Back to Journals » International Journal of Nanomedicine » Volume 16
Carbon Dots: A Future Blood–Brain Barrier Penetrating Nanomedicine and Drug Nanocarrier
Authors Zhang W, Sigdel G , Mintz KJ, Seven ES , Zhou Y, Wang C, Leblanc RM
Received 6 May 2021
Accepted for publication 1 July 2021
Published 23 July 2021 Volume 2021:16 Pages 5003—5016
DOI https://doi.org/10.2147/IJN.S318732
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Wei Zhang,1,* Ganesh Sigdel,1,* Keenan J Mintz,1 Elif S Seven,1 Yiqun Zhou,1 Chunyu Wang,2,3 Roger M Leblanc1
1Department of Chemistry, University of Miami, Coral Gables, FL, 33146, USA; 2Department of Biological Sciences, Rensselaer Polytechnic Institute, Troy, NY, 12180, USA; 3Department of Chemistry and Chemical Biology, Rensselaer Polytechnic Institute, Troy, NY, 12180, USA
*These authors contributed equally to this work
Correspondence: Roger M Leblanc Email [email protected]
Abstract: Drug delivery across the blood–brain barrier (BBB) is one of the biggest challenges in modern medicine due to the BBB’s highly semipermeable property that limits most therapeutic agents of brain diseases to enter the central nervous system (CNS). In recent years, nanoparticles, especially carbon dots (CDs), exhibit many unprecedented applications for drug delivery. Several types of CDs and CD-ligand conjugates have been reported successfully penetrating the BBB, which shows a promising progress in the application of CD-based drug delivery system (DDS) for the treatment of CNS diseases. In this review, our discussion of CDs includes their classification, preparations, structures, properties, and applications for the treatment of neurodegenerative diseases, especially Alzheimer’s disease (AD) and brain tumor. Moreover, abundant functional groups on the surface, especially amine and carboxyl groups, allow CDs to conjugate with diverse drugs as versatile drug nanocarriers. In addition, structure of the BBB is briefly described, and mechanisms for transporting various molecules across the BBB and other biological barriers are elucidated. Most importantly, recent developments in drug delivery with CDs as BBB-penetrating nanodrugs and drug nanocarriers to target CNS diseases especially Alzheimer’s disease and brain tumor are summarized. Eventually, future prospects of the CD-based DDS are discussed in combination with the development of artificial intelligence and nanorobots.
Keywords: carbon dots, blood–brain barrier, drug delivery, brain tumor, central nervous system diseases
Graphical Abstract:
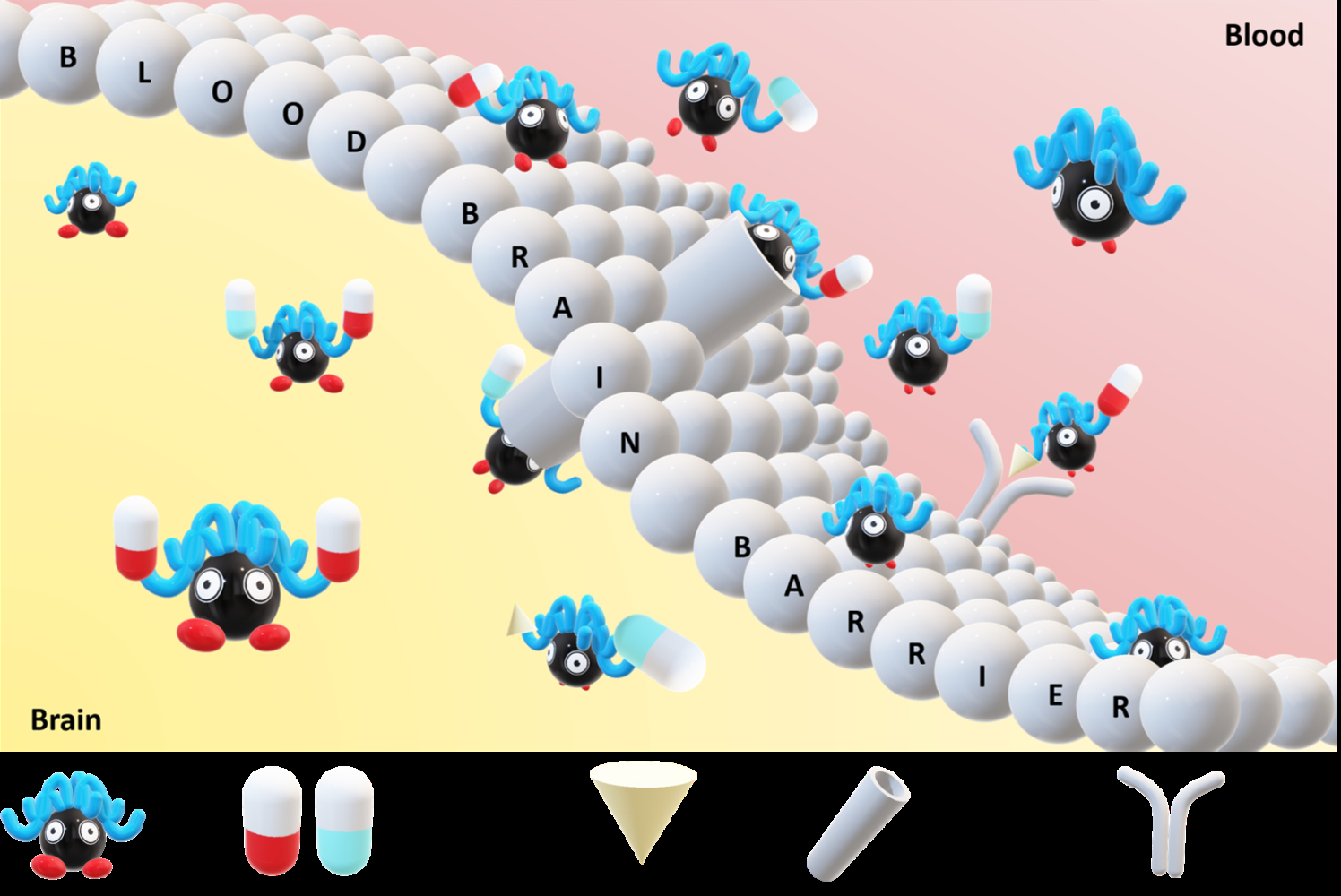
Introduction
There is a long-lasting question in modern medicine that is how therapeutic agents can penetrate the blood–brain barrier (BBB) to reach and function in the central nervous system (CNS). Due to the BBB’s highly semipermeable property, most therapeutic agents of brain diseases are precluded from entering into the CNS.1,2 Alternative strategies such as photodynamic and photothermal therapies have shown possibilities of bypassing the BBB and are used clinically for the treatment of a wide range of brain diseases. However, the side effects of photosensitization and damages to skin tissues raise other concerns.3 In recent years, the development of nanoparticles (NPs), especially multifunctional smart NPs, has exhibited many unprecedented properties for the application of NP-mediated drug delivery due to their capacities to noninvasively overcome the BBB for the treatment of CNS diseases.4 The biggest advantage to apply smart NPs is that they can be engineered to react in a predictable and specific way to the external and internal stimuli, which improves the control of the drug delivery process. Moreover, studies have shown that drug delivery efficiency can be greatly enhanced through covalent conjugation of NPs and various ligands. Some ligands such as niosome and glutathione peptide can improve solubility and stability of the NP-based drug delivery systems (DDS). Also, utilization of certain ligands, such as transferrin, can benefit for targeted therapy and the penetration of biological barriers such as the BBB and cell membranes.5,6
Among all the NPs, carbon dots (CDs) are one of the most promising candidates to penetrate the BBB due to their special characteristics, such as high biocompatibility, nontoxicity, abundant surface functional groups, excellent photoluminescence (PL), and nanoscale size.7–9 Additionally, precursors, synthetic approaches and post-synthesis treatments can also greatly affect their BBB penetration capacity. Multiple studies have reported that CDs can cross the BBB with or without cargo molecules.10–12 In other words, CDs have exhibited a great potential as versatile drug nanocarriers to cross the BBB and treat the CNS diseases. In this review, the classification and multiple synthetic approaches of CDs are introduced. The special relationships between the precursors and the resulted properties of CDs are also discussed. The structures of the BBB and the mechanisms to penetrate the BBB are included. The ability of CDs and CD conjugated derivatives to cross the biological barriers and the applications of CDs as drug nanocarriers to deliver drugs for the treatment of neurodegenerative disorders, such as Alzheimer’s disease (AD) and brain tumor, are discussed. Eventually, our humble perspectives on the future research on this topic are presented.
To cultivate literature for this review’s main topic, search terms including “carbon dots”, “carbon nanodots”, “graphene quantum dots”, “carbon nitride dots”, “blood-brain barrier”, “drug delivery”, “Alzheimer’s disease”, and “brain cancer” were applied to Google Scholar.
Carbon Dots
CDs refer to a broad class of carbon-based nanomaterials, which can be generally divided into carbon nanodots, polymer dots, graphene quantum dots (GQDs) and carbon nitride dots (CNDs). Syntheses of CDs consist of top-down and bottom-up approaches with a variety of reaction conditions and precursors.13 The most popular methodology to prepare CDs is microwave irradiation due to short reaction time and uniform heating.10 In 2009, Zhu et al were the first to utilize this technique to prepare CDs from polyethylene glycol (PEG) and sucrose.14 Later, Wang et al used the same technique with a series of carbohydrates to synthesize CDs.15 Citric acid is a common precursor in the microwave-mediated CD syntheses, and it is often paired with diverse dopants, especially nitrogen dopants, such as ethylenediamine,16 phenylenediamine,17 and urea,18 to yield highly photoluminescent CDs. Hydrothermal/solvothermal approaches rival microwave-mediated reactions as the most popular methods to fabricate CDs. For instance, Zhao et al used benzenetetramine to prepare CDs with emission wavelength above 600 nm.19 A variety of precursors have been used to synthesize CDs hydrothermally or solvothermally, such as carbohydrates,20 proteins,21,22 and many organic acids including amino acids and citric acids.23–26 Meanwhile, ultrasonication represents a gentler method compared with microwave irradiation or hydrothermal/solvothermal approaches, although examples of this technique are rare.27 For example, Zhou et al reported a unique type of excitation-independent yellow-emissive CDs (Y-CDs) with citric acid and o-phenylenediamine (OPD) applied as precursors.28 Moreover, it was also reported by Ajmal et al to produce CDs from glucose through probe sonication.29
There are common optical properties among CDs with minor variations. Carbon nanodots usually possess excitation-dependent emission which for a long time was confined to the blue-green region of light but has been recently extended to the orange-red and even near-IR (NIR) region of light.30,31 Interestingly, the orange-red and NIR PL of CDs are much less to be excitation-dependent than the blue-green PL. In contrast, GQDs are less varied in their PL properties due to their much more well-defined structures than carbon nanodots.32 Nonetheless, their emissions can be tuned (mostly from blue to orange) by heteroatoms and the size of sp2 carbon systems in the particle.33–35 CNDs have similar properties to generic N-doped carbon nanodots. They possess a higher fluorescence quantum yield (QY) than typical CDs and usually have emission wavelengths skewed towards the blue light.36,37
In addition, the main difference among different CDs resides in the structure (Scheme 1A), specifically the core. The cores of carbon nanodots are usually amorphous.30 Some studies have suggested a sp2/sp3 hybrid structure for carbon nanodots, while others showed a more organic/polymer-like structure.38 GQDs are, by definition, particles composed of a few stacked graphene sheets.12 CNDs typically possess an s-triazine or other carbon nitride structures in their cores.37 While each class of CDs owns a different core structure, their surfaces are believed to be composed of simple functional groups or small organic molecules.
 |
Scheme 1 (A) Graphical representation of the overall structure of CDs; (B) number of references per year based on search results using “carbon dots” and “blood-brain barrier” from Google Scholar. |
Furthermore, synthetic routes and selection of precursors play an important role in determination of the structure and optical properties of CDs. Zhou et al synthesized CDs from citric acid and OPD with a microwave-assisted method (700 W, 7 min).17 The synthesized CDs displayed abundant surface functional moieties including -COOH, C=N within a ring system, and C=O, which were revealed by X-ray photoelectron spectroscopy (XPS) with elemental percentages varied with different particle sizes. In addition, excitation-dependent PL emissions were observed in three fractions of the CDs separated by size-exclusion chromatography (SEC). Zhou et al also utilized the same precursors but a different ultrasonication-mediated method to obtain the aforementioned Y-CDs.28 However, after separation by SEC, only one fraction was obtained from Y-CDs. Moreover, XPS characterization demonstrated that Y-CDs possessed C=C, C-O, C=O, and pyrrolidonyl nitrogen on the surface.39 Most surprisingly, XPS measurement and fluorescence spectroscopy revealed that Y-CDs have a uniquely high carbon content (93.4%) and an excitation-independent PL. Furthermore, under microwave irradiation (700 W, 7 min), citric acid and urea were applied by Liyanage et al to fabricate CNDs that owned an excitation-dependent PL and diverse surface functional groups, such as -COOH, -OH, C-O and C=N.37 In comparison, remarkably distinct structures and optical properties were obtained in the CDs prepared using the same precursors or synthetic approaches, which suggests the importance of both factors in determination of the structure and optical properties of CDs. Furthermore, Table 1 summarizes a few classic CD synthetic methodologies and resulting surface structures and optical properties. Except for surface functional moieties and excitation-dependency of PL, it can be also observed from Table 1 that CDs synthesized using top-down approaches such as laser ablation or bottom-up approaches such as ultrasonication generally show low QY. In contrast, bottom-up approaches including microwave-assisted and hydrothermal/solvothermal methods more often lead to higher fluorescence QY.
 |
Table 1 Summary of Common CDs Synthesis Approaches, Starting Materials, Reaction Conditions, and Properties |
As previously mentioned, CDs have been widely explored for many applications in the field of nanomedicine since their discovery.13 Initially, CDs were imaged in human breast cancer cells in 2007 by Sun and coworkers.40 To assess their cytotoxicity and biocompatibility, healthy or cancer cells were exposed to CD solutions with a series of concentrations. After incubation in CD solutions, cell viability was measured, which revealed low cytotoxicity of all CDs exhibited in Table 2.7,41–46 The stability of CDs was also evaluated by investigating their surface charges, sizes, and structures after incubation with cells or after storage under different pH conditions. Similar zeta potentials and sizes indicated a good stability of the surface and physiological states of CDs even after entering cells. And atomic force microscopy (AFM) images demonstrated that CDs were stable under different pH conditions for more than 7 days.42,44,46 In 2009, CDs’ biocompatibility was assessed for the first time in mice. Three groups of mice were exposed to CD aqueous solutions and survived during four-week experiment period without showing any clinical symptom or violent behavior. Normal hepatic and renal functions were also confirmed by using serum biochemistry assays.47 In the same year, Sun and coworkers provided the first example of in vivo bioimaging using CDs.48 Attention to CDs’ applications in traversal of the BBB is illustrated by Scheme 1B. Although mentioned before 2010, they did not gain much attention until mid-2010s, when attention to this topic began growing almost exponentially.
 |
Table 2 CDs or CD Conjugates Reported Able to Penetrate the BBB |
The Blood–Brain Barrier
The BBB is a unique and complex multicellular structural barrier in the CNS consisting of a highly semipermeable membrane of endothelial cells that only allow O2, CO2, water and small molecules to pass while restricting the entrance of pathogens and most macromolecules into the CNS.49 The BBB is mainly comprised of pericytes, astrocytes, basement membrane, neurons, and tight junctions (TJs) between endothelial cells as shown in Figure 1.4
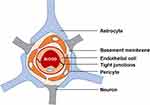 |
Figure 1 Graphical illustration of the structure of the BBB. |
The mechanism to penetrate the BBB can be divided into passive and active routes. Small, lipophilic molecules overcome the BBB by passive diffusion. On the other hand, large, hydrophilic and highly charged molecules are generally transported via active routes including receptor- and adsorption-mediated endocytosis and carrier-mediated penetration.50 Influx and efflux transporters, such as GLUT1 (a glucose transporter), ASCT2 (a neutral amino acid transporter), L1 (an amino acid transporter), CNT2 (an adenosine transporter), and MCT1 (a monocarboxylate transporter), are main transporters for carrying ions and nutrients across the BBB.51,52
Many CNS disorders and malignancies have no satisfactory treatments due to that the BBB hinders the passage of drugs from blood stream to the brain, which motivates developments of novel strategies for neuro-drug delivery into the CNS, such as the use of NPs as nanocarriers for drug delivery across the BBB.4,53 Among various NPs, lipid, polymeric and metal-based NPs are the most popular drug nanocarriers considering their surface features, particle sizes and shapes.54 Moreover, polymeric NPs that originated from human serum albumin, poly(alkyl cyanoacrylate), poly(lactide-co-glycolide) and chitosan exhibited improved control of the drug delivery process.54
Penetration of Biological Barriers with Carbon Dots
The Blood–Brain Barrier
As discussed above, NPs with certain features have demonstrated the ability to cross the BBB and deliver drugs into the CNS.55 Compared with metal-based NPs, CDs received wide attention due to their low cytotoxicity and high biocompatibility.4 Also, the synthetic approaches of CDs are facile, and the large surface-area-to-volume ratio makes them possible to have a high drug loading capacity.7 In addition, due to their excellent PL, distribution of CDs can be tracked in both in vitro and in vivo experiments.7,30,41,42 Furthermore, some advantageous surface properties such as low charge and amphiphilicity endow certain CDs with the potential to cross the BBB.7
To verify the ability of CDs and CD-conjugated derivatives to cross the BBB, both in vitro and in vivo models have been developed. Lu et al used polyethylenimine (PEI) as the precursor to synthesize nitrogen-doped CDs (N-CDs) with a one-pot hydrothermal method and tested their ability to cross the BBB by using an in vitro model made of rat microvascular endothelial cells and astrocytes.41 The strong blue PL of N-CDs under UV irradiation demonstrated that in a concentration-dependent manner, N-CDs were translocated through the BBB. The transwell model in N-CDs study is a good example of in vitro studies. Advantages of this biomimetic model include adjustable parameters and limited variations, but the main disadvantage is that it is still a poor mimic of the BBB in a live animal. To overcome this limitation, as shown in Figure 2A and B, zebrafish and mice are widely applied as in vivo models for the BBB-related studies. For instance, CNDs and their conjugates were reported by Liyanage et al to penetrate the BBB to reach the spinal cord central canal of zebrafish. Moreover, Zhou et al in their study also mentioned a type of gel-like CDs which could cross the BBB using a zebrafish model.56 Zebrafish has a high degree of similarity to human CNS physiology, including all major components of neural system such as transmitters, hormones, and receptors.57 In addition, in comparison to mice, zebrafish reproduces a large number of offspring, which allows reproduction of experiments.4 Moreover, zebrafish offspring can be accommodated in a limited space and are cost-effective.57 Most importantly, since the bodies of zebrafish embryos and larvae are transparent, any fluorescently labeled activity in blood and brain can be tracked in real time.58
 |
Figure 2 The BBB penetration of CDs in animal models recorded by fluorescence imaging. (A) Preparation of CDs from carbon powder, conjugation of CDs with transferrin by EDC/NHS coupling reaction, intravascular injection into the heart of zebrafish, and confocal imaging of the CNS zone; (B) preparation of CDs from OPD and HNO3, an MTT assay performed on C6 glioma cells incubated with CDs, injection of CDs into rats through tail vein, and fluorescence imaging of the brain.Notes: Reproduced with permission from Li S, Peng Z, Dallman J, et al. Crossing the blood–brain–barrier with transferrin conjugated carbon dots: a zebrafish model study. Colloids Surf. B. 2016; 145: 251–256. Copyright 3016, with permission from Elsevier.9 Reproduced with permission from Liu Y, Liu J, Zhang J, et al. Noninvasive brain tumor imaging using red emissive carbonized polymer dots across the blood–brain barrier. ACS Omega. 2018; 3: 7888–7896. Copyright © 2018 American Chemical Society.42 |
Nonetheless, mechanisms for crossing the BBB with CDs remain poorly understood, and they are important areas of investigation for the development of future CDs or CD conjugates to penetrate the BBB for drug delivery. TJs between endothelial cells hold a gap of 4–6 nm,59 making it possible for the CDs with a size less than 4 nm to pass the gap by passive diffusion. Another restriction of the gap is electric charge. There are anionic areas in TJs which attract more cationic molecules and CDs.60 For instance, the aforementioned N-CDs derived from PEI exhibited a positively charged surface.41 The particle size of 2.6 nm and positively charged surface helped N-CDs successfully penetrate the BBB by passive diffusion. Another essential factor for CDs to overcome the BBB by passive diffusion is amphiphilicity. The lipid barrier inside the BBB is hydrophobic. However, after crossing the BBB, molecules need to be hydrophilic to move through the brain fluid in the CNS and that is the reason why the amphiphilic Y-CDs were able to cross the lipid barrier and move through the brain fluid.7 Generally speaking, due to many intrinsic properties of CDs, passive diffusion is favored as the mainstream explanation of the BBB penetration.
Additionally, receptor-mediated endocytosis is a classic strategy for assisting CDs to cross the BBB. As shown in Figure 2A, Li et al showed that B-CDs alone cannot pass the BBB, but after conjugation with human transferrin, the B-CD conjugate was able to cross the BBB via receptor-mediated endocytosis.9 Another strategy is to synthesize self-targeting CDs using compounds that are ligands for specific transporters and the obtained CDs will inherit similar surface functional groups from their precursors. This synthetic approach can eliminate additional steps of surface modifications. Seven et al showed CDs prepared from glucose (GluCDs) could cross the BBB via GLUT1-mediated transport, which was confirmed by two in vivo models, zebrafish and rat.8 In this study, it was also shown that GluCDs could carry fluorescein across the BBB. Moreover, Mintz et al synthesized CDs using tryptophan as a precursor.43 Since tryptophan can cross the BBB via LAT1-mediated transport, the synthesized CDs (T-CDs) with tryptophan moiety present on the surface could be recognized as tryptophan to successfully penetrate the BBB. Thus, CDs are promising drug delivery platforms for CNS diseases and disorders.
Other Biological Barriers
Studies showed that the NPs with sizes comparable to CDs (1–10 nm) had a high efficiency to cross biological barriers such as skin, tissues, alveolar air-blood barrier, intestinal barrier, blood-milk barrier, and blood-placenta barrier.61 In a recent work, CDs derived from Escherichia coli (CDs-WT) showed the ability to permeate mouse skin and tissues.62 When CDs were injected into mice, strong fluorescence signals in intestinal region were observed up to 12 hours after injection. The fluorescence signals disappeared after 24 hours of injection indicating a rapid excretion of CDs.
Han et al synthesized CDs by oxidizing conductive carbon NPs and the prepared CDs (2–3 nm size) were able to penetrate intestinal wall, gonad vesicle wall and germ-cell membrane.63 This novel CD system helped in imaging DNA and RNA structures during cell division. In another study, Shoval et al applied surface-functionalized CDs (8 nm in size) to successfully penetrate ocular including epithelial layer, cornea, eye cavity and lens.64 Wang et al studied the penetration mechanism of two different sizes of GQDs on a Madin Darby Canine Kidney (MDCK) cell monolayer.65 The result revealed that 3-nm GQDs had a good membrane permeability, while 12-nm GQDs were moderately transported through the MDCK membrane. In addition, Table 3 summarizes different CD species with their sizes and abilities to cross various biological barriers.
 |
Table 3 CD Reported Able to Cross the Biological Barrier |
Application of Carbon Dots in Treating CNS Diseases
CDs have demonstrated abilities to cross the BBB. Also, Table 2 shows that there are abundant amine (-NH2) and carboxyl (-COOH) groups on the surfaces of CDs, which can be used to conjugate with diverse CNS drugs. In addition, CDs are nontoxic and, therefore, are safe to be used for biological applications. Therefore, CDs are ideal nanocarriers to deliver drugs into the CNS to treat AD and brain tumor.
Alzheimer’s Diseases
Neurodegeneration is the progressive and irreversible process of neuron and neuronal function loss, which is often associated with deposition of protein aggregates. Common neurodegenerative disorders include Alzheimer’s disease (AD), Parkinson’s disease (PD) and Huntington’s disease (HD).66 Based on the Alzheimer’s Disease Fact Sheet from the National Institutes of Health, more than 5 million Americans are living with AD, and AD is the 6th leading cause of death in the US. With the number of AD cases expected to increase tremendously in our rapidly aging population, it is urgent to seek effective therapies.
Moreover, the pathogenetic mechanisms of AD are poorly understood, and there are no effective treatments. The cause of AD is multifactorial including aggregations of amyloid-beta (Aβ),67 hyperphosphorylated tau proteins,68 and metal ion dyshomeostasis.69 Current therapies only focus on managing symptoms, while drug developments usually target a single pathogenetic mechanism. In addition, the BBB is a major obstacle for drugs targeting AD. For example, less than 1% of antibody drugs for AD clinical trials can cross the BBB, resulting in huge dosage and high cost. Taken together, developments of multifunctional drugs and drug carriers could be a promising strategy for AD treatment.
Reducing the accumulation of Aβ in CNS is one of the most frequently used strategies to treat AD. It is worth noting that Y-CDs showed the capability to inhibit generations of amyloid precursor proteins (APP) and Aβ in cells (Figure 3), which allows them to serve as both nanomedicine and drug nanocarriers for the treatment of AD.7 Liu et al found that GQDs could also prevent Aβ aggregation.70 By linking with tramiprosate, GQDs became a more effective inhibitor of Aβ aggregation.71 Gong et al loaded glycine-proline-glutamate on CDs and the functionalized CDs showed the ability to inhibit Aβ aggregation and diminish proinflammatory cytokines.72 Similarly, the branched PEI-loaded CDs synthesized by Chung et al exhibited cationic surface and were able to suppress the aggregation of Aβ.73 More examples of conjugating CDs with drugs to diagnose or treat AD are summarized in Table 4. Furthermore, copper ion was implicated in Aβ aggregation. Chung et al used polymerized OPD-derived CDs to coordinate with copper ions and diminish Aβ aggregation.74
 |
Figure 3 Confocal images of (A) Y-CDs aqueous dispersion (0.1 mg mL−1) and (B) Y-CDs coated by 3-amino-1-propanol or DEA (1.5 mg mL−1) across the BBB. The red arrows indicate the spinal cord central canal of zebrafish; (C) cellular uptake and in vitro toxicity of Y-CDs; Representative micrographs (left) of Chinese hamster ovary (CHO) cells treated with 10 µM of Y-CDs, scale-5 µm; Quantification of CHO cell viability following 48-h treatment of different concentrations of Y-CDs (right). Data expressed as mean ± SEM; (D) in vitro efficacy of Y-CDs. The CHO cells stably overexpressing APP were incubated in different concentrations of Y-CD aqueous dispersions for 24 h. Representative micrographs (top) of CHO cells treated with vehicle and 10 µM of Y-CDs, scale-20 µm. The CHO cells overexpressing human APP751 were treated with Y-CDs and fixed at 24 h. APP mean fluorescence intensity (MFI) per cell was quantified using NIH Image J software with more than 300 cells counted per treatment following Y-CD treatments (bottom left). Secreted Aβ monomers in cell culture media were quantified following Y-CD treatments (bottom right). Data expressed as mean ± SEM of two independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 based on the ANOVA (analysis of variance) and Tukey’s post hoc test compared to vehicle (0 μM) control.Notes: Reproduced from Zhou Y, Liyanage PY, Devadoss D, et al. Nontoxic amphiphilic carbon dots as promising drug nanocarriers across the blood–brain barrier and inhibitors of β-amyloid. Nanoscale. 2019; 11: 22387–22397, with permission from the Royal Society of Chemistry.7 |
 |
Table 4 Diagnosis and Treatment of Neurodegenerative Diseases with CD Conjugates |
Brain Tumor
Brain tumor is a challenging public health issue, due to the lack of effective treatment and a large number of deaths worldwide each year. Some conventional treatment options include surgery, chemotherapy and radiation therapy. Among them, chemotherapy is widely used to treat brain malignancies such as medulloblastomas, gliomas, CNS lymphomas and brain metastases.75 However, these conventional methods are not very effective, considering the difficulty to remove the entire tumor, lack of tumor-specific drugs and their inability to cross the BBB, and the genetic and cellular heterogeneity of tumor tissues.76,77
Although it is difficult to create DDS specifically targeting tumor cells, great progress has been made. Saul et al introduced a dual-ligand approach to enhance the selectivity of targeting nanocarriers.78 In this approach, some common targeting ligands were used instead of unique ligands for specific ligand-receptor pairing. Doxorubicin-loaded liposomes were developed as a DDS model to selectively target tumor cells. Zheng et al developed CDs (CD-Asp) with D-glucose (Glu) and L-aspartic acid (Asp) as precursors.79 The in vitro study, confirmed by bioimaging, demonstrated a high selectivity and targeting ability of CD-Asp towards C6 glioma cells. Later, Qiao et al optimized the ratio of Glu to Asp to improve the targeting ability of CD-Asp towards brain glioma cells.80 Flow cytometry and confocal laser scanning microscopy (CLSM) studies revealed that the highest targeting ability of CDs-Asp to glioma cells was achieved at a Glu/Asp molar ratio of 7:3.
Moreover, due to the overexpression of transferrin receptors on tumor cells and endothelial cells in the BBB, after conjugation with transferrin, CDs can be specifically led to cancerous cells across the BBB.81 Hettiarachchi et al designed a triple-conjugated CD-based DDS for targeting glioblastoma with transferrin, epirubicin and temozolomide altogether conjugated to the aforementioned B-CDs as presented in Figure 4A. 82 As a result, a much lower concentration of triple-conjugated system (C-dots-transferrin-epirubicin-temozolomide (C-DT)) was required to reduce tumor cell viability compared to non-transferrin systems (NT) and dual-conjugated systems, namely CDs-transferrin-temozolomide (C-TT) and CDs-transferrin-epirubicin (C-ET) (as shown in Figure 4B). Therefore, it is clear that CDs can be simultaneously loaded with multiple therapeutic agents, which can exert a synergistic effect on antitumor efficiency.
 |
Figure 4 (A) Schematic illustration of a triple-conjugated system composed of transferrin, epirubicin and temozolomide on the carboxylic acid-functionalized C-dots (CDs). The drawings are not according to the exact scale and ratio; (B) cytotoxicity profiles of the three transferrin-conjugated samples on each cell line compared to that of non-treated (NT) sample. Brain tumor cell lines SJGBM2, CHLA266, CHLA200 and U87 were plated into 96 well plates 24 h prior to drug treatment. The cells were then treated with three transferrin-conjugated samples and NT sample. After 72 h incubation, viability was examined by using CellTiter 96® Aqueous One Solution Cell Proliferation Assay.Notes: Reproduced from Hettiarachchi SD, Graham RM, Mintz KJ, et al. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale. 2019; 11: 6192–6205, with permission from the Royal Society of Chemistry.82 |
Furthermore, CNDs were reported to be able to selectively target pediatric glioblastoma cells.83 Studies showed that conjugation of gemcitabine (GM) to CNDs effectively killed CNS cancerous cells, but a high concentration of CND-GM conjugate was needed. However, after further conjugating CND-GM with transferrin (Tf), the new conjugate (CND-GM-Tf) could target CNS cancerous cells at an extremely low concentration. Additionally, both CND-GM and CND-GM-Tf were found to be able to cross the BBB, which allows CNDs to become a promising BBB-penetrating drug nanocarrier targeting brain tumors.
Conclusion and Future Prospects
In the report from the World Health Organization in 2016, neurological disorders accounted for nearly 276 million disability-adjusted life-years and around 9 million deaths globally.84 With the number of neurological disorder cases expected to increase tremendously, there is an urgent need for effective therapies. However, due to the highly selective semipermeable property of the BBB, most macromolecules, including drugs, are restricted from entering the CNS, which becomes a major obstacle for the treatment of neurological disorders. In recent years, the development of nanomaterials, especially NP-mediated drug delivery, shows a promising progress to overcome the BBB. Therefore, in this review, we focused on the introduction of CDs and their ability to cross the BBB. The relationships between the use of a wide variety of precursors and preparation methods, resulted physicochemical characteristics of CDs, their abilities to cross the BBB and efficacy in the treatment of ADs are summarized. Moreover, the abundant surface functional groups of CDs provide a great potential to conjugate CDs with single or multiple ligands or drugs to serve as CD-based DDS able to overcome the BBB to treat neurological disorders and brain tumor such as the glioblastoma widely studied by Leblanc and his co-workers.
As CD-based DDS is still in infancy, there is a huge room for its development in the following aspects: (1) currently, most NPs showed the ability to cross the BBB relying on specific ligands for ligand-mediated endocytosis. However, the potential of self-targeting CDs as BBB-penetrating drug delivery platforms or therapeutic agents remains to be explored considering a large number of potential precursors and available sites for conjugation with multiple drug molecules compared to non-self-targeting CDs; (2) CDs are promising drug nanocarriers to reach the CNS but, for future clinical studies, mechanisms of the BBB penetration need to be better understood in addition to drug loading capacity and release kinetics. Most proposed mechanisms have not been rigorously investigated. A better understanding of these complex mechanisms is crucial for developing better solutions to the long-standing challenges in CNS drug delivery. Also, CDs can be directly tracked in vitro and in vivo due to their intrinsic PL, which will assist to unveil the BBB penetration mechanisms of CDs; (3) in addition, it should be noted that there may be concerns about the toxicity and possible inflammatory effects of CDs. Several studies showed high biocompatibility and negligible toxicity of CDs. On the other hand, CD-induced inflammation is still not well known. A few pioneering studies regarding the CD-induced inflammation have been conducted in vitro, showing an immune response in only very high concentrations of CDs.85 Therefore, it is also important to optimize the dosage of CDs in future studies; (4) moreover, most studies on drug delivery with CDs as nanomedicine or nanocarriers to target AD and brain tumors are in vitro. More in vivo models for AD and brain tumors are needed to fully test the potential of CDs for treating CNS diseases and disorders; (5) Also, most reported CDs possess PL in short-wavelength region. Regardless of their high fluorescence QY, blue emission interferes with the autofluorescence of some tissues or organs in animal studies, which makes it difficult to track the drugs loaded on CDs, especially in vivo. In contrast, long-emissive CDs are more desirable. According to literature references,86,87 the isomers of phenylenediamine have been widely applied to successfully synthesize CDs with long emission wavelengths which can be better tracked in biological tissues.
Furthermore, artificial intelligence (AI) is playing an unprecedentedly important role in the development of modern nanomedicine. Machine learning has been applied to assist synthesizing and studying nanomaterials, including CDs, through its effective learning ability based on a vast database.88,89 The machine learning-assisted synthesis of highly fluorescent, photostable, and environmentally stable CDs was reported lately, which enhanced their optical properties significantly.90,91 Also, machine learning has shown a better ability to predict, analyze, and differentiate properties of CDs compared with classical statistical methods, which will promote the research of nanomaterials and nanomedicine.92–94 Overall, the combination of machine learning and chemistry provides a novel way to design and synthesize nanomaterials in the future. Another promising future prospect is to apply CD-based nanomedicine to medical robotics to achieve rapid targeted drug delivery. Miniature untethered medical robots have recently received an increasing attention due to their invasiveness, accessibility into unprecedented limited region inside human body as well as technological advances of actuation, sensing, and fabrication in micro and nanoscales.95 However, propulsion and biocompatibility are the main obstacles of the development of medical robotic. To solve these challenges, Singh et al summarized a group of sperm cell-driven microrobots, namely spermbots, which are naturally biocompatible and utilize flagellar movement of sperm cells for propulsion.96 Additionally, along with recent developments in biological cell-driven biohybrid systems, diverse biological microswimmer (eg, bacteria and algae) populations were deeply studied.97 A stable and specific bacteria-attachment methodology was developed by Singh et al via specific and strong biotin-streptavidin binding to enhance the propulsion performance of a bacteria-driven biohybrid microswimmer.98 These surface patterning and attachment methodologies can be also applied to other biohybrid microdevices/systems. Thus, with both obstacles overcome, in the near future, biological cell-driven medical robots seem to be an excellent “vehicle” to achieve rapid targeted delivery of CD-based nanomedicine.
Abbreviations
Aβ, amyloid-beta; AD, Alzheimer’s disease; AFM, atomic force microscopy; AI, artificial intelligence; APP, amyloid precursor proteins; Asp, aspartic acid; BBB, blood–brain barrier; B-CD, black CD; CD, carbon dot; CHO, Chinese hamster ovary; CLSM, confocal laser scanning microscopy; CND, carbon nitride dot; CNS, central nervous system; DDS, drug delivery system; Glu, glucose; GM, gemcitabine; GQD, graphene quantum dot; HD, Huntington’s disease; MDCK, Madin Darby Canine Kidney; MFI, mean fluorescence intensity; N-CD, nitrogen-doped CDs; NIR, near-infrared; NP, nanoparticle; OPD, o-phenylenediamine; PD, Parkinson’s disease; PEG, polyethylene glycol; PEI, polyethylenimine; PL, photoluminescence; QY, quantum yield; SEC, size-exclusion chromatography; T-CD, tryptophan CD; Tf, transferrin; TJ, tight junction; XPS, X-ray photoelectron spectroscopy; Y-CD, yellow-emissive CDs.
Acknowledgment
Professor Roger M. Leblanc thanks the support from National Science Foundation under the grants 1809060 and 2041413.
Disclosure
The authors declare no conflicts of interest.
References
1. Burgess A, Hynynen K. Drug delivery across the blood–brain barrier using focused ultrasound. Expert Opin Drug Deliv. 2014;11:711–721. doi:10.1517/17425247.2014.897693
2. Sarrazin JL, Bonneville F, Martin-Blondel G. Brain infections. Diagn Interv Imaging. 2012;93:473–490. doi:10.1016/j.diii.2012.04.020
3. Dhas N, Kudarha R, Garkal A, et al. Molybdenum-based hetero-nanocomposites for cancer therapy, diagnosis and biosensing application: current advancement and future breakthroughs. J Control Release. 2021;330:257–283.
4. Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Control Release. 2018;270:290–303. doi:10.1016/j.jconrel.2017.12.015
5. Gharbavi M, Amani J, Kheiri-Manjili H, Danafar H, Sharafi A. Niosome: a promising nanocarrier for natural drug delivery through blood-brain barrier. Adv Pharmacol Sci. 2018;2018:6847971–6847985.
6. Nosrati H, Tarantash M, Bochani S, et al. Glutathione (GSH) peptide conjugated magnetic nanoparticles as blood–brain barrier shuttle for MRI-monitored brain delivery of paclitaxel. ACS Biomater Sci Eng. 2019;5:1677–1685. doi:10.1021/acsbiomaterials.8b01420
7. Zhou Y, Liyanage PY, Devadoss D, et al. Nontoxic amphiphilic carbon dots as promising drug nanocarriers across the blood–brain barrier and inhibitors of β-amyloid. Nanoscale. 2019;11:22387–22397. doi:10.1039/C9NR08194A
8. Seven ES, Zhou Y, Seven YB, Mitchell GS, Leblanc RM. Crossing blood-brain barrier with carbon quantum dots. FASEB J. 2019;33:
9. Li S, Peng Z, Dallman J, et al. Crossing the blood–brain–barrier with transferrin conjugated carbon dots: a zebrafish model study. Colloids Surf B Biointerfaces. 2016;145:251–256. doi:10.1016/j.colsurfb.2016.05.007
10. Kappe CO. Controlled microwave heating in modern organic synthesis. Angew Chem Int Ed. 2004;43:6250–6284.
11. de Medeiros TV, Manioudakis J, Noun F, Macairan JR, Victoria F, Naccache R. Microwave-assisted synthesis of carbon dots and their applications. J Mater Chem C. 2019;7:7175–7195. doi:10.1039/C9TC01640F
12. Tajik S, Dourandish Z, Zhang K, et al. Carbon and graphene quantum dots: a review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020;10(26):15406–15429. doi:10.1039/D0RA00799D
13. Tejwan N, Saha SK, Das J. Multifaceted applications of green carbon dots synthesized from renewable sources. Adv Colloid Interface Sci. 2020;275:102046–102063. doi:10.1016/j.cis.2019.102046
14. Zhu H, Wang X, Li Y, Wang Z, Yang F, Yang X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem Commun. 2009;34:5118–5120.
15. Wang X, Qu K, Xu B, Ren J, Qu X. Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J. Mater. Chem. 2011;21(8):2445–2450. doi:10.1039/c0jm02963g
16. Simões EF, da Silva JCE, Leitão JM. Peroxynitrite and nitric oxide fluorescence sensing by ethylenediamine doped carbon dots. Sens Actuators B Chem. 2015;220:1043–1049. doi:10.1016/j.snb.2015.06.072
17. Zhou Y, Zahran EM, Quiroga BA, et al. Size-dependent photocatalytic activity of carbon dots with surface-state determined photoluminescence. Appl Catal B. 2019;248:157–166. doi:10.1016/j.apcatb.2019.02.019
18. Simões EF, Leitão JM, da Silva JCE. Carbon dots prepared from citric acid and urea as fluorescent probes for hypochlorite and peroxynitrite. Microchim Acta. 2016;183:1769–1777. doi:10.1007/s00604-016-1807-6
19. Zhao P, Li X, Baryshnikov G, et al. One-step solvothermal synthesis of high-emissive amphiphilic carbon dots via rigidity derivation. Chem Sci. 2018;9:1323–1329. doi:10.1039/C7SC04607C
20. Seven ES, Sharma SK, Meziane D, et al. Close-packed Langmuir monolayers of saccharide-based carbon dots at the air–subphase interface. Langmuir. 2019;35(20):6708–6718. doi:10.1021/acs.langmuir.9b00920
21. Mintz KJ, Guerrero B, Leblanc RM. Photoinduced electron transfer in carbon dots with long-wavelength photoluminescence. J Phys Chem C. 2018;122(51):29507–29515. doi:10.1021/acs.jpcc.8b06868
22. Ghosh SA, Kumar VB, Fixler D, Dubinsky Z, Gedanken A, Iluz D. Nitrogen-doped carbon dots prepared from bovine serum albumin to enhance algal astaxanthin production. Algal Res. 2017;23:161–165. doi:10.1016/j.algal.2017.01.011
23. Yang X, Wang Y, Shen X, et al. One-step synthesis of photoluminescent carbon dots with excitation-independent emission for selective bioimaging and gene delivery. J Colloid Interface Sci. 2017;492:1–7. doi:10.1016/j.jcis.2016.12.057
24. Sun Z, Chen Z, Luo J, et al. A yellow-emitting nitrogen-doped carbon dots for sensing of vitamin B12 and their cell-imaging. Dyes Pigm. 2020;176:108227–108234. doi:10.1016/j.dyepig.2020.108227
25. Xu YL, Bai RB, Qi CY, et al. Fluorescence “off-on” probe for L-cysteine detection based on nitrogen doped carbon dots. J Fluoresc. 2019;29:819–825. doi:10.1007/s10895-019-02408-x
26. Zhou M, Zhou Z, Gong A, Zhang Y, Li Q. Synthesis of highly photoluminescent carbon dots via citric acid and tris for iron (III) ions sensors and bioimaging. Talanta. 2015;143:107–113. doi:10.1016/j.talanta.2015.04.015
27. Tejwan N, Saini AK, Sharma A, Singh TA, Kumar N, Das J. Metal-doped and hybrid carbon dots: a comprehensive review on their synthesis and biomedical applications. J Control Release. 2021;330:132–150. doi:10.1016/j.jconrel.2020.12.023
28. Zhou Y, Mintz K, Oztan C, et al. Embedding carbon dots in superabsorbent polymers for additive manufacturing. Polymers. 2018;10:921–932. doi:10.3390/polym10080921
29. Ajmal M, Yunus U, Graham RM, Leblanc RM. Design, synthesis, and targeted delivery of fluorescent 1, 2, 4-triazole–peptide conjugates to pediatric brain tumor cells. ACS Omega. 2019;4:22280–22291. doi:10.1021/acsomega.9b01903
30. Mintz KJ, Zhou Y, Leblanc RM. Recent development of carbon quantum dots regarding their optical properties, photoluminescence mechanism, and core structure. Nanoscale. 2019;11:4634–4652. doi:10.1039/C8NR10059D
31. Shi X, Meng H, Sun Y, et al. Far-red to near-infrared carbon dots: preparation and applications in biotechnology. Small. 2019;15(48):1901507–1901523. doi:10.1002/smll.201901507
32. Zheng P, Wu N. Fluorescence and sensing applications of graphene oxide and graphene quantum dots: a review. Chem Asian J. 2017;12:2343–2353. doi:10.1002/asia.201700814
33. Shen S, Wang J, Wu Z, Du Z, Tang Z, Yang J. Graphene quantum dots with high yield and high quality synthesized from low cost precursor of aphanitic graphite. Nanomaterials. 2020;10:375–385. doi:10.3390/nano10020375
34. Zhu S, Song Y, Zhao X, Shao J, Zhang J, Yang B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res. 2015;8:355–381. doi:10.1007/s12274-014-0644-3
35. Wang H, Wu X, Dong W, Lee SL, Yuan Q, Gan W. One-step preparation of single-layered graphene quantum dots for the detection of Fe3+. Spectrochim Acta A. 2020;226::117626–117632. doi:10.1016/j.saa.2019.117626
36. Zhou J, Yang Y, Zhang CY. A low-temperature solid-phase method to synthesize highly fluorescent carbon nitride dots with tunable emission. Chem Commun. 2013;49(77):8605–8607. doi:10.1039/c3cc42266f
37. Liyanage PY, Graham RM, Pandey RR, et al. Carbon nitride dots: a selective bioimaging nanomaterial. Bioconjug Chem. 2018;30:111–123. doi:10.1021/acs.bioconjchem.8b00784
38. Xia C, Zhu S, Feng T, Yang M, Yang B. Evolution and synthesis of carbon dots: from carbon dots to carbonized polymer dots. Adv Sci. 2019;6(23):1901316–1901338. doi:10.1002/advs.201901316
39. Mintz KJ, Bartoli M, Rovere M, et al. A deep investigation into the structure of carbon dots. Carbon. 2021;173:433–447. doi:10.1016/j.carbon.2020.11.017
40. Cao L, Wang X, Meziani MJ, et al. Carbon dots for multiphoton bioimaging. J Am Chem Soc. 2007;129:11318–11319. doi:10.1021/ja073527l
41. Lu S, Guo S, Xu P, et al. Hydrothermal synthesis of nitrogen-doped carbon dots with real-time live-cell imaging and blood–brain barrier penetration capabilities. Int J Nanomed. 2016;11:6325–6336. doi:10.2147/IJN.S119252
42. Liu Y, Liu J, Zhang J, et al. Noninvasive brain tumor imaging using red emissive carbonized polymer dots across the blood–brain barrier. ACS Omega. 2018;3:7888–7896. doi:10.1021/acsomega.8b01169
43. Mintz KJ, Mercado G, Zhou Y, et al. Tryptophan carbon dots and their ability to cross the blood-brain barrier. Colloids Surf B Biointerfaces. 2019;176:488–493. doi:10.1016/j.colsurfb.2019.01.031
44. Niu Y, Tan H, Li X, et al. Protein–carbon dot nanohybrid-based early blood–brain barrier damage theranostics. ACS Appl Mater Interfaces. 2020;12:3445–3452. doi:10.1021/acsami.9b19378
45. Ji Z, Ai P, Shao C, et al. Manganese-doped carbon dots for magnetic resonance/optical dual-modal imaging of tiny brain glioma. ACS Biomater Sci Eng. 2018;4:2089–2094. doi:10.1021/acsbiomaterials.7b01008
46. Wang S, Li C, Qian M. Augmented glioma-targeted theranostics using multifunctional polymer-coated carbon nanodots. Biomaterials. 2017;141:29–39. doi:10.1016/j.biomaterials.2017.05.040
47. Yang ST, Wang X, Wang H, et al. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J Phys Chem C. 2009;113(42):18110–18114. doi:10.1021/jp9085969
48. Yang ST, Cao L, Luo PG, et al. Carbon dots for optical imaging in vivo. J Am Chem Soc. 2009;131:11308–11309. doi:10.1021/ja904843x
49. Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi:10.1038/nrn1824
50. Xu L, Zhang H, Wu Y. Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chem Neurosci. 2014;5:2–13. doi:10.1021/cn400182z
51. Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412–a020435. doi:10.1101/cshperspect.a020412
52. Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi:10.1016/j.neuron.2008.01.003
53. Saraiva C, Praca C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J Control Release. 2016;235:34–47. doi:10.1016/j.jconrel.2016.05.044
54. Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood–brain barrier by nanoparticles. J Control Release. 2012;161:264–273. doi:10.1016/j.jconrel.2011.08.017
55. Du J, Xu N, Fan J, Sun W, Peng X. Carbon dots for in vivo bioimaging and theranostics. Small. 2019;15:1805087–1805102. doi:10.1002/smll.201805087
56. Zhou Y, Mintz KJ, Cheng L, et al. Direct conjugation of distinct carbon dots as lego-like building blocks for the assembly of versatile drug nanocarriers. J Colloid Interface Sci. 2020;576:412–425. doi:10.1016/j.jcis.2020.05.005
57. Panula P, Sallinen V, Sundvik M, et al. Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish. 2006;3:235–247. doi:10.1089/zeb.2006.3.235
58. Aceto J, Nourizadeh-Lillabadi R, Marée R, et al. Zebrafish bone and general physiology are differently affected by hormones or changes in gravity. PLoS One. 2015;10:e0126928–e01226969. doi:10.1371/journal.pone.0126928
59. Cai Q, Wang L, Deng G, Liu J, Chen Q, Chen Z. Systemic delivery to central nervous system by engineered PLGA nanoparticles. Am J Transl Res. 2016;8(2):749–764.
60. Bilensoy E. Cationic nanoparticles for cancer therapy. Expert Opin Drug Deliv. 2010;7:795–809. doi:10.1517/17425247.2010.485983
61. Jia J, Wang Z, Yue T, Su G, Teng C, Yan B. Crossing biological barriers by engineered nanoparticles. Chem Res Toxicol. 2020;33:1055–1060. doi:10.1021/acs.chemrestox.9b00483
62. Qin K, Zhang D, Ding Y, et al. Applications of hydrothermal synthesis of Escherichia coli derived carbon dots in in vitro and in vivo imaging and p-nitrophenol detection. Analyst. 2020;145:177–183. doi:10.1039/C9AN01753D
63. Han G, Zhao J, Zhang R, et al. Membrane-penetrating carbon quantum dots for imaging nucleic acid structures in live organisms. Angew Chem Int Ed. 2019;58(21):7087–7091. doi:10.1002/anie.201903005
64. Shoval A, Markus A, Zhou Z, et al. Anti-VEGF-aptamer modified C-dots—a hybrid nanocomposite for topical treatment of ocular vascular disorders. Small. 2019;15:1902776–1902785. doi:10.1002/smll.201902776
65. Wang XY, Lei R, Huang HD, et al. The permeability and transport mechanism of graphene quantum dots (GQDs) across the biological barrier. Nanoscale. 2015;7:2034–2041. doi:10.1039/C4NR04136D
66. Clark LF, Kodadek T. The immune system and neuroinflammation as potential sources of blood-based biomarkers for Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease. ACS Chem Neurosci. 2016;7:520–527. doi:10.1021/acschemneuro.6b00042
67. Ohnishi S, Takano K. Amyloid fibrils from the viewpoint of protein folding. Cell Mol Life Sci. 2004;61:511–524. doi:10.1007/s00018-003-3264-8
68. Mudher A, Lovestone S. Alzheimer’s disease - do tauists and baptists finally shake hands? Trends Neurosci. 2002;25:22–26. doi:10.1016/S0166-2236(00)02031-2
69. Savelieff MG, Lee S, Liu Y, Lim MH. Untangling amyloid-β, tau, and metals in Alzheimer’s disease. ACS Chem Biol. 2013;8:856–865. doi:10.1021/cb400080f
70. Liu Y, Xu LP, Dai W, Dong H, Wen Y, Zhang X. Graphene quantum dots for the inhibition of β amyloid aggregation. Nanoscale. 2015;7:19060–19065. doi:10.1039/C5NR06282A
71. Liu Y, Xu LP, Wang Q, Yang B, Zhang X. Synergistic inhibitory effect of GQDs–tramiprosate covalent binding on amyloid aggregation. ACS Chem Neurosci. 2017;9:817–823. doi:10.1021/acschemneuro.7b00439
72. Gong X, Zhang Q, Gao Y, Shuang S, Choi MMF, Dong C. Phosphorus and nitrogen dual-doped hollow carbon dot as a nanocarrier for doxorubicin delivery and biological imaging. ACS Appl Mater Interfaces. 2016;8:11288–11297. doi:10.1021/acsami.6b01577
73. Chung YJ, Kim K, Lee BI, Park CB. Carbon nanodot-sensitized modulation of Alzheimer’s β-amyloid self-assembly, disassembly, and toxicity. Small. 2017;13:1700983–1700991. doi:10.1002/smll.201700983
74. Chung YJ, Lee BI, Park CB. Multifunctional carbon dots as a therapeutic nanoagent for modulating Cu(II)-mediated β-amyloid aggregation. Nanoscale. 2019;11:6297–6306. doi:10.1039/C9NR00473D
75. Graham CA, Cloughesy TF. Brain tumor treatment: chemotherapy and other new developments. Semin Oncol Nurs. 2004;20:260–272. doi:10.1016/S0749-2081(04)00090-7
76. Gutman RL, Peacock G, Lu DR. Targeted drug delivery for brain cancer treatment. J Control Release. 2000;65:31–41. doi:10.1016/S0168-3659(99)00229-1
77. Dirks PB. Brain tumor stem cells: bringing order to the chaos of brain cancer. J Clin Oncol. 2008;26:2916–2924. doi:10.1200/JCO.2008.17.6792
78. Saul JM, Annapragada AV, Bellamkonda RV. A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. J Control Release. 2006;114:277–287. doi:10.1016/j.jconrel.2006.05.028
79. Zheng M, Ruan S, Liu S, et al. Self-targeting fluorescent carbon dots for diagnosis of brain cancer cells. ACS Nano. 2015;9:11455–11461. doi:10.1021/acsnano.5b05575
80. Qiao L, Sun T, Zheng X, Zheng M, Xie Z. Exploring the optimal ratio of D-glucose/L-aspartic acid for targeting carbon dots toward brain tumor cells. Mater Sci Eng C. 2018;85:1–6. doi:10.1016/j.msec.2017.12.011
81. Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi:10.1124/pr.54.4.561
82. Hettiarachchi SD, Graham RM, Mintz KJ, et al. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale. 2019;11:6192–6205. doi:10.1039/C8NR08970A
83. Liyanage PY, Zhou Y, Al-Youbi AO, et al. Pediatric glioblastoma target-specific efficient delivery of gemcitabine across the blood–brain barrier via carbon nitride dots. Nanoscale. 2020;12:7927–7938. doi:10.1039/D0NR01647K
84. Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi:10.1016/S1474-4422(18)30499-X
85. Lategan K, Fowler J, Bayati M, Fidalgo de Cortalezzi M, Pool E. The effects of carbon dots on immune system biomarkers, using the murine macrophage cell line RAW 264.7 and human whole blood cell cultures. Nanomaterials. 2018;8:388–401. doi:10.3390/nano8060388
86. Jiang K, Feng X, Gao X, et al. Preparation of multicolor photoluminescent carbon dots by tuning surface states. Nanomaterials (Basel). 2019;9:529–540. doi:10.3390/nano9040529
87. Zhang T, Zhu J, Zhai Y, et al. A novel mechanism for red emission carbon dots: hydrogen bond dominated molecular states emission. Nanoscale. 2017;9:13042–13051. doi:10.1039/C7NR03570E
88. Singh AV, Maharjan RS, Kanase A, et al. Machine-learning-based approach to decode the influence of nanomaterial properties on their interaction with cells. ACS Appl Mater Interfaces. 2021;13:1943–1955. doi:10.1021/acsami.0c18470
89. Dager A, Uchida T, Maekawa T, Tachibana M. Synthesis and characterization of mono-disperse carbon quantum dots from fennel seeds: photoluminescence analysis using machine learning. Sci Rep. 2019;9:14004–14015. doi:10.1038/s41598-019-50397-5
90. Han Y, Tang B, Wang L, et al. Machine-learning-driven synthesis of carbon dots with enhanced quantum yields. ACS Nano. 2020;14:14761–14768. doi:10.1021/acsnano.0c01899
91. Pandit S, Banerjee T, Srivastava I, Nie S, Pan D. Machine learning-assisted array-based biomolecular sensing using surface-functionalized carbon dots. ACS Sens. 2019;4:2730–2737. doi:10.1021/acssensors.9b01227
92. Xu Z, Wang Z, Liu M, Yan B, Ren X, Gao Z. Machine learning assisted dual-channel carbon quantum dots-based fluorescence sensor array for detection of tetracyclines. Spectrochim Acta A. 2020;232:118147–118155. doi:10.1016/j.saa.2020.118147
93. Singh AV, Rosenkranz D, Ansari MHD, et al. Artificial intelligence and machine learning empower advanced biomedical material design to toxicity prediction. Adv Intell Syst. 2020;2:2000084–2000102. doi:10.1002/aisy.202000084
94. Singh AV, Ansari MHD, Rosenkranz D, et al. Artificial intelligence and machine learning in computational nanotoxicology: unlocking and empowering nanomedicine. Adv Healthc Mater. 2020;9:1901862–1901880. doi:10.1002/adhm.201901862
95. Vikram Singh A, Sitti M. Targeted drug delivery and Imaging using mobile milli/microrobots: a promising future towards theranostic pharmaceutical design. Curr. Pharm. Des. 2016;22:1418–1428. doi:10.2174/1381612822666151210124326
96. Singh AV, Ansari MHD, Mahajan M, et al. Sperm cell driven microrobots—emerging opportunities and challenges for biologically inspired robotic design. Micromachines (Basel). 2020;11(4):448–464. doi:10.3390/mi11040448
97. Singh AV, Kishore V, Santomauro G, Yasa O, Bill J, Sitti M. Mechanical coupling of puller and pusher active microswimmers influences motility. Langmuir. 2020;36(19):5435–5443. doi:10.1021/acs.langmuir.9b03665
98. Singh AV, Sitti M. Patterned and specific attachment of bacteria on biohybrid bacteria-driven microswimmers. Adv Healthc Mater. 2016;5:2325–2331. doi:10.1002/adhm.201600155
99. Xu H, Yan L, Nguyen V, Yu Y, Xu Y. One-step synthesis of nitrogen-doped carbon nanodots for ratiometric pH sensing by femtosecond laser ablation method. Appl Surf Sci. 2017;414:238–243. doi:10.1016/j.apsusc.2017.04.092
100. Singh V, Kashyap S, Yadav U, et al. Nitrogen doped carbon quantum dots demonstrate no toxicity under in vitro conditions in a cervical cell line and in vivo in Swiss albino mice. Toxicol Res. 2019;8:395–406. doi:10.1039/C8TX00260F
101. Yang Z, Xu M, Liu Y, et al. Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale. 2014;6:1890–1895. doi:10.1039/C3NR05380F
102. Xu Q, Pu P, Zhao J, et al. Preparation of highly photoluminescent sulfur-doped carbon dots for Fe(iii) detection. J Mater Chem A. 2015;3:542–546. doi:10.1039/C4TA05483K
103. Ma Z, Ming H, Huang H, Liu Y, Kang Z. One-step ultrasonic synthesis of fluorescent N-doped carbon dots from glucose and their visible-light sensitive photocatalytic ability. New J Chem. 2012;36:861–864. doi:10.1039/c2nj20942j
104. Wang H, Sun P, Cong S, et al. Nitrogen-doped carbon dots for “green” quantum dot solar cells. Nanoscale Res Lett. 2016;11:27–32. doi:10.1186/s11671-016-1231-1
105. Ahmed A, Ghallab EH, Shehata M, Zekri ARN, Ahmed OS. Impact of nano-conjugate on Drosophila for early diagnosis of Alzheimer’s disease. Nanotechnology. 2020;31:365102–365113. doi:10.1088/1361-6528/ab7535
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
