Back to Journals » Journal of Inflammation Research » Volume 15
Carbon 60 Dissolved in Grapeseed Oil Inhibits Dextran Sodium Sulfate-Induced Experimental Colitis
Authors Lazcano-Silveira R, Jia X , Liu K, Liu H, Li X, Hui M
Received 23 March 2022
Accepted for publication 14 May 2022
Published 25 July 2022 Volume 2022:15 Pages 4185—4198
DOI https://doi.org/10.2147/JIR.S366886
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Rayko Lazcano-Silveira,1 Xiaoxiao Jia,1 Kaixuan Liu,2 Honggang Liu,2 Xinrong Li,2 Mizhou Hui1
1College of Life Sciences, Northeast Agricultural University, Harbin, Heilongjiang, People’s Republic of China; 2College of Animal Science and Technology, Qingdao Agricultural University, Qingdao, Shandong, People’s Republic of China
Correspondence: Mizhou Hui, College of Life Sciences, Northeast Agricultural University, 50 Mucai Street, Xiangfang District, Harbin, Heilongjiang, 150038, People’s Republic of China, Tel +86 13484005199, Email [email protected]
Introduction: Carbon 60 (C60) and its derivatives have various biological applications. In our laboratory, we have demonstrated that C60 dissolved in grape seed oil (C60-Oil) has antioxidant and anti-inflammatory properties; however, the effectiveness of this formulation to treat diseases of the intestinal tract and specifically ulcerative colitis has not been studied. In this study, we intend to explore the effects of C60-Oil against experimental ulcerative colitis induced by Dextran Sulfate Sodium (DSS) in rats and a human colorectal cell line, HT-29.
Methods: The rats were randomly distributed into three groups: a negative control group with no induced damage and two other groups were treated with DSS to induce UC for seven days: one as untreated control and the other group treated with C60-Oil 3 mg/kg/day. We quantified the clinical manifestations of the disease, body weight, colon weight, microscopic damage score, and colonic content of IL-6, TNF-alpha, IL-1B, and IL-10. As part of the cell studies, HT-29 cells were pretreated with C60-Oil at different concentrations (0.1, 1, 5, 10, 50, 30 μg/mL) and then stimulated with DSS (10 μg/mL). We measured the levels of IL-8 and NO secreted in the medium and the intracellular levels of ROS.
Results: Oral treatment with C60-Oil significantly prevented the change in body weight, reduced most of the clinical signs of the disease, colon weight, microscopic damage score, and considerably improved the profile of cytokines analyzed. The pretreatment of HT-29 cells also protected the cells from the action of DSS as it reduced the levels of IL-8, NO, and ROS.
Conclusion: According to our results, we can suggest C60-Oil, as a formulation with pharmacological potential for treating ulcerative colitis.
Keywords: carbon 60, DSS, experimental colitis, inflammation, oxidative stress
Introduction
Ulcerative colitis (UC) is one of the most common Inflammatory Bowel Diseases (IBD). Historically UC has been associated with developed and industrialized western countries, but a rise has been seen in many Asian countries.1 The main clinical manifestations of UC include blood in the stool, fecal urgency, diarrhea, mucus in the stool, weight loss, abdominal pain, and asthenia.2 UC is characterized by massive neutrophil infiltration that presents with hyperemia, hemorrhage, and ulceration. Activated neutrophils produce and release reactive oxygen species such as hydroxyl radicals and superoxide anions, which cause tissue damage. Other inflammatory mediators (cytokines and chemokines) also play an important role in developing and progressing this disease entity.3
There is no known cure yet for UC, the therapeutic strategy to treat UC includes using specific anti-inflammatory drugs such as aminosalicylates, corticosteroids, and antibodies that act on tumor necrosis factor-alpha (TNFα).4 However, these drugs can cause serious adverse effects such as opportunistic infections, diabetes mellitus, hypertension, ocular effects (glaucoma and cataracts), psychiatric complications, hypothalamic-pituitary-adrenal axis suppression, increased fracture risk, and some nephrotoxic events.5 Its treatment does not include the use of classic non-steroidal anti-inflammatory drugs (NSAIDs) because these agents can cause gastrointestinal toxicity, including mucosal injury.6,7 For these reasons, the search for other safe and effective therapeutic strategies for UC management is justified.
It is known that together with the various applications in the area of nanotechnology, carbon 60 (fullerene, C60) and its derivatives have many biological applications.8,9 Fullerenes can also be considered an effective antioxidant agent due to their redox properties and their remarkable ability to absorb free radicals.10 Studies have shown that the carbon 60 dissolved in vegetable oils is a powerful antioxidant that has various therapeutic effects; the oral administration of C60 dissolved in olive oil in repeated doses to rats not only does not entail chronic toxicity but almost doubles their life expectancy in these animals.11
In previous results obtained in our laboratory, soon to be published, the pharmacological potential of C60 dissolved in grape seed oil (C60-Oil) as an anti-inflammatory and antioxidant is demonstrated. C60-Oil inhibits the release of TNF-α, the migration and production of ROS by human neutrophils; it also has a significant scavenging effect on superoxide free radicals and DPPH free radicals, and oral administration of C60-Oil strongly reduced the serum C-Reactive Protein (CRP) in dogs. In these studies, we also demonstrate that these functions are due to C60; with the administration of grapeseed oil only, the results that we highlight were not seen.
The main objective of the research is to analyze whether C60-Oil can serve as a therapy to prevent the alterations observed in experimental models of UC induced by Sodium Dextran Sulfate (DSS) in rats and in human intestinal epithelial cells. The study focuses on the macroscopic, microscopic and biochemical changes in UC in response to C60-Oil as treatment. The results could suggest that this compound can be part of the daily food intake in all people with a family history of ulcerative colitis or in all people in general as preventive dietary therapy.
Materials and Methods
C60-Oil Preparation, Others Chemicals and Reagents
C60 dry powder (Suzhou Dade Carbon Nano Technology Co., Ltd., Suzhou, Jiangsu China), grape seed oil (Pinli Food Co., Ltd., Beijing, China). C60 dry powder with a purity of >0.99 was dissolved in grape seed oil (hereinafter C60-Oil). To prepare C60-Oil stock solution with a 3 mg/mL concentration, C60-Oil was placed in a magnetic stirrer and mixed thoroughly for 9 days at room temperature. The stock solution was sterilized by passing through a 0.22-µM filter and then stored. Dextran sulfate sodium (DSS) was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Rnase-free neutral formalin fixative was purchased from Shanghai Shangbao Biotechnology Co., Ltd. (Shanghai, China).
Animal Experimental Design
Six-week-old male Wistar rats (16–20g) were purchased from Beijing Weitonglihua Laboratory Animal Technology Co., Ltd. (Beijing, China). The rats were kept in a vivarium at a temperature of 23±1°C and exposed to 12 hour light/dark cycles, with free access to water and food. The rats were allowed to adapt to the vivarium environment for one week before starting the experiment. The animals were divided into three groups of 6 rats each: Control group, untreated animals; the DSS group and the C60 group, both groups formed by the animals who had free access to water containing 5% DSS, the latter group was treated with intragastric administration of C60-Oil 1mL per kg of animal weight, corresponding to 3mg of C60 per kg in each dose. The duration of the experiment was seven days. The Institutional Animal Care and Use Committee of Qingdao Agricultural University approved the protocol of this study. All experimental animal tests were carried out according to the 2016 China Laboratory Animal Standards and other related regulations in the Animal Welfare Law.
Bodyweight Measurement and Disease Activity Index
The changes in body weight of the rats were measured and recorded daily by using a device coupled to a Sartorius weight (QUINTIX2102-1S, Gottingen, Germany). The disease activity index (DAI) was evaluated to assess the severity of UC. The consistency of the stool and the existence of blood in the stool, as common clinical signs of this disease, were recorded daily according to previous methods.12 DAI score was calculated as a sum of parameters listed in Table 1.
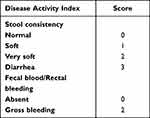 |
Table 1 Disease Activity Index of DSS-Induced Ulcerative Colitis |
Preparation of Distal Colon Samples
One day after the end of the experiment, all the rats were euthanized by cervical dislocation. The colon samples were collected according to an established method;13 briefly, 10 cm of colon samples were taken at the starting point of 3 cm from the anus. The samples were washed by PBS injection. Then, the samples were divided into two 5 cm segments. The proximal 5 cm segments were formalin-fixed for histological examinations, and the distal colon samples were weighed and homogenized in 0.1 mM Tris-HCl buffer (pH=7) at a ratio of 1g tissue vs 9 mL buffer. The homogenates were then centrifuged at 10,000 rpm for 10 minutes, and the supernatant was stored at minus 80°C for measurement of colonic cytokines levels.
Histological Damage Score Evaluation
The colonic tissue samples were fixed in 10% Neutral formalin and impregnated into paraffin to prepare 5 μm sections. After being stained with hematoxylin-eosin, they were examined under the light microscope (Nade Trinocular Head Microscope XSZ-N107CCD, Hangzhou, Zhejiang, China). The histological lesions were scored using the scale described by Molina-Cuevas et al14 (Table 2).
 |
Table 2 Criteria Used to Determine Ulcerative Colitis Histological Scoring |
Cell Culture
HT-29 cells (Procell Life Science & Technology Co., Ltd., Wuhan, Hebei, China) were cultured in McCoy’s 5A culture medium (Procell Life Science & Technology Co., Ltd., Wuhan, Hebei, China) supplemented with 10% Fetal Bovine serum (FBS, Sangon Biotech Co., Ltd., Shanghai, China) and 1% Penicillin-Streptomycin (P/S, Gibco, Thermo Fisher Scientific, Shanghai, China). Other reagents used in the treatment of the cells culture were: Phosphate-buffered saline (PBS, Sangon Biotech Co., Ltd., Shanghai, China), Trypsin (Gibco, Thermo Fisher Scientific, Shanghai, China), Dextran sulfate sodium (SDS, Shanghai Macklin Biochemical Co., Ltd, Shanghai, China).
Cell Viability Studies
For viability studies, 1×104 cells were seeded in each well of a 96-well plate. They were incubated at 37°C and 5% CO2. The cells were treated with C60-Oil or DSS for 24, 48 or 72 hours depending on the study. Viability percentages were measured according to the Cell counting Kit-8 colorimetric assay (CCK-8, Sigma-Aldrich, Darmstadt, Hesse, Germany). Morphological changes of cells were observed under an inverted electronic microscope (ZEISS Axio Vert. A1 FL, Oberkochen, Germany).
Measurement of Cytokine Levels and Nitric Oxide
Cytokine levels throughout the experiment were determined using enzyme-linked immunosorbent assay (ELISA) Kits. Kits for Cytokine-6 (IL-6), Tumor necrosis factor-alpha (TNF-α), and Cytokine-10 (IL-10) (Shanghai Bohu Biological Technology Co., Ltd., Shanghai, China), and Cytokine-1beta (lL-1β) (Ruixin Biological Co., Ltd., Quanzhou, Fujian, China) was used. In the case of cell experiments, 2×105 cells were seeded in each well of a 24-well plate. Depending on the experimental group, the cells were treated with different concentrations of C60-Oil for 24 hours and then stimulated with DSS for 12 hours at 37°C and 5% CO2. The supernatants were collected and preserved at −20°C until measurements were made. IL-8 levels were measured using a commercial kit (Shanghai Bohu Biotechnology Co., Ltd. Shanghai, China). For quantifying nitric oxide (NO) levels, a detection kit (Beyotime Biotechnology, Shanghai, China) was used. All samples were tested in duplicate. Optical density was measured using an automatic plate reader (Biotek, ELx808, Beijing, China). The measurements on the colonic homogenates are expressed in pg/g of tissue and on the cell culture medium in mg/mL.
Determination of Intracellular Levels of ROS
For this assay, 2×105 cells were seeded in each well of a black 6-well plate. They were incubated at 37°C and 5% CO2. The cells of the experimental group were pretreated with C60-Oil at a concentration of 5μg/mL for 30 minutes and then stimulated with DSS for one hour. The cells’ culture medium was replaced by serum-free medium, and they were treated for 30 minutes with 10μM of 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA, Solarbio Science & Technology Co., Ltd., Beijing, China), dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich, Darmstadt, Hesse, Germany). Subsequently, the cells were washed twice with serum-free medium and finally PBS was added for the subsequent measurement of the generation of intracellular ROS in an inverted fluorescence microscope (ZEISS Axio Vert.A1, Oberkochen, Germany). After taking representative images of each experimental group, the images were analyzed with the software ImageJ (1.53k, Wisconsin, United States) to examine the intensity of the pixels and make an approximate quantitative study of the intensity of fluorescence in the samples.
Statistical Analysis
The data was processed in the software GraphPad Prism8.0.1 (San Diego, California, United States) for statistical analysis. Data are shown as the mean ± standard deviation. Significant differences between each sample were determined using the Student’s t-test. Mann–Whitney U-test were used to compare the DAI and the histological damage scores. Values of p<0.05, p<0.01, and p<0.001 were considered significant.
Results
Effect of C60-Oil on Body Weight of Wistar Rats
In the first four days of experimentation, no significant differences were observed in the weight of the animals (Figure 1). All the rats in which the administration of DSS induced colitis did not show a weight gain; at the end of the experiment, the average weight did not present differences with the initial weight. From day five onwards, the differences turned out to be statistically significant with the control group rats (p<0.05; p<0.01), which did gain weight throughout the study. The administration of C60-Oil had a positive effect on the weight of the rats that were also administered DSS. C60-Oil restored the weight gain in the animals that from the 6th day presented significant differences with the rats that were only administered DSS (p<0.05).
Effect of C60-Oil on Disease Activity Index of DSS-Induced Ulcerative Colitis
DSS administration produced a rapid onset of classic UC symptoms. DSS-treated rats showed higher DAI scores than control group rats, statistically significant (p < 0.01). No animal in the control group presented diarrhea or rectal bleeding, with a score of zero. Intragastric treatment with C60-Oil significantly decreased the DAI score (p <0.05); in the rats to which it was administered, the appearance of the symptoms of the disease was reduced. Figure 2 shows all the values of the DAI score.
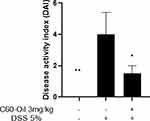 |
Figure 2 Disease activity index (DAI) of the model of DSS-induced colitis in Wistar rats. The data are presented as the mean ± SD *p<0.05;**p<0.01 statistical comparison with DSS group. |
Effect of C60-Oil on DSS-Induced Colonic Inflammation
A characteristic of intestinal inflammation is the thickening of the large intestine, depending on the degree of inflammation, swelling, congestion, or edema of the colon is evident. These characteristics can be quantified by measuring the 5cm weight of the distal colon in the rats (Figure 3). Rats receiving DSS demonstrated significantly increased colon weight than rats in the control group (p < 0.05). Treatment with C60-Oil significantly decreased colonic weight (p < 0.05), indicating that this treatment attenuated intestinal inflammation in rats caused by DSS.
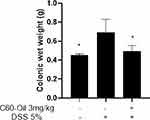 |
Figure 3 Wet weight of 5 cm of distal colon samples of Wistar rats of Control, DSS and C60 groups. Values are presented as the mean ± standard deviation.*p<0.05 compared with DSS group. |
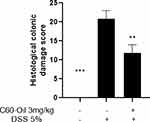
Effect of C60-Oil on Colonic Histological Damage
Previously described microscopic scoring evaluated the magnitude of colonic histological damage in the DSS-induced UC (Figure 4). Colonic tissue samples from Control group rats exhibited typical mucosal tissue architecture with regular and well-defined crypts; the thickness of the outer submucosal and muscular layer was also expected, corresponding to a histological damage score of zero (Figure 5A). In contrast, colonic tissue samples from rats treated with DSS alone showed alterations in the mucosal epithelium and architecture, with partial or complete loss of intestinal crypts, in some cases, ulcerations, and marked infiltration of immune cells in the lamina propria and submucosa (Figure 5B). The histological damage score in this group was significantly higher than the numbers in the Control group (p<0.001). Compared to the DSS group, C60-Oil treatment significantly attenuated DSS-induced colonic lesions (p<0.01), showing a reduction in mucosal changes and cellular infiltrate (Figure 5C).
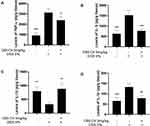
Effect of C60-Oil on Colonic Tissue Cytokine Content
As part of the molecular studies, the content of specific cytokines related to the intestinal inflammatory process was analyzed in the colonic homogenates of rats (Figure 6). The levels of TNF-α, IL-6, and IL-1β, all pro-inflammatory cytokines, were increased; in the case of IL-10, interleukin with anti-inflammatory properties, the levels were reduced; in both cases compared to the Control group. The administration with C60-Oil showed statistically significant improvements in the profile of the cytokines analyzed, decreased the levels of inflammatory mediators, and increased those of the inhibitor of the inflammatory process analyzed.
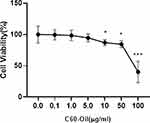
Effects of C60-Oil on Cytotoxicity on HT-29 Cells
To analyze the effect of C60-Oil on HT-29 cells, the effect of this compound on cell viability was analyzed. Cells were exposed for 48 hours to different doses of this compound (0.1, 1, 5, 10, 50, 100 μg/mL), followed by assays using CCK-8, as described in materials and methods; data are expressed in % relative to untreated cells (Figure 7). It was observed that concentrations greater than 5 μg/mL significantly reduced the percentage of viability; at lower concentrations, cell viability was not modified. Therefore, it was decided to use these concentrations of C60-Oil (0.1, 1, 5 μg/mL) for subsequent experiments.
Cytoprotective Effects of C60-Oil on Induced HT-29 DSS Cells
Studies have been found in the literature that has also used DSS as a proinflammatory inducing effect in different cell lines, so before continuing with the tests with C60-Oil, we search for the optimal dose of DSS. For this, the effect of different concentrations of DSS on the cell viability of HT-29 cells was analyzed. The cells were stimulated for 72 hours with doses of 0.1, 1.0, 10, 50 μg/mL of DSS, and through CCK-8 was possible to estimate the number of cells. As seen in Figure 8, it was possible to determine that concentrations of DSS greater than 10 μg/mL significantly reduced cell viability. This concentration was used for subsequent experiments to examine the effects of C60 oil on HT-29 cells.
As a preliminary study to determine the protective effects of C60-Oil on HT-29 cells against the action of DSS, using the CCK-8 assay, the number of cells after treatment with both substances was estimated (Figure 9). Different concentrations of C60-Oil (0.1, 1, 5 μg/mL) were used to pre-treat the cells for 24h before stimulation with DSS (10 μg/mL) for another 24h, then cell proliferation was estimated.
Cell pretreatment with all concentrations of C60-Oil prevented DSS-induced damage; a significant increase in cell number was observed compared to cells that were only treated with DSS. Observations of cell density in each culture under the electron microscope corroborate these results (Figure 10).
Effects of C60-Oil on the Inhibition of IL-8 and NO Secretion in HT-29 DSS Induced Cells
Once the cytoprotective effect of C60-Oil had been determined against stimulation with DSS, we analyzed its effect on IL-8, a pro-inflammatory immune response marker, and NO levels, which positively correlated with the severity of IBD. ELISA determined the concentrations of both molecules secreted into the culture medium (Figure 11). We observed that DSS significantly induced IL-8 and NO secretion compared to the levels seen in unstimulated cells. This increase was prevented by pretreatment of the cells with C60-Oil.
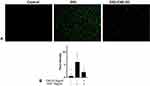
Effects of C60-Oil on ROS Levels in HT-29 Cells-DSS Induced
To demonstrate the antioxidant capacity of C60-Oil, we evaluated its effect on the reduction of intracellular ROS produced by stimulation with DSS. Cells were pretreated for 30 minutes with 5 µg/mL C60-Oil and subsequently stimulated with DSS (10 µg/mL) for 1 hour. Intracellular ROS levels were estimated using the DCFH-DA assay and fluorescence microscopy (Figure 12).
By observing the fluorescence microscopy images, it is possible to note that the stimulation with DSS causes a high production of intracellular ROS; pretreatment with C60-Oil considerably reduced the effects of DSS. Analyzing the intensity of the pixels in the images, the fluorescence level of each image obtained can be compared numerically.
Discussion
Currently, there is no curative surgical or pharmacological treatment for UC. Due to the failure of currently available medications to treat this disease, 10–20% of patients require colectomy. Since the main character in UC is inflammation, new therapies are necessary that limit and control the scope of this inflammatory process and that also meet the characteristics of ideal drugs: practical, easy to administer, free from side effects, and it is aimed at several etiopathogenic factors and has a low cost.
Previous experimental studies have shown that applying an aqueous colloidal solution of C60 fullerene to rats that underwent acetic acid-induced UC restored the integrity of the colonic mucosa and the epithelial barrier.15,16 However, the effect of C60 dissolved in any vegetable oil has not been studied on the digestive tract epithelium.
C60-Oil, at the cellular and molecular level, has shown potential results in the treatment of inflammatory diseases related to the release of reactive oxygen spices and the release of TNF-alpha from neutrophils. Based on the results of these previous studies carried out in our laboratory, we can suggest a possible protective effect of C60-Oil in UC.
The administration of DSS in experimental animals has been widely accepted as a suitable model for translating to UC in humans to identify new therapies and is one of the most widely used. Its reproducibility, accessibility, similarity with the clinical and histological picture of UC in humans have been the determining factors that have prompted its choice as a model to be used.
DSS alters the epithelial barrier causing acute clinical symptoms, weight loss, erosion of the colon, and loss of crypts; it induces severe oxidative damage and consequent inflammation, characteristics that are observed in UC since the increased permeability allows the massive interaction of the intestinal flora with the system immune system of the host, a primary factor in the initiation of the disease.17 It is also known that DSS increases the formation of the mutagenic oxidative DNA damage biomarker 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oxo dG) in colonic cells of rats in vivo.18 This oxidative damage is a common characteristic of apoptosis. DSS-induced ROS generation has been proposed as a key to the activation of p38 MAPK and NF-κB and signaling cascades related to the induction of pro-inflammatory genes, including IL-1β, TNF-α, IL-6, and IL-8.19,20
In the present study, the DSS induced-UC in the rats of the DSS group caused marked clinical symptoms (abnormal body weight, presence of diarrhea, and rectal bleeding), macroscopic and microscopic damage of the colonic mucosa, as well as an increase in levels of pro-inflammatory cytokines. In contrast, the animals in the control group did not present such symptoms nor damage to the colonic tissue, and the levels of pro-inflammatory cytokines were significantly lower than the DSS group. In the experiment with HT-29 cells, DSS administration caused a reduction in cell viability and increased IL-8, NO, and intracellular ROS levels; all these indicators with significant differences with the group of control cells not treated with DSS. The damage indicators resulting from the action of DSS measured in the present study, both in the animal experiment and in the cell experiment, behaved as described by other authors for the model, which validates the present results.17,21
The C60-Oil treatment in the animals and the HT-29 cells protected the action of DSS in both cases. In the animal experiment, C60-Oil significantly reduced clinical symptoms and histological lesion scores; it also increased the viability of HT-29 cells. It should be noted that this formulation was toxic to cells at high concentrations, greater than 10mg/mL.
Pro-inflammatory cytokines play an essential role in the pathogenesis of UC, especially TNF-alpha, IL-6, IL-1B, and IL-8. TNF-α is a pro-inflammatory mediator that plays an integral role in the pathogenesis of inflammatory bowel disease, stimulates adhesion molecules that lead to neutrophil recruitment, promotes tissue remodeling, and increases cell permeability resulting in impaired barrier function colonic epithelium.22 In 2007, a group of researchers demonstrated with the drug triptolide that by inhibiting IL-6, it is possible to prevent inflammation in a rodent model of ulcerative colitis.23 IL-1B also promotes inflammatory infiltration, edema, and necrosis of colon tissue; a recent study promotes treatment with blockers of this cytokine to inhibit the course of this disease.24 High levels of IL-8 in the rectal dialysate of patients with ulcerative colitis also show a biological role for IL-8 in colonic inflammation;25 treatment with an IL-8 antagonist, together with a probiotic, shows good therapeutic potential since it confers protection against IBD, particularly in UC.26 On the contrary, mutations, and polymorphisms in the gene for IL-10, anti-inflammatory cytokine, significantly increase the risk of suffering from ulcerative colitis.27
In both experiments, C60-Oil improved the indicators in the cytokine profile. The significantly high levels of TNF-alpha, IL-6, IL-1B, and IL-8 and the reduced IL-10 values observed are explained by the dysregulation of the immune response of the colon mucosa as a result of the destruction of the epithelial barrier caused by the administration of DSS. Our results show that treatment with C60-Oil suppresses the increase in proinflammatory cytokines, TNF-alpha, IL-6, IL-1B, and IL-8, also raises IL-10 levels. These values in the levels of the cytokines studied, modulated by C60-Oil, can be explained by the ability of C60 fullerenes to scavenge free radicals, restore antioxidant protection of tissues, and inhibit inflammatory infiltration. In studies with HT-29 cells, we evidenced the effectiveness of C60-Oil to reduce and restore NO and ROS levels to normal levels. Although we did not show the mechanism involved in generating these molecules in DSS-stimulated cells, our results imply that C60-Oil can inhibit IL-8 expression by blocking DSS-induced ROS generation, as seen in other studies.28
Similar results were obtained by Byelinska et al that reported the application of an aqueous colloid solution of C60 at a lower concentration effectively treated DSS-induced ulcerative colitis; this formulation reduced inflammation in the colon and restored the integrity of the mucosa.15 Another group of researchers using another aqueous solution of C60 in another experimental model of UC, demonstrated its effectiveness for treating the disease, indicating that this molecule does not cause apparent toxicity in treated rodents.16
Taken together, the treatment with C60-Oil effectively treated DSS-induced ulcerative colitis and also had a protective effect against DSS-stimulated cell damage. The results of this study indicate that C60 dissolved in grapeseed oil could be successfully delivered into the cell interior, thus supporting the assumption that C60 not dissolved in aqueous colloidal solutions may also be a promising candidate for drug for the treatment of ulcerative colitis and other intestinal diseases.
Conclusion
Treatment with C60 dissolved in grape seed oil significantly reduced damage markers resulting from DSS administration as an experimental model of ulcerative colitis in rats and HT-29 cells. This treatment prevented weight loss, macroscopic and histological damage to the colonic tissue, and at the molecular level, managed to reduce the levels of inflammatory cytokines, both in animals and in cells; in the cells model, provided cytoprotection and reduced NO and ROS levels. The results suggest that a treatment based on C60 dissolved in grapeseed oil could be used to treat ulcerative colitis.
Acknowledgments
This research was carried out in the laboratories of the Science and Technology Center of Qingdao Agricultural University. We would like to thank all the faculty in the institution for supporting this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yang SK. How Does the Epidemiology of Inflammatory Bowel Disease Differ between East and West? A Korean Perspective. Inflamm Intest Dis. 2017;2(2):95–101.
2. Nobrega VG, Silva INN, Brito BS, Silva J, Silva M, Santana GO. The Onset of Clinical Manifestations in Inflammatory Bowel Disease Patients. Arq Gastroenterol. 2018;55(3):290–295.
3. Muthas D, Reznichenko A, Balendran CA, et al. Neutrophils in ulcerative colitis: a review of selected biomarkers and their potential therapeutic implications. Scand J Gastroenterol. 2017;52(2):125–135.
4. Ben-Horin S, Andrews JM, Katsanos KH, et al. Combination of corticosteroids and 5-aminosalicylates or corticosteroids alone for patients with moderate-severe active ulcerative colitis: a global survey of physicians’ practice. World J Gastroenterol. 2017;23(16):2995–3002.
5. Curkovic I, Egbring M, Kullak-Ublick GA. Risks of inflammatory bowel disease treatment with glucocorticosteroids and aminosalicylates. Dig Dis. 2013;31(3–4):368–373.
6. Klein A, Eliakim R. Non Steroidal Anti-Inflammatory Drugs and Inflammatory Bowel Disease. Pharmaceuticals. 2010;3(4):1084–1092.
7. Smolović B, Vučković L, Borozan S, Vukčevič B. Nonsteroidal anti-inflammatory drug-induced colopathy: an uncommon cause of positive immunochemical faecal occult blood test in the program for colorectal cancer screening. Vojnosanitetski pregled. 2021;78:1.
8. Innocenzi P, Stagi L. Carbon-based antiviral nanomaterials: graphene, C-dots, and fullerenes. A perspective. Chem Sci. 2020;11(26):6606–6622.
9. Zhang Y, Zhang H, Zou Q, Xing R, Jiao T, Yan X. An injectable dipeptide-fullerene supramolecular hydrogel for photodynamic antibacterial therapy. J Mater Chem B. 2018;6(44):7335–7342.
10. Gudkov SV, Guryev EL, Gapeyev AB, et al. Unmodified hydrated capital ES, Cyrillic 60 fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen species and protect mice against injuries caused by radiation-induced oxidative stress. Nanomedicine. 2019;15(1):37–46.
11. Baati T, Bourasset F, Gharbi N, et al. The prolongation of the lifespan of rats by repeated oral administration of [60]fullerene. Biomaterials. 2012;33(19):4936–4946.
12. Qian B, Wang C, Zeng Z, Ren Y, Li D, Song JL. Ameliorative Effect of Sinapic Acid on Dextran Sodium Sulfate- (DSS-) Induced Ulcerative Colitis in Kunming (KM) Mice. Oxid Med Cell Longev. 2020;2020:8393504.
13. Balaha M, Kandeel S, Elwan W. Garlic oil inhibits dextran sodium sulfate-induced ulcerative colitis in rats. Life Sci. 2016;146:40–51.
14. Molina-Cuevas V, Ravelo-Calzado Y, Zamora-Rodriguez Z, et al. [Effects in rats of bee-wax alcohols (D-002) on ulcerative colitis induced by dextran sulfate and ethanol]. Rev Peru Med Exp Salud Publica. 2017;34(2):176–182. Chinese.
15. Byelinska IV, Kuznietsova HM, Dziubenko NV, et al. Effect of capital ES, Cyrillic 60 fullerenes on the intensity of colon damage and hematological signs of ulcerative colitis in rats. Mater Sci Eng C Mater Biol Appl. 2018;93:505–517.
16. Liao X, Zhao Z, Li H, et al. Fullerene nanoparticles for the treatment of ulcerative colitis. Sci China Life Sci. 2021;1:54.
17. Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:1525 11–15 25 14.
18. Tardieu D, Jaeg JP, Cadet J, Embvani E, Corpet DE, Petit C. Dextran sulfate enhances the level of an oxidative DNA damage biomarker, 8-oxo-7,8-dihydro-2’-deoxyguanosine, in rat colonic mucosa. Cancer Lett. 1998;134(1):1–5.
19. Epstein J, Docena G, MacDonald TT, Sanderson IR. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1beta and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br J Nutr. 2010;103(6):824–832.
20. Jiang Y, Lu X, Man C, et al. Lactobacillus acidophilus induces cytokine and chemokine production via NF-kappaB and p38 mitogen-activated protein kinase signaling pathways in intestinal epithelial cells. Clin Vaccine Immunol. 2012;19(4):603–608.
21. Lee Y-M, Lee K-S, Kim D-K. Aqueous Extract of Schizandra chinensis Suppresses Dextran Sulfate Sodiuminduced Generation of IL-8 and ROS in the Colonic Epithelial Cell Line HT-29. Natural Product Sci. 2009;15(4):185–191.
22. Sands BE, Kaplan GG. The role of TNFalpha in ulcerative colitis. J Clin Pharmacol. 2007;47(8):930–941.
23. Zhang H, Chen W. Interleukin 6 inhibition by triptolide prevents inflammation in a mouse model of ulcerative colitis. Exp Ther Med. 2017;14(3):2271–2276.
24. Yin Q, Pi X, Jiang Y, et al. An immuno-blocking agent targeting IL-1beta and IL-17A reduces the lesion of DSS-induced ulcerative colitis in mice. Inflammation. 2021;44(5):1724–1736.
25. Keshavarzian A, Fusunyan RD, Jacyno M, Winship D, MacDermott RP, Sanderson IR. Increased interleukin-8 (IL-8) in rectal dialysate from patients with ulcerative colitis: evidence for a biological role for IL-8 in inflammation of the colon. Am J Gastroenterol. 1999;94(3):704–712.
26. Walana W, Ye Y, Li M, et al. IL-8 antagonist, CXCL8 (3-72)K11R/G31Pcoupled with probiotic exhibit variably enhanced therapeutic potential in ameliorating ulcerative colitis. Biomed Pharmacother. 2018;103:253–261.
27. Moran CJ, Walters TD, Guo CH, et al. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflamm Bowel Dis. 2013;19(1):115–123.
28. Ruan W, Engevik M, Chang-Graham A, Hyser J, Versalovic J. 7 Lactobacillus reuteri suppresses pro-inflammatory driven reactive oxygen species in vitro in human intestinal epithelial cells and in vivo in a TNBS colitis mouse model. Inflamm Bowel Dis. 2020;26(Supplement_1):S41–S41.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.