-LET radiation to tumors. Through our comprehensive review of physical and biological properties of CIRT, associated dosimetric and treatment planning concerns, and available clinical data to date, we posit that CIRT is well tolerated and leads to excellent disease control in HCC with minimal impact on normal liver function. CIRT might have a unique and distinct niche in the treatment of HCC with liver-directed therapies that will need to be defined in the coming years.
Disclosure
Dr Kabir Mody reports personal fees, research funding, advisory board, and consulting from/for AstraZeneca, consulting and research funding for/from Incyte and BTG Consulting, advisory board and research funding for/from Exelixis, Taiho, and Genentech, advisory board, personal fees, and research funding for/fromIpsen, personal fees from Tempus and QED, and research funding from Senhwa Biosciences and Natera, outside the submitted work. Dr Beau Toskich reports being an advisor for Boston Scientific, Sirtex Medical, Johnson and Johnson, AstraZeneca, Genentech, Eisai, and Turnstone Biologics, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Momin BR, Pinheiro PS, Carreira H, Li C, Weir HK. Liver cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123(Suppl 24):5059–5078. doi:10.1002/cncr.30820
3. Liu Z, Jiang Y, Yuan H, et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the global burden of disease study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70(4):674–683. doi:10.1016/j.jhep.2018.12.001
4. Delis SG, Dervenis C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J Gastroenterol. 2008;14(22):3452–3460. doi:10.3748/wjg.14.3452
5. Couto OF, Dvorchik I, Carr BI. Causes of death in patients with unresectable hepatocellular carcinoma. Dig Dis Sci. 2007;52(11):3285–3289. doi:10.1007/s10620-007-9750-3
6. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
7. Sandroussi C, Dawson LA, Lee M, et al. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int. 2010;23(3):299–306. doi:10.1111/j.1432-2277.2009.00980.x
8. Park S, Yoon WS, Rim CH. Indications of external radiotherapy for hepatocellular carcinoma from updated clinical guidelines: diverse global viewpoints. World J Gastroenterol. 2020;26(4):393–403. doi:10.3748/wjg.v26.i4.393
9. Hsieh C-E, Venkatesulu BP, Lee C-H, et al. Predictors of radiation-induced liver disease in eastern and western patients with hepatocellular carcinoma undergoing proton beam therapy. Int J Radiat Oncol Biol Phys. 2019;105(1):73–86. doi:10.1016/j.ijrobp.2019.02.032
10. Tanaka N, Matsuzaki Y, Chuganji Y, Osuga T, Kuramoto K, Tsujii H. Proton irradiation for hepatocellular carcinoma. Lancet. 1992;340(8831):1358. doi:10.1016/0140-6736(92)92546-R
11. Ray S, Cekanaviciute E, Lima IP, Sørensen BS, Costes SV. Comparing photon and charged particle therapy using DNA damage biomarkers. Int j Part Ther. 2018;5(1):15–24. doi:10.14338/IJPT-18-00018.1
12. Zeitlin C, La Tessa C. The role of nuclear fragmentation in particle therapy and space radiation protection. Front Oncol. 2016;6:65. doi:10.3389/fonc.2016.00065
13. Ebner DK, Tsuji H, Yasuda S, Yamamoto N, Mori S, Kamada T. Respiration-gated fast-rescanning carbon-ion radiotherapy. Jpn J Clin Oncol. 2017;47(1):80–83. doi:10.1093/jjco/hyw144
14. Tsujii H, Kamada T. A review of update clinical results of carbon ion radiotherapy. Jpn J Clin Oncol. 2012;42(8):670–685. doi:10.1093/jjco/hys104
15. Karger CP, Peschke P. RBE and related modeling in carbon-ion therapy. Phys Med Biol. 2017;63(1):01tr02. doi:10.1088/1361-6560/aa9102
16. Noda K. Beam delivery method for carbon-ion radiotherapy with the heavy-ion medical accelerator in Chiba. Int J Part Ther. 2016;2(4):481–489. doi:10.14338/IJPT-15-00041.1
17. Toskich B, Vidal LL, Olson MT, et al. Pathologic response of hepatocellular carcinoma treated with yttrium-90 glass microsphere radiation segmentectomy prior to liver transplantation: a validation study. J Vasc Interv Radiol. 2021;32(4):518–526 e511. doi:10.1016/j.jvir.2020.12.019
18. Vouche M, Habib A, Ward TJ, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60(1):192–201. doi:10.1002/hep.27057
19. Gabr A, Riaz A, Johnson GE, et al. Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imaging. 2021;48(2):580–583. doi:10.1007/s00259-020-04976-8
20. Sanford NN, Pursley J, Noe B, et al. Protons versus photons for unresectable hepatocellular carcinoma: liver decompensation and overall survival. Int J Radiat Oncol Biol Phys. 2019;105(1):64–72. doi:10.1016/j.ijrobp.2019.01.076
21. Hagiwara Y, Niimi A, Isono M, et al. 3D-structured illumination microscopy reveals clustered DNA double-strand break formation in widespread γH2AX foci after high LET heavy-ion particle radiation. Oncotarget. 2017;8(65):109370–109381. doi:10.18632/oncotarget.22679
22. Wang H, Wang X, Zhang P, Wang Y. The Ku-dependent non-homologous end-joining but not other repair pathway is inhibited by high linear energy transfer ionizing radiation. DNA Repair (Amst). 2008;7(5):725–733. doi:10.1016/j.dnarep.2008.01.010
23. Ballarini F, Altieri S, Bortolussi S, Giroletti E, Protti N. A model of radiation-induced cell killing: insights into mechanisms and applications for hadron therapy. Radiat Res. 2013;180(3):307–315. doi:10.1667/RR3285.1
24. Gerelchuluun A, Manabe E, Ishikawa T, et al. The major DNA repair pathway after both proton and carbon-ion radiation is NHEJ, but the HR pathway is more relevant in carbon ions. Radiat Res. 2015;183(3):345–356. doi:10.1667/RR13904.1
25. Kavanagh BD. Introduction to clinical radiation oncology. Radiology. 1998;209(1):168. doi:10.1148/radiology.209.1.168
26. Yasuda S, Kato H, Imada H, et al. Long-term results of high-dose 2-fraction carbon ion radiation therapy for hepatocellular carcinoma. Adv Radiat Oncol. 2020;5(2):196–203.
27. Kato H, Yasuda S, Yamada S, et al. Two-fraction carbon ion radiotherapy for hepatocellular carcinoma. J Clin Oncol. 2007;25(18_suppl):15134. doi:10.1200/jco.2007.25.18_suppl.15134
28. Kato H, Tsujii H, Miyamoto T, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004;59(5):1468–1476.
29. Kato H, Yamada S, Yasuda S, et al. Two-fraction carbon ion radiotherapy for hepatocellular carcinoma: preliminary results of a phase I/II clinical trial. J Clin Oncol. 2005;23(16_suppl):4124. doi:10.1200/jco.2005.23.16_suppl.4124
30. Shibuya K, Ohno T, Katoh H, et al. A feasibility study of high-dose hypofractionated carbon ion radiation therapy using four fractions for localized hepatocellular carcinoma measuring 3 cm or larger. Radiother Oncol. 2019;132:230–235.
31. Kasuya G, Kato H, Yasuda S, et al. Progressive hypofractionated carbon-ion radiotherapy for hepatocellular carcinoma: combined analyses of 2 prospective trials. Cancer. 2017;123(20):3955–3965.
32. Shibuya K, Ohno T, Terashima K, et al. Short-course carbon-ion radiotherapy for hepatocellular carcinoma: a multi-institutional retrospective study. Liver Int. 2018;38(12):2239–2247.
33. Combs SE, Habermehl D, Ganten T, et al. Phase I study evaluating the treatment of patients with hepatocellular carcinoma (HCC) with carbon ion radiotherapy: the PROMETHEUS-01 trial. BMC Cancer. 2011;11(1):1–8. doi:10.1186/1471-2407-11-67
34. Komatsu S, Fukumoto T, Demizu Y, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer. 2011;117(21):4890–4904. doi:10.1002/cncr.26134
35. Abe T, Saitoh J-I, Kobayashi D, et al. Dosimetric comparison of carbon ion radiotherapy and stereotactic body radiotherapy with photon beams for the treatment of hepatocellular carcinoma. Radiat Oncol. 2015;10:187. doi:10.1186/s13014-015-0491-8
36. Skinner HD, Sharp HJ, Kaseb AO, et al. Radiation treatment outcomes for unresectable hepatocellular carcinoma. Acta Oncol. 2011;50(8):1191–1198. doi:10.3109/0284186X.2011.592147
37. Chiesa C, Mira M, Bhoori S, et al. Radioembolization of hepatocarcinoma with (90) Y glass microspheres: treatment optimization using the dose-toxicity relationship. Eur J Nucl Med Mol Imaging. 2020;47(13):3018–3032. doi:10.1007/s00259-020-04845-4
38. Chadha AS, Gunther JR, Hsieh CE, et al. Proton beam therapy outcomes for localized unresectable hepatocellular carcinoma. Radiother Oncol. 2019;133:54–61. doi:10.1016/j.radonc.2018.10.041
39. Skinner HD, Hong TS, Krishnan S. Charged-particle therapy for hepatocellular carcinoma. Semin Radiat Oncol. 2011;21(4):278–286. doi:10.1016/j.semradonc.2011.05.007
40. Okamoto M, Shiba S, Okazaki S, et al. Feasibility and safety of repeated carbon ion radiotherapy for locally advanced unresectable pancreatic cancer. Cancers. 2021;13(4):665. doi:10.3390/cancers13040665
41. Hu J, Huang Q, Gao J, et al. Clinical outcomes of carbon-ion radiotherapy for patients with locoregionally recurrent nasopharyngeal carcinoma. Cancer. 2020;126(23):5173–5183. doi:10.1002/cncr.33197
42. Venkatesulu BP, Giridhar P, Malouf TD, Trifletti DM, Krishnan S. A systematic review of the role of carbon ion radiation therapy in recurrent rectal cancer. Acta Oncologica. 2020;59(10):1218–1223. doi:10.1080/0284186X.2020.1769184
43. Mizoe J-E, Hasegawa A, Takagi R, Bessho H, Onda T, Tsujii H. Carbon ion radiotherapy for skull base chordoma. Skull Base. 2009;19(3):219–224. doi:10.1055/s-0028-1114295
44. Kuo H-T, Que J, Lin L-C, Yang -C-C, Koay L-B, Lin C-H. Impact of tumor size on outcome after stereotactic body radiation therapy for inoperable hepatocellular carcinoma. Medicine. 2017;96(50):e9249–e9249.
45. Que J, Kuo H-T, Lin L-C, et al. Clinical outcomes and prognostic factors of cyberknife stereotactic body radiation therapy for unresectable hepatocellular carcinoma. BMC Cancer. 2016;16(1):451. doi:10.1186/s12885-016-2512-x
46. Kwon JH, Bae SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10(1):1–10. doi:10.1186/1471-2407-10-475
47. Toramatsu C, Katoh N, Shimizu S, et al. What is the appropriate size criterion for proton radiotherapy for hepatocellular carcinoma? A dosimetric comparison of spot-scanning proton therapy versus intensity-modulated radiation therapy. Radiat Oncol. 2013;8(1):48. doi:10.1186/1748-717X-8-48
48. Kim DY, Park J-W, Kim TH, et al. Risk-adapted simultaneous integrated boost-proton beam therapy (SIB-PBT) for advanced hepatocellular carcinoma with tumour vascular thrombosis. Radiother Oncol. 2017;122(1):122–129. doi:10.1016/j.radonc.2016.12.014
49. Shiba S, Abe T, Shibuya K, et al. Carbon ion radiotherapy for 80 years or older patients with hepatocellular carcinoma. BMC Cancer. 2017;17(1):721. doi:10.1186/s12885-017-3724-4
50. Schlaff CD, Krauze A, Belard A, O’Connell JJ, Camphausen KA. Bringing the heavy: carbon ion therapy in the radiobiological and clinical context. Radiat Oncol. 2014;9(1):88. doi:10.1186/1748-717X-9-88
51. Shiba S, Shibuya K, Katoh H, et al. No deterioration in clinical outcomes of carbon ion radiotherapy for sarcopenia patients with hepatocellular carcinoma. Anticancer Res. 2018;38(6):3579–3586. doi:10.21873/anticanres.12631
52. Shiba S, Shibuya K, Okamoto M, et al. Clinical impact of hypofractionated carbon ion radiotherapy on locally advanced hepatocellular carcinoma. Radiat Oncol. 2020;15(1):195. doi:10.1186/s13014-020-01634-z
53. Shiba S, Shibuya K, Katoh H, et al. A comparison of carbon ion radiotherapy and transarterial chemoembolization treatment outcomes for single hepatocellular carcinoma: a propensity score matching study. Radiat Oncol. 2019;14(1):137. doi:10.1186/s13014-019-1347-4
54. Imada H, Kato H, Yasuda S, et al. Compensatory enlargement of the liver after treatment of hepatocellular carcinoma with carbon ion radiotherapy - relation to prognosis and liver function. Radiother Oncol. 2010;96(2):236–242. doi:10.1016/j.radonc.2010.03.025
55. Habermehl D, Debus J, Ganten T, et al. Hypofractionated carbon ion therapy delivered with scanned ion beams for patients with hepatocellular carcinoma – feasibility and clinical response. Radiat Oncol. 2013;8(1):59. doi:10.1186/1748-717X-8-59
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
